Abstract
1 The influence of hormonal and neuronal factors on adrenoceptors mediating increased cardiac force and rate of contraction were studied in rat isolated atria. The pharmacological properties of these receptors were deduced from the relative potencies of agonists and from the effects of selective α- and β-adrenoceptor antagonists. The numbers and affinities of α- and β-adrenoceptors were also determined by radioligand binding to ventricular membrane fragments.
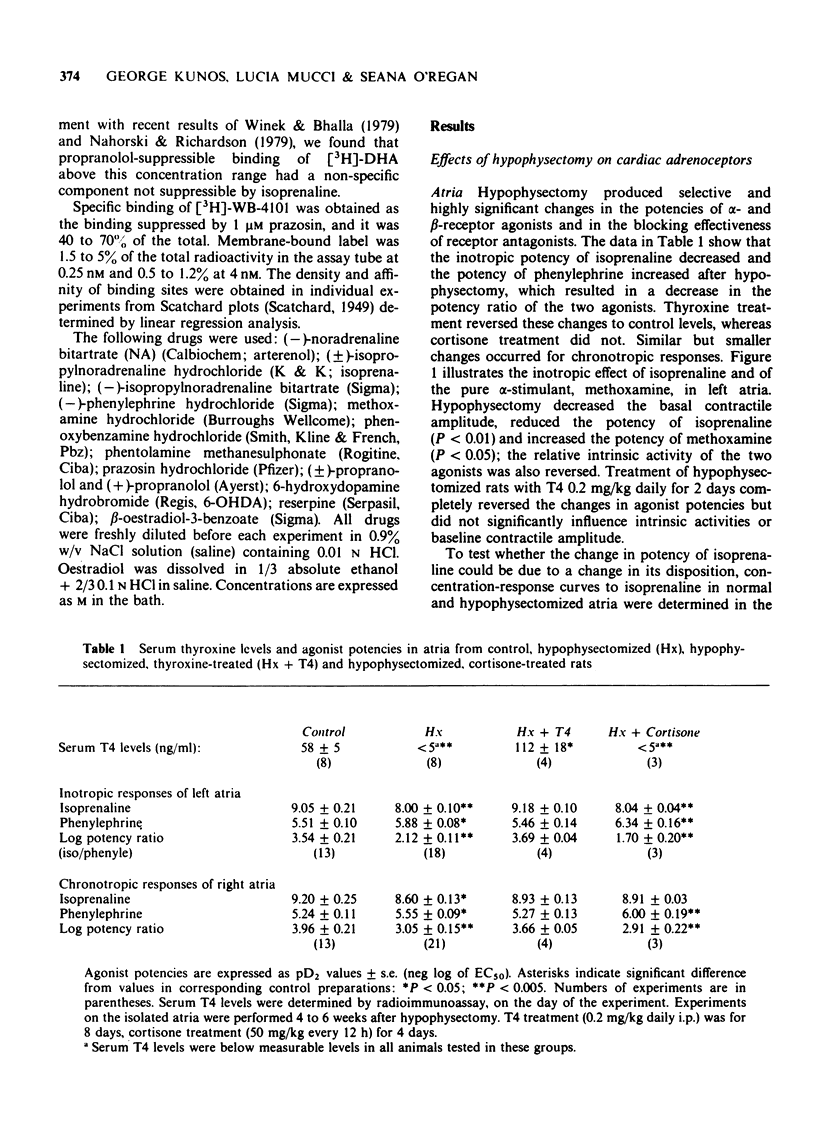
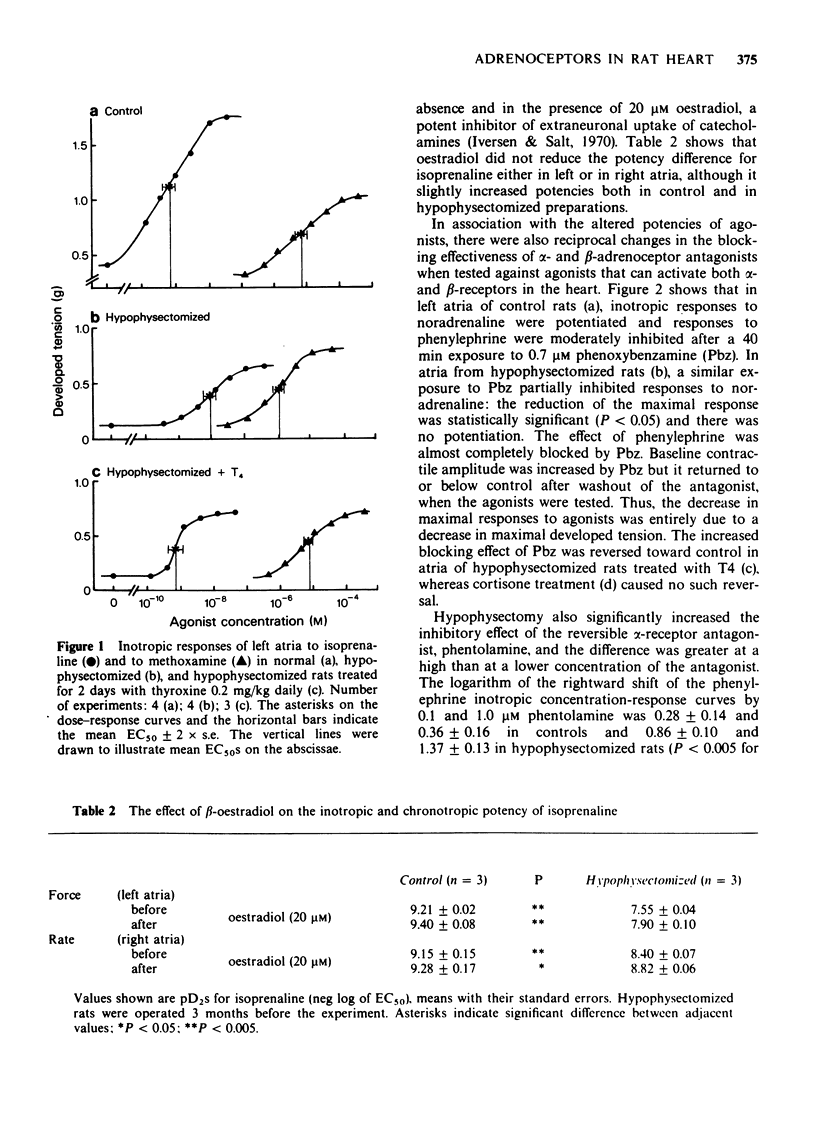
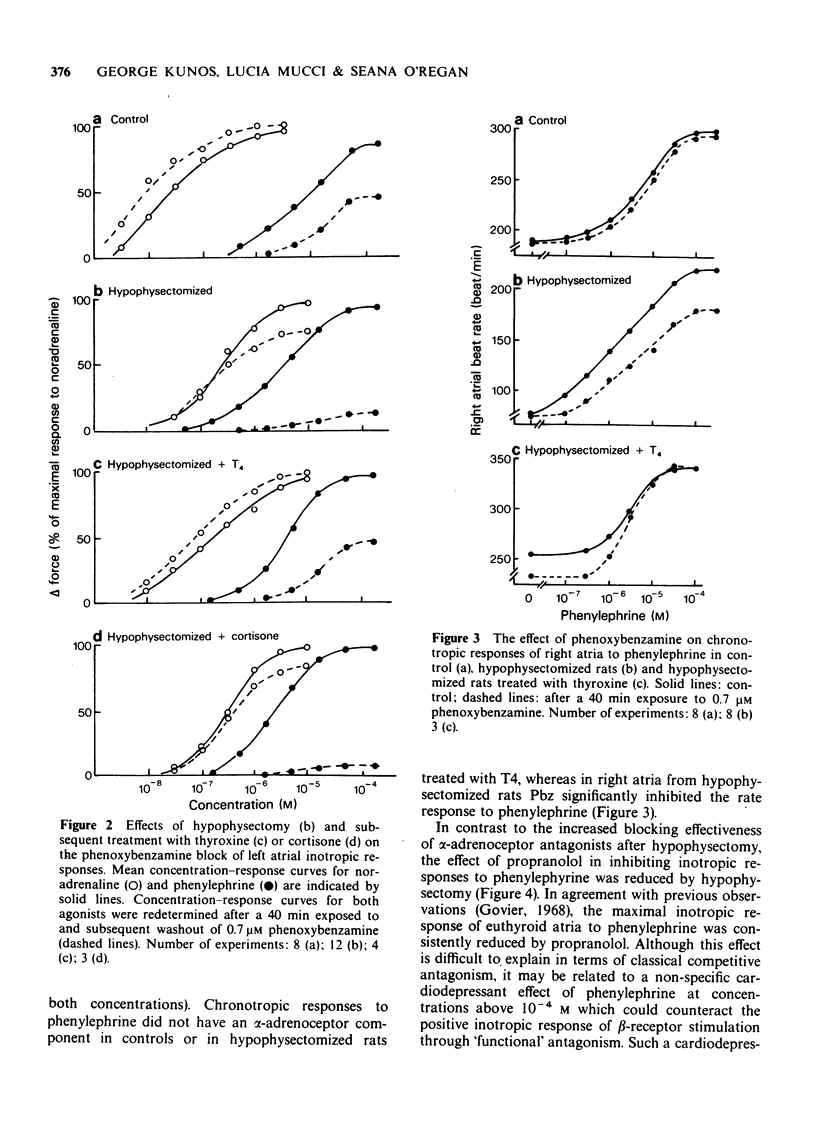
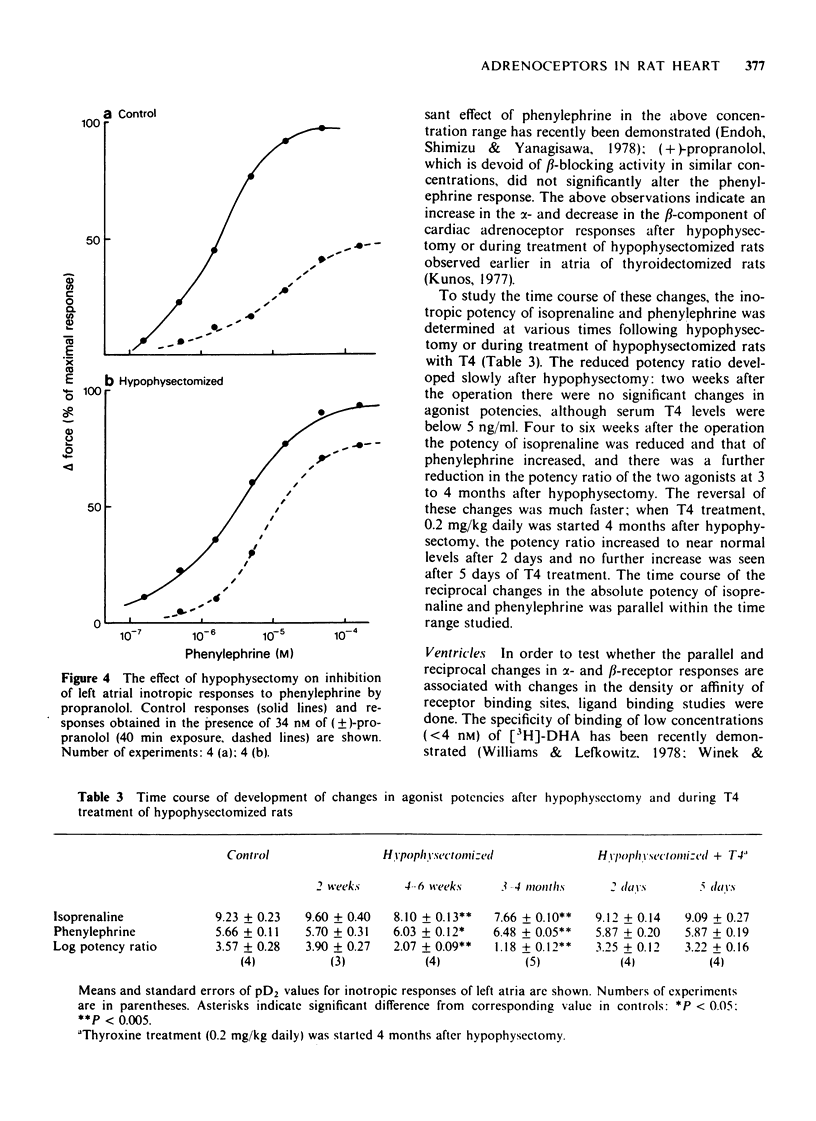
2 Hypophysectomy reduced the inotropic potency of isoprenaline and increased the potency of phenylephrine and methoxamine in left atria. The effect of phenylephrine was inhibited by propranolol less effectively and by phentolamine or phenoxybenzamine more effectively in hypophysectomized than in control rats. The difference in block was smaller at low than at high antagonist concentrations. Similar but smaller changes were observed for chronotropic responses of right atria.
3 The decreased β- and increased α-receptor response after hypophysectomy was similar to that observed earlier in thyroidectomized rats (Kunos, 1977). These changes developed slowly after hypophysectomy (>2 weeks), they were both reversed within 2 days of thyroxine treatment (0.2 mg/kg daily), but were not affected by cortisone treatment (50 mg/kg every 12 h for 4 days).
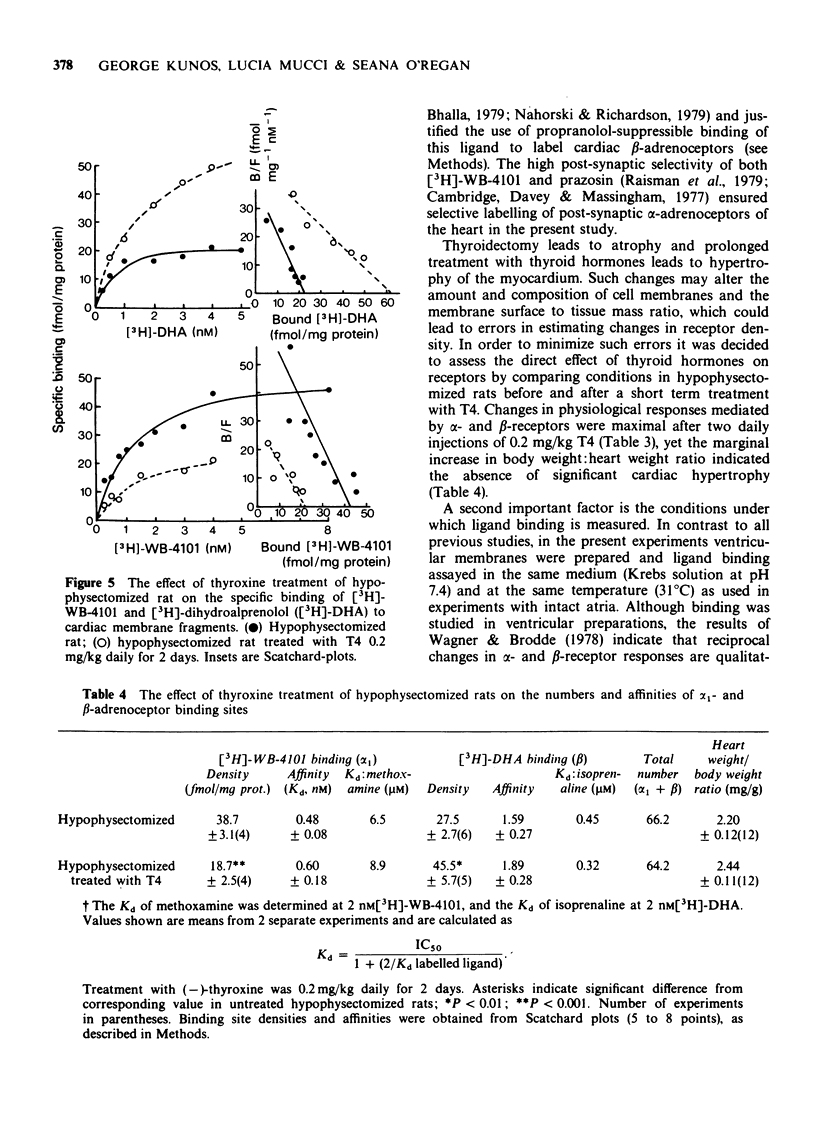
4 Treatment of hypophysectomized rats for 2 days with thyroxine increased the density of [3H]-dihydroalprenolol ([3H]-DHA) binding sites from 27.5 ± 2.7 to 45.5 ± 5.7 fmol/mg protein and decreased the density of [3H]-WB-4101 binding sites from 38.7 ± 3.1 to 18.7 ± 2.5 fmol/mg protein. The affinity of either type of binding site for agonists or antagonist was not significantly altered by thyroxine treatment and the sum total of α1- and β-receptors remained the same.
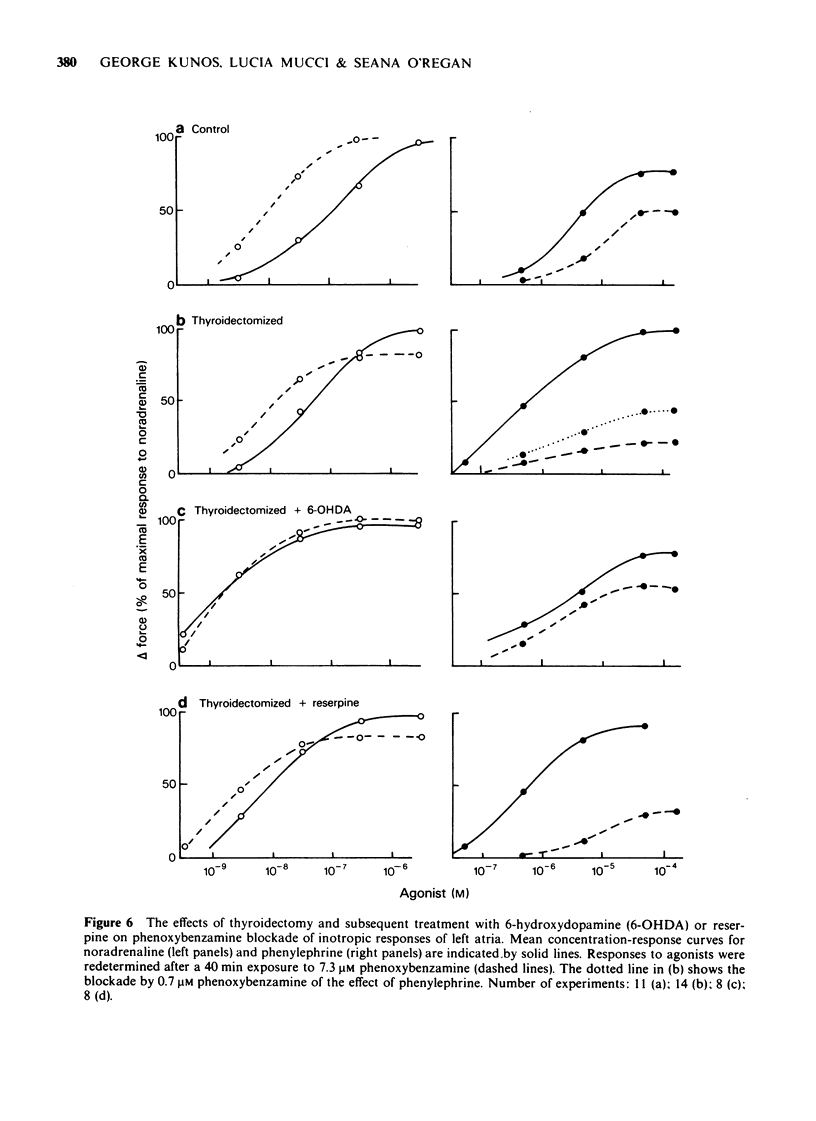
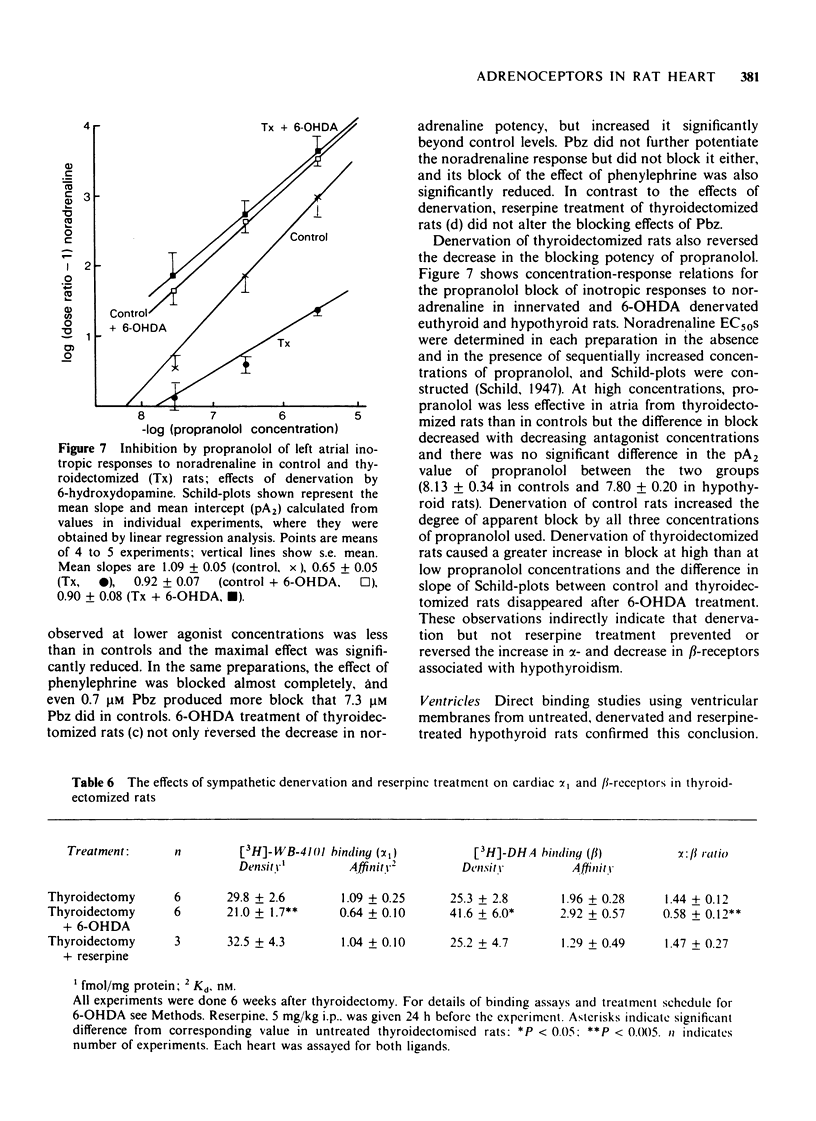
5 Sympathetic denervation of thyroidectomized rats by 6-hydroxydopamine increased the inotropic potency of isoprenaline and noradrenaline and the blocking effect of propranolol, and decreased the potency of phenylephrine and the blocking effect of phenoxybenzamine to or beyond values observed in euthyroid controls. The density of [3H]-DHA binding sites was higher and that of [3H]-WB-4101 binding sites was lower in the denervated than in the innervated hypothyroid myocardium. Depletion of endogenous noradrenaline stores by reserpine did not significantly alter the adrenoceptor response pattern of the hypothyroid preparations and did not influence the density or affinity of [3H]-DHA and [3H]-WB-4101 binding sites.
6 These results indicate that thyrotropin or steroids do not contribute to the reciprocal changes in the sensitivity of cardiac α1- and β-adrenoceptors in altered thyroid states. These thyroid hormone-dependent changes are probably due to a parallel, reciprocal change in the numbers but not the affinities of α1- and β-adrenoceptors. Reciprocal regulation of cardiac α1- and β-adrenoceptors by thyroid hormones requires intact sympathetic innervation but not the presence of normal stores of the neurotransmitter.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonaccio M. J., Smith C. B. Effects of chronic pretreatment with small doses of reserpine upon adrenergic nerve function. J Pharmacol Exp Ther. 1974 Mar;188(3):654–667. [PubMed] [Google Scholar]
- Axelrod J. Relationship between catecholamines and other hormones. Recent Prog Horm Res. 1975;31:1–35. doi: 10.1016/b978-0-12-571131-9.50005-0. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Kung L. S. beta-Adrenergic receptors in rat heart: effects of thyroidectomy. Eur J Pharmacol. 1977 May 15;43(2):207–208. doi: 10.1016/0014-2999(77)90134-0. [DOI] [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Cambridge D., Davey M. J., Massingham R. Prazosin, a selective antagonist of post-synaptic alpha-adrenoceptors [proceedings]. Br J Pharmacol. 1977 Mar;59(3):514P–515P. [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T., Marinetti G. V. Thyroxine and propylthiouracil effects of vivo on alpha and beta adrenergic receptors in rat heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):984–991. doi: 10.1016/0006-291x(77)91615-1. [DOI] [PubMed] [Google Scholar]
- Endoh M., Shimizu T., Yanagisawa T. Characterization of adrenoceptors mediating positive inotropic responses in the ventricular myocardium of the dog. Br J Pharmacol. 1978 Sep;64(1):53–61. doi: 10.1111/j.1476-5381.1978.tb08640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govier W. C. Myocardial alpha adrenergic receptors and their role in the production of a positive inotropic effect by sympathomimetic agents. J Pharmacol Exp Ther. 1968 Jan;159(1):82–90. [PubMed] [Google Scholar]
- Guicheney P., Garay R. P., Levy-Marchal C., Meyer P. Biochemical evidence for presynaptic and postsynaptic alpha-adrenoceptors in rat heart membranes: positive homotropic cooperativity of presynaptic binding. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6285–6289. doi: 10.1073/pnas.75.12.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPSU V. K. Effects of experimental alterations of the thyroid function on the adrenal medulla of the mouse. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 48):1–87. [PubMed] [Google Scholar]
- Haeusler G., Haefely W., Thoenen H. Chemical sympathectomy of the cat with 6-hydroxydopamine. J Pharmacol Exp Ther. 1969 Nov;170(1):50–61. [PubMed] [Google Scholar]
- Hashimoto H., Nakashima M. Influence of thyroid hormone on the positive inotropic effects mediated by alpha- and beta-adrenoceptors in isolated guinea pig atria and rabbit papillary muscles. Eur J Pharmacol. 1978 Aug 15;50(4):337–347. doi: 10.1016/0014-2999(78)90139-5. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Salt P. J. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970 Nov;40(3):528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Mechanism of cocaine potentiation of responses to amines. Br J Pharmacol. 1969 Mar;35(3):428–439. doi: 10.1111/j.1476-5381.1969.tb08284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson S., Marinetti G. V., Shaw A. Hormone action at the membrane level. VII. Stimulation of dihydroalprenolol binding to beta-adrenergic receptors in isolated rat heart ventricle slices by triiodothyronine and thyroxine. Biochim Biophys Acta. 1978 May 3;540(2):320–329. doi: 10.1016/0304-4165(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Kunos G. Adrenoceptors. Annu Rev Pharmacol Toxicol. 1978;18:291–311. doi: 10.1146/annurev.pa.18.040178.001451. [DOI] [PubMed] [Google Scholar]
- Kunos G., Hoffman B., Kwok Y. N., Kan W. H., Mucci L. Dihydroergocryptine binding and alpha-adrenoreceptors in smooth muscle. Nature. 1979 Mar 15;278(5701):254–256. doi: 10.1038/278254a0. [DOI] [PubMed] [Google Scholar]
- Kunos G., Nickerson M. Effects of sympathetic innervation and temperature on the properties of rat heart adrenoceptors. Br J Pharmacol. 1977 Apr;59(4):603–614. doi: 10.1111/j.1476-5381.1977.tb07728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G. Thyroid hormone-dependent interconversion of myocardial alpha- and beta-adrenoceptors in the rat. Br J Pharmacol. 1977 Jan;59(1):177–189. doi: 10.1111/j.1476-5381.1977.tb06992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G., Vermes-Kunos I., Nickerson M. Effects of thyroid state on adrenoceptor properties. Nature. 1974 Aug 30;250(5469):779–781. doi: 10.1038/250779a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labrie F., Pelletier G., Labrie R., Ho-Kim M. A., Delgado A., MacIntosh B., Fortier C. Liaison transcortine-corticostérone et contrôle de l'activité hypophyso-surrénalienne chez le rat. Interactions hypophyse-thyroïde-surrénales-gonades. Ann Endocrinol (Paris) 1968 Jan-Feb;29(1):29–43. [PubMed] [Google Scholar]
- Landsberg L., Axelrod J. Influence of pituitary, thyroid, and adrenal hormones on norepinephrine turnover and metabolism in the rat heart. Circ Res. 1968 May;22(5):559–571. doi: 10.1161/01.res.22.5.559. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Richardson A. Pitfalls in the assessment of the specific binding of(-)[3H]-dihydroalprenolol to beta-adrenoceptors [proceedings]. Br J Pharmacol. 1979 Jul;66(3):469P–470P. [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Hagino Y. Evidence for the existence of alpha adrenergic receptor in isolated rat atria. Jpn J Pharmacol. 1972 Apr;22(2):227–233. doi: 10.1254/jjp.22.227. [DOI] [PubMed] [Google Scholar]
- Popovic W. J., Brown J. E., Adamson J. W. Modulation of in vitro erythropoiesis. Studies with euthyroid and hypothyroid dogs. J Clin Invest. 1979 Jul;64(1):56–61. doi: 10.1172/JCI109463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiksaitis H. G., Kunos G. Adrenoceptor-mediated activation of liver glycogen phosphorylase: effects of thyroid state. Life Sci. 1979 Jan 1;24(1):35–41. doi: 10.1016/0024-3205(79)90277-7. [DOI] [PubMed] [Google Scholar]
- Raisman R., Briley M., Langer S. Z. Specific labelling of postsynaptic alpha 1 adrenoceptors in rat heart ventricle by 3H-WB 4101. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;307(3):223–226. doi: 10.1007/BF00505937. [DOI] [PubMed] [Google Scholar]
- Rosenqvist U. Inhibition of noradrenaline-induced lipolysis in hypothyroid subjects by increased -adrenergic responsiveness. An effect mediated through the reduction of cyclic AMP levels in adipose tissue. Acta Med Scand. 1972 Nov;192(5):353–359. doi: 10.1111/j.0954-6820.1972.tb04830.x. [DOI] [PubMed] [Google Scholar]
- Sharma V. K., Banerjee S. P. alpha-adrenergic receptor in rat heart. Effects of thyroidectomy. J Biol Chem. 1978 Aug 10;253(15):5277–5279. [PubMed] [Google Scholar]
- Story D. D., Briley M. S., Langer S. Z. The effects of chemical sympathectomy with 6-hydroxydopamine on alpha-adrenoceptor and muscarinic cholinoceptor binding in rat heart ventricle. Eur J Pharmacol. 1979 Aug 15;57(4):423–426. doi: 10.1016/0014-2999(79)90505-3. [DOI] [PubMed] [Google Scholar]
- Tedesco J. L., Flattery K. V., Sellers E. A. Effects of thyroid hormones and cold exposure on turnover of norepinephrine in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1977 Jun;55(3):515–522. doi: 10.1139/y77-073. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Chen A. Effect of L-triiodothyronine on (--)3H-dihydroalprenolol binding and cyclic AMP response to (--)adrenaline in cultured heart cells. Nature. 1978 Sep 14;275(5676):138–140. doi: 10.1038/275138a0. [DOI] [PubMed] [Google Scholar]
- Tu T., Nash C. W. The influence of prolonged hyper- and hypothyroid states on the noradrenaline content of rat tissues and on the accumulation and efflux rates of tritiated noradrenaline. Can J Physiol Pharmacol. 1975 Feb;53(1):74–80. doi: 10.1139/y75-009. [DOI] [PubMed] [Google Scholar]
- Wagner J., Brodde O. E. On the presence and distribution of alpha-adrenoceptors in the heart of various mammalian species. Naunyn Schmiedebergs Arch Pharmacol. 1978 May;302(3):239–254. doi: 10.1007/BF00508293. [DOI] [PubMed] [Google Scholar]
- Wagner J., Reinhardt D. Characterization of the adrenoceptors mediating the positive ino- and chronotropic effect of phenylephrine on isolated atria from guinea pigs and rabbits by means of adrenolytic drugs. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(3):295–306. doi: 10.1007/BF00501237. [DOI] [PubMed] [Google Scholar]
- Wildenthal K. Studies of fetal mouse hearts in organ culture: influence of prolonged exposure to triiodothyronine on cardiac responsiveness to isoproterenol, glucagon, theophylline, acetylcholine and dibutyryl cyclic 3',5'-adenosine monophosphate. J Pharmacol Exp Ther. 1974 Aug;190(2):272–279. [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J., Watanabe A. M., Hathaway D. R., Besch H. R., Jr Thyroid hormone regulation of beta-adrenergic receptor number. J Biol Chem. 1977 Apr 25;252(8):2787–2789. [PubMed] [Google Scholar]
- Williams R. S., Lefkowitz R. J. Alpha-adrenergic receptors in rat myocardium. Identification by binding of [3H]dihydroergocryptine. Circ Res. 1978 Nov;43(5):721–727. doi: 10.1161/01.res.43.5.721. [DOI] [PubMed] [Google Scholar]
- Winek R., Bhalla R. [3H]Dihydroalprenolol binding sites in rat myocardium: relationship between a single binding site population and the concentration of radioligand. Biochem Biophys Res Commun. 1979 Nov 14;91(1):200–206. doi: 10.1016/0006-291x(79)90603-x. [DOI] [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K., Molinoff P. B. beta-adrenergic receptors in rat liver: effects of adrenalectomy. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1343–1347. doi: 10.1073/pnas.73.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]


