Abstract
The development of mucosal adjuvants is still a critical need in vaccinology. In the present work, we show that bis(3′,5′)-cyclic dimeric GMP (cdiGMP), a second messenger that modulates cell surface properties of several microorganisms, exerts potent activity as a mucosal adjuvant. BALB/c mice were immunized intranasally with the model antigen β-galactosidase (β-Gal) coadministered with cdiGMP. Animals receiving cdiGMP as an adjuvant showed significantly higher anti-β-Gal immunoglobulin G (IgG) titers in sera than controls (i.e., 512-fold [P < 0.05]). Coadministration of cdiGMP also stimulated efficient β-Gal-specific secretory IgA production in the lung (P < 0.016) and vagina (P < 0.036). Cellular immune responses were observed in response to both the β-Gal protein and a peptide encompassing its major histocompatibility complex class I-restricted epitope. The IgG1-to-IgG2a ratio of anti-β-Gal antibodies and the observed profiles of secreted cytokines suggest that a dominant Th1 response pattern is promoted by mucosal coadministration of cdiGMP. Finally, the use of cdiGMP as a mucosal adjuvant also led to the stimulation of in vivo cytotoxic T-lymphocyte responses in C57BL/6 mice intranasally immunized with ovalbumin and cdiGMP (up to 30% of specific lysis). The results obtained indicate that cdiGMP is a promising tool for the development of mucosal vaccines.
Bacteria are an extremely diverse group of living organisms, which are adapted to different environments including the human body. Despite their intrinsic diversity, pathogenic bacteria share common gateways into the human body (i.e., mucosal surfaces). Thus, the mucosal immune system serves as the first line of defense against bacteria and viruses. Therefore, significant efforts have been invested on the development of mucosal vaccination strategies that are able to promote efficient immune responses at both systemic and mucosal levels. The implementation of a mucosal vaccination strategy should not only result in disease prevention but also block early infection, thereby reducing the likelihood of horizontal transmission to susceptible hosts. In addition, vaccination by the mucosal route reduces the risk of cross contamination, is associated with an easy administration logistic, and is widely accepted by the public. However, most antigens are poorly immunogenic when administered by this route. This is in part due to enzymatic degradation, structural modification resulting from extreme pH, and/or mechanical removal. Mucosal adjuvants can be exploited to overcome this problem. Unfortunately, there are only a few molecules exhibiting this property.
Until recently, the discovery of new adjuvants has not been an extremely successfully process, which was driven mainly by the rules of serendipity. However, recent advances in our understanding of the immune system, particularly with respect to early proinflammatory signals, have led to the identification of new potential targets for immune modulation (4-6, 11, 24, 39). The establishment of improved techniques for total chemical synthesis has also allowed the production of well-defined molecules. This is expected to facilitate the generation of new vaccines exhibiting an adequate safety-and-efficacy profile that are able to stimulate immune responses according to specific needs (21, 36, 37). Nevertheless, there is no vaccine formulation containing a mucosal adjuvant on the market that is approved for human use. Thus, there is an urgent need for new candidate adjuvants. Bacteria can communicate through small “hormone-like” organic compounds, which are called autoinducers. Bis(3′,5′)-cyclic dimeric GMP (cdiGMP) represents one of these cell-to-cell signaling systems that allow bacteria to regulate gene expression via cell density by a mechanism called quorum sensing (29, 30). cdiGMP was first identified in Gluconacetobacter xylinus, where it regulates the production of cellulose through the modulation of cellulose synthase activity (31). It was shown previously that increased levels of cdiGMP correlate with enhanced bacterial aggregation (34, 35, 40, 41) and biofilm formation (13). Karaolis et al. also showed previously that cdiGMP can act on eukaryotes as a danger signal (17). This prompted us to evaluate its potential as an adjuvant (9). The coadministration of cdiGMP with β-galactosidase (β-Gal) to mice by the subcutaneous route resulted in the stimulation of strong humoral and cellular immune responses, which were characterized by a balanced Th1/Th2 pattern. Subsequently, Karaolis et al. showed that cdiGMP stimulates the expression of costimulatory molecules, maturation markers, and cytokines by human dendritic cells (18). These results suggested that cdiGMP might also exert immunomodulatory activities when administered by mucosal routes. Therefore, in the present work, we evaluated the potential of cdiGMP as a mucosal adjuvant. To this end, immunization studies were performed using β-Gal and ovalbumin (Ova) as model antigens. Antigen coadministration with cdiGMP by the intranasal route resulted in the stimulation of strong humoral and cellular immune responses at both systemic and mucosal levels. The results obtained suggest that cdiGMP represents a promising tool for the development of mucosal vaccines.
MATERIALS AND METHODS
Synthesis of cdiGMP.
cdiGMP was synthesized by cyclization according to established protocols (14, 32, 44). The resulting compound was purified by reversed-phase high-performance liquid chromatography at room temperature using a Phenomex column (50 by 2 mm, 4 μm, C18) and a gradient of 25 mM triethylammonium formic acid (solvent A [pH 6.8]) to acetonitrile (solvent B) from 0% to 20% solvent B within 20 min at a flow rate of 0.7 ml/min. Subsequently, the sodium form of cdiGMP was obtained by Dowex-Na+ ion exchange. The structure of water-soluble cdiGMP was confirmed by 1H und 13P nuclear magnetic resonance and matrix-assisted laser desorption-ionization mass spectrometry. To rule out lipopolysaccharide (LPS) contamination, the synthesized compound was tested using the HEK-Blue LPS detection kit (InvivoGen) according to the manufacturer's instructions. Even when tested at 40 μg/ml, no LPS contamination was detectable in the cdiGMP batches (detection limit, 0.3 ng/ml). The final lyophilized compound was stored at −20°C. For the immunization studies, cdiGMP was dissolved in sterile water. The resulting solution was stable for at least 6 and 2 months at −20°C and 4°C, respectively.
Immunization protocols.
Groups of female BALB/c (H-2d) or C57BL/6 (H-2b) mice (n = 5), 6 to 8 weeks of age, were intranasally immunized on days 0, 14, and 28 with 30 μg of β-Gal (Roche, Germany) or 50 μg of Ova (Roche, Germany) alone or coadministered with cdiGMP (1 or 5 μg/dose). The solution was prepared in phosphate-buffered saline (PBS) 30 min before administration (final volume, 20 μl; 10 μl per nostril). To facilitate animal manipulations and to standardize the immunization protocols, mice were mildly anesthetized (1 min) with Isoflo (Abbott Animal Health) according to the manufacturer's instructions. The optimal amount of the adjuvant used was determined in preliminary studies. Animals in the negative control group received only PBS. Animal permission was given by the local government of Lower Saxony (Germany) (no. 509.42502/07-04.01).
Sample collection.
Serum samples were collected on days −1, 13, 27, and 42. Blood was taken from the retro-orbital complex to ensure that the lungs were not injured (e.g., as may happen after heart puncture), thereby affecting the quality of the bronchoalveolar lavage samples. Samples were then centrifuged to remove red blood cells (10 min at 3,000 × g), and sera were stored at −20°C until processing. On day 42, mice were sacrificed, and bronchoalveolar lavage samples and vaginal lavage samples were obtained by flushing the organs with 1 ml of PBS supplemented with 50 mM EDTA, 0.1% bovine serum albumin, and 10 mM phenyl-methane-sulfonyl fluoride. After centrifugation to eliminate debris (10 min at 3,000 × g), supernatant fluids were collected and stored at −20°C until processing for the detection of secretory immunoglobulin A (IgA) (sIgA). Spleens were dissected, and the splenocytes were collected and used for monitoring of the stimulation of antigen-specific cellular responses. Antibodies were investigated in individual animals, whereas cellular responses were analyzed using pools of spleen cells as previously described (1).
Detection of β-Gal-specific IgG in serum.
The β-Gal-specific antibodies in serum samples were determined by enzyme-linked immunosorbent assay (ELISA) using microtiter plates coated with 100 μl/well of β-Gal or Ova (5 μg/ml in 0.05 M carbonate buffer [pH 9.6]) as previously described (2). Briefly, 96-well Nunc (Roskilde, Denmark) Immuno MaxiSorp assay plates were coated with 5 μg/ml β-Gal or Ova in coating buffer (bicarbonate [pH 8.2]). After overnight incubation at 4°C, plates were blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at 37°C. Serial twofold dilutions of sera in 3% BSA-PBS were added (100 ml/well), and plates were incubated for 2 h at 37°C. After six washes with 1% BSA-PBS-0.05% Tween 20, secondary antibodies were added: biotinylated chain-specific goat anti-mouse IgG (Sigma) or, to determine IgG subclass, biotinylated rat anti-mouse IgG1 and IgG2a (Pharmingen, San Diego, CA). Plates were further incubated for 2 h at 37°C. After six washes, 100 μl of peroxidase-conjugated streptavidin (Pharmingen) was added to each well, and plates were incubated at room temperature for 1 h. After another six washes, reactions were developed using ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer (pH 4.35) containing 0.01% H2O2. Endpoint titers were expressed as absolute values of the last dilution, which gave an optical density at 405 nm of two times above the values of the negative controls after 15 and 30 min of incubation, as previously described (8).
Determination of β-Gal-specific sIgA.
The amount of antigen-specific sIgA present in the lavages was determined by ELISA using biotinylated alpha-chain-specific goat anti-mouse IgA (Sigma), as described above (2). To compensate for variations in the efficiency of recovery of secretory antibodies among animals, the results were normalized and expressed as endpoint titers of antigen-specific IgA per μg of total IgA present in the sample. In brief, plates coated with 2 μg/ml of goat anti-mouse IgA (Sigma Chemie) as capture antibody were incubated with serial twofold dilutions of either lavage samples or, for the standard curve, purified mouse IgA (Dianova, Hamburg, Germany) for 1 h. After serial washes with PBS plus 0.1% Tween 20, plates were incubated for 1 h with the secondary antibody biotinylated goat anti-mouse IgA (Sigma), washed six times, and developed as described above.
Measurement of cellular proliferation.
Spleen cells (5 × 105 cells/well) of vaccinated mice were aseptically removed, and cell suspensions were prepared. Erythrocytes were lysed by 2 min of incubation in lysis buffer (0.15 M NH4Cl, 1.0 M KHCO3, 0.1 mM EDTA [pH 7.2]). Cells were washed twice and adjusted to 2 × 106 cells/ml in complete RPMI medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Splenocytes were then seeded with a volume of 100 μl per well in quadruplicates in a flat-bottomed 96-well microtiter plate (Sarstedt Inc., Newton, NC) and cultured for 4 days in the presence of different concentrations of β-Gal (Sigma), 5 μg/ml concanavalin A, or medium alone. Eighteen hours before harvesting, 1 μCi [3H]thymidine (Amersham International, Freiburg, Germany) was added to each well (2). Cells were harvested on paper filters (Filtermat A; Wallac, Freiburg, Germany) using a cell harvester (Inotech, Wohlen, Switzerland), and the incorporation of [3H]thymidine into the DNA of proliferating cells was determined using a scintillation counter (Wallac 1450; Micro-Trilux). The results are expressed as counts per minute (cpm).
ELISPOT assay.
To determine the amounts of gamma interferon (IFN-γ)-, interleukin-2 (IL-2)-, and IL-4-secreting cells, murine IFN-γ, IL-2, and IL-4 enzyme-linked immunospot (ELISPOT) kits (BD Pharmingen) were used according to the manufacturer's instructions. Cells (1 × 106 or 5 × 105 cells/well) were incubated for 16 h in the absence or in the presence of a β-Gal peptide (TPHARIGL), which was known to encompass an Ld-restricted cytotoxic T-lymphocyte (CTL) epitope (for IFN-γ) or β-Gal protein (for IL-2 and IL-4) at a concentration of 10 μM. Cells were then removed, and the plates were processed according to the manufacturer's instructions. Colored spots were counted with a CTL ELISPOT reader and analyzed using ImmunoSpot image analyzer software v3.2.
Cytometric bead array.
To quantify the cytokines secreted by splenocytes restimulated in vitro using β-Gal, supernatants were collected on days 2 and 4 and stored at −70°C. The contents of IFN-γ, TNF-α, IL-2, IL-4, and IL-5 were determined using a cytometric bead array according to the manufacturer's instructions (Becton Dickinson, San Jose, CA). Supplied standards were used to generate a standard curve.
Determination of lymphocyte-mediated cytotoxicity in vivo.
Suspensions of splenocytes from naïve C57BL/6 mice were depleted of red cells and split into two equal portions. One was labeled with a high concentration (1 μM) of carboxy fluorescein succinimidyl ester (CFSE) (Molecular Probes) and pulsed for 1 h at 37°C with a peptide from Ova (amino acids 257 to 264) encompassing an immunodominant major histocompatibility complex (MHC) class I-restricted epitope at a concentration of 15 μg/ml. The other was labeled with a low concentration (0.1 μM) of CFSE and further incubated for 1 h at 37°C without peptide. Equal numbers of each cell population were mixed. A total of 2 × 107 cells was adoptively transferred by intravenous injection into the mice immunized with 50 μg of Ova protein coadministered with 5 μg cdiGMP by intranasal injection on days 1, 14, and 28. Cells from spleen were analyzed by flow cytometry after 16 h and 40 h with a FACSCalibur apparatus by using BD Cell Quest Pro software. Specific lysis was distinguished by the loss of the peptide-pulsed CFSEhi population in comparison with the control CFSElo population (12). The following formula was used to calculate the percentage of specific lysis: 100 − {[(% CFSEhi in immunized mice/% CFSElo in immunized mice)/(% CFSEhi in control mice/% CFSElo in control mice)] × 100}.
Statistical analysis.
The statistic significance of the differences observed between the different experimental groups was analyzed using the Student unpaired t test and the nonparametric Mann-Whitney test. Differences were considered significant at a P value of <0.05.
RESULTS
Intranasal immunization using cdiGMP as a mucosal adjuvant results in the induction of strong humoral immune responses at systemic and mucosal levels.
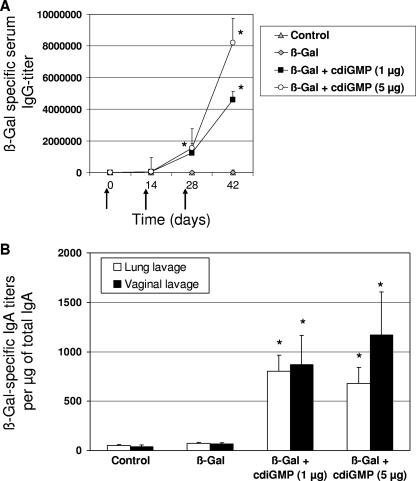
To analyze the capacity of cdiGMP to act as a mucosal adjuvant in vivo, mice were immunized with the model antigen β-Gal (30 μg/dose) alone or coadministered with cdiGMP by the intranasal route. The use of cdiGMP as a mucosal adjuvant resulted in significantly increased β-Gal-specific IgG titers in comparison to animals vaccinated with β-Gal alone (Fig. 1A). Interestingly, mice immunized with β-Gal coadministered with either 1 or 5 μg of cdiGMP showed significantly higher antigen-specific IgG titers even after a single boost than controls receiving β-Gal alone. However, the highest titers were observed in animals receiving 5 μg of cdiGMP per dose.
FIG. 1.
Humoral immune responses stimulated in mice vaccinated using cdiGMP as a mucosal adjuvant. (A) Kinetic analysis of anti-β-Gal IgG responses in sera from BALB/c mice (n = 5) immunized on days 1, 14, and 28 (indicated by arrows) with PBS (control), β-Gal (30 μg/dose), or β-Gal coadministered with cdiGMP (1 and 5 μg/dose) intranasally. (B) Analysis of antigen-specific secretory IgA in lung and vaginal lavage samples of immunized mice. Results are expressed as β-Gal-specific IgA titers per μg of total IgA. The standard error of the mean is indicated by vertical lines. Differences were statistically significant at a P value of <0.05 (*) with respect to mice receiving antigen alone. Data from one representative experiment out of three independent experiments are shown.
The capacity of cdiGMP to stimulate mucosal immune responses was then further evaluated. To this end, antigen-specific secretory IgA titers were measured in lung and vaginal lavage samples from vaccinated animals (Fig. 1B). As expected, intranasal immunization with β-Gal coadministered with cdiGMP induced strong antigen-specific sIgA responses both in the lung and in the vagina. In contrast, antigen-specific sIgA was not detected in lavages from mice receiving β-Gal alone (P < 0.05).
Immunization using cdiGMP as a mucosal adjuvant stimulates β-Gal-specific cellular immune responses.
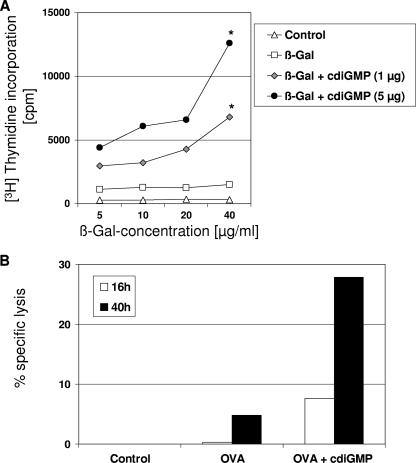
Lymphoproliferative assays were carried out to evaluate the capacity of cdiGMP to promote cellular immune responses. Immune cells isolated from spleens 42 days after the first immunization were restimulated in vitro with the β-Gal protein. Proliferative responses were observed in cells from mice receiving β-Gal with either 1 or 5 μg of cdiGMP (Fig. 2A). In contrast, almost no responses were observed when splenocytes from mice immunized with the β-Gal protein alone were tested. To further analyze the in vivo influence of cdiGMP on the stimulation of cytotoxic cells, Ova peptide-loaded spleen cells from naïve C57BL/6 mice were injected to Ova-vaccinated mice according to the in vivo CTL protocol described previously by Hermans et al. (12). In animals receiving Ova coadministered with 5 μg cdiGMP, approximately 30% of the peptide-loaded cells were lysed, whereas in mice vaccinated with the antigen alone, only a weak (5%) Ova-specific lysis was observed (Fig. 2B).
FIG. 2.
Cellular immune responses stimulated in mice using cdiGMP as a mucosal adjuvant. (A) Proliferative responses stimulated in mice immunized using cdiGMP as an adjuvant. Spleen cells from vaccinated animals were restimulated with different concentrations of β-Gal for 96 h. Cellular proliferation was assessed by the determination of the [3H]thymidine incorporated into the DNA of replicating cells. Results are averages of triplicates, and they are expressed as counts per minute (cpm). The results obtained in cdiGMP-vaccinated animals were statistically significant with respect to those observed in mice receiving β-Gal alone, at a P value of <0.05 (*). Data from one representative experiment out of four independent experiments are shown. (B) Assessment of the effect of coadministration of cdiGMP on CTL responses as measured by the VITAL assay (in vivo CTL) (see Materials and Methods). Lysis of CFSE+ Ova peptide-loaded splenocytes was monitored relative to that of unloaded splenocytes in spleens taken from mice that had been immunized three times with 50 μg of Ova alone or coadministered with 5 μg cdiGMP by intranasal application 4 weeks after the last immunization. The percentage of specific lysis for each group of two animals is shown.
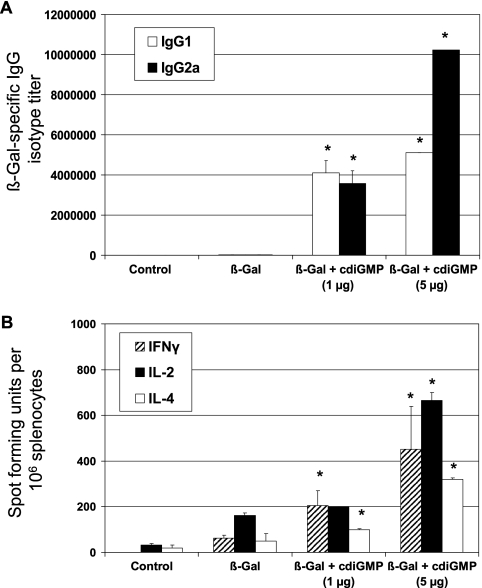
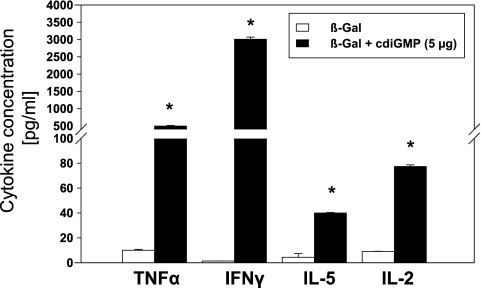
To assess the effects on T-helper responses resulting from using cdiGMP as a mucosal adjuvant, the subclass distribution of β-Gal-specific serum IgG was first analyzed. Intranasal immunization of mice with β-Gal in combination with cdiGMP resulted in a shift from a balanced Th1/Th2 to a dominant Th1 response pattern upon increasing the cdiGMP concentration from 1 to 5 μg/dose (Fig. 3A). Up to 640- and 320-fold increments in antigen-specific IgG2a and IgG1, respectively, were observed. To further confirm these data, the presence of β-Gal-specific IFN-γ-, IL-2-, and IL-4-secreting cells was assessed by ELISPOT (Fig. 3B). Splenocytes from immunized mice secreted predominantly IFN-γ (sevenfold increment with respect to cells from BALB/c mice) in response to β-Gal antigen, followed by IL-4 (6.5-fold) and IL-2 (fourfold). These results were in agreement with those for the cytokines secreted by β-Gal-restimulated splenocytes. Intranasal coadministration of cdiGMP (5 μg/dose) also resulted in an enhanced secretion of TNF-α (50-fold), IFN-γ (>2,000-fold), IL-5, and IL-2 (more than eightfold) in comparison to spleen cells recovered from animals receiving antigen alone (Fig. 4). The analysis of proinflammatory cytokines such as IL-6 and anti-inflammatory cytokines such as IL-10 showed 24-fold and 16-fold increases, respectively, with respect to the control group, which was immunized with β-Gal alone (data not shown).
FIG. 3.
Evaluation of the T-helper responses stimulated in mice vaccinated using cdiGMP as a mucosal adjuvant. (A) Analysis of β-Gal-specific IgG isotypes in sera of immunized mice. Anti-β-Gal-specific IgG isotypes in mice immunized with PBS, β-Gal plus cdiGMP (1 and 5 μg), or β-Gal alone by the intranasal route were determined by ELISA. Results are expressed as endpoint titers. The IgG isotype titers represent the means of data for five animals per experimental group. (B) Detection of IFN-γ-, IL-2-, and IL-4-secreting cells. Spleen cells (1 × 106 and 5 × 105 cells/well) recovered from vaccinated mice were incubated for 24 or 48 h in the presence of a peptide encompassing the immunodominant Ld-restricted epitope of β-Gal (TPHPARIGL), which is specific for MHC class I presentation (for IFN-γ) or the β-Gal protein (for IL-2 and IL-4). The numbers of IFN-γ-, IL-2-, and IL-4-producing cells were then determined by ELISPOT. Results are presented as spot-forming units per 106 cells, which were subtracted from the values obtained from nonstimulated cells. The standard error of the mean of triplicates is indicated by vertical lines. Differences were statistically significant at a P value of <0.05 with respect to mice receiving antigen alone (*). Data from one representative experiment out of four independent experiments are shown.
FIG. 4.
Analysis of the cytokines secreted by the splenocytes of mice vaccinated using cdiGMP as a mucosal adjuvant. The cytokines secreted by spleen cells recovered from vaccinated animals after restimulation with β-Gal were evaluated by cytometric bead array. Data from one representative experiment out of three are shown. Differences were statistically significant at a P value of <0.05 with respect to the values observed by testing cells from animals vaccinated with β-Gal alone (*).
DISCUSSION
Vaccination remains the most cost-efficient strategy to prevent infectious diseases. There is also increasing interest in the therapeutic use of vaccines against infection, cancer, and chronic inflammatory diseases (7, 28). Most pathogens enter the host via the mucosa. Thus, the induction of humoral and cellular immune responses at both systemic and mucosal levels represents a major goal in the development of vaccines against infectious agents. In fact, the efficient stimulation of local immune responses at the portal of entry would allow prevention not only against disease but also against infection. In addition, vaccination by the mucosal route is more accepted by the public, and it is associated with a lower risk of appearance of side effects (38).
The incorporation of a limited number of well-defined protective antigens into the vaccine formulation has also led to a significantly improved safety profile. However, subunit vaccines are usually less immunogenic than whole-cell vaccines (23). Therefore, adjuvants should be incorporated into the formulation. On the other hand, it is important to consider that adjuvants not only enhance the strength of the elicited responses but also exhibit immunomodulatory properties. In this context, the stimulation of the right type of immune response is a key aspect in order to achieve protection and avoid immune pathological reactions (43).
In the present work, we investigated whether the responses elicited after mucosal vaccination can be strengthened and modulated by using the new candidate adjuvant cdiGMP, which was demonstrated to exert immunostimulatory activities when administered by the parenteral route (9, 18). To achieve this aim, animals were immunized with model antigens coadministered with cdiGMP by the intranasal route. The presence of cdiGMP in the formulation resulted in a significantly improved stimulation of antigen-specific immune responses at both systemic and mucosal levels. More specifically, high titers of β-Gal-specific IgG were detected in sera of vaccinated mice even after a single boost. Furthermore, the production of β-Gal-specific secretory IgA was efficiently stimulated not only locally in the lungs but also at distant mucosal territories, such as in vaginal secretions. Cellular responses were also stronger in mice receiving β-Gal coadministered with cdiGMP than in animals receiving the antigen alone. Similar results were obtained after immunization of C57BL/6 mice with Ova, thereby demonstrating that the adjuvant activity of cdiGMP is not restricted to a particular mouse haplotype (data not shown). Of note, the use of cdiGMP as a mucosal adjuvant promoted MHC class I-restricted immune responses, as shown by the analysis of CTL in vivo.
This vaccination strategy promoted a shift from a balanced Th1/Th2 to a more Th1-dominated helper response pattern, as demonstrated by the improved expression of IFN-γ, IL-2, IL-4, and IL-5 (Fig. 4). A deeper analysis showed that mice have enhanced expression of cytokines and chemokines, which play a role as attractants of naïve and effector T cells and in T-cell differentiation. By using a 20-plex cytokine of Biosource and the QIAGEN Luminex system, we observed an increased secretion of macrophage inflammatory protein 1α (MIP-1α) (CCL3) (29,800 ng/ml), IL-17 (2,978 ng/ml), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (18,400 ng/ml) in supernatant fluids of restimulated spleen cells from mice vaccinated with β-Gal plus cdiGMP. In contrast, spleen cells recovered from mice vaccinated with β-Gal alone showed no or only marginal secretion of MIP-1α (73.5 pg/ml), IL-17 (10.5 pg/ml), and GM-CSF (11.5 pg/ml).
MIP-1α affects the magnitude and polarity of T-cell responses. Alternative pathways for Th1 and Th2 polarization, in which a parallel exists between CCL3-CCR5 and IL-12, and CCL2 and the capacity of IL-4 or IL-10 to polarize Th-cell responses, have been proposed (25). Thus, the enhanced secretion of MIP-1α after the coadministration of cdiGMP could explain, at least in part, the induction of IL-12 and IFN-γ. These molecules also influence T-cell differentiation, affecting natural killer (NK) (3, 15, 16, 33) and NKT (26) cell activities at inflammatory sites (10, 27, 33). On the other hand, IL-17 is involved in the recruitment of neutrophils, cytokine expression, and dendritic cell maturation (19-22, 38, 42, 45). IL-17 is also able to induce GM-CSF via the RafI-MEK-extracellular signal-regulated kinase pathway (20). This can explain the observed increment in GM-CSF, which might in turn be crucial for the maturation of various cell subsets and T-cell polarization.
In conclusion, our results have demonstrated that the coadministration of antigens with cdiGMP by the intranasal route results in the stimulation of strong humoral and cellular immune responses at systemic and mucosal levels. Recent studies showed that cdiGMP also activates human monocyte-derived dendritic cells (18). This further supports the usefulness of cdiGMP in humans, suggesting that this signaling molecule is indeed a promising adjuvant for the development of mucosal vaccines.
Acknowledgments
We are particularly grateful to K. Planck-Schumacher, K. Watzke, and E. Reinhard for their outstanding technical help.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Borsutzky, S., T. Ebensen, C. Link, P. D. Becker, V. Fiorelli, A. Cafaro, B. Ensoli, and C. A. Guzman. 2006. Efficient systemic and mucosal responses against the HIV-1 Tat protein by prime/boost vaccination using the lipopeptide MALP-2 as adjuvant. Vaccine 24:2049-2056. [DOI] [PubMed] [Google Scholar]
- 2.Borsutzky, S., V. Fiorelli, T. Ebensen, A. Tripiciano, F. Rharbaoui, A. Scoglio, C. Link, F. Nappi, M. Morr, S. Butto, A. Cafaro, P. F. Muhlradt, B. Ensoli, and C. A. Guzman. 2003. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur. J. Immunol. 33:1548-1556. [DOI] [PubMed] [Google Scholar]
- 3.Braun, S. E., K. Chen, R. G. Foster, C. H. Kim, R. Hromas, M. H. Kaplan, H. E. Broxmeyer, and K. Cornetta. 2000. The CC chemokine CK beta-11/MIP-3 beta/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J. Immunol. 164:4025-4031. [DOI] [PubMed] [Google Scholar]
- 4.Burdin, N., B. Guy, and P. Moingeon. 2004. Immunological foundations to the quest for new vaccine adjuvants. BioDrugs 18:79-93. [DOI] [PubMed] [Google Scholar]
- 5.Cavallo, F., A. Astolfi, M. Iezzi, F. Cordero, P. L. Lollini, G. Forni, and R. Calogero. 2005. An integrated approach of immunogenomics and bioinformatics to identify new tumor associated antigens (TAA) for mammary cancer immunological prevention. BMC Bioinformatics 6(Suppl. 4):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Guilmi, A. M., and A. Dessen. 2002. New approaches towards the identification of antibiotic and vaccine targets in Streptococcus pneumoniae. EMBO Rep. 3:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drabner, B., and C. A. Guzman. 2001. Elicitation of predictable immune responses by using live bacterial vectors. Biomol. Eng. 17:75-82. [DOI] [PubMed] [Google Scholar]
- 8.Ebensen, T., S. Paukner, C. Link, P. Kudela, C. de Domenico, W. Lubitz, and C. A. Guzman. 2004. Bacterial ghosts are an efficient delivery system for DNA vaccines. J. Immunol. 172:6858-6865. [DOI] [PubMed] [Google Scholar]
- 9.Ebensen, T., K. Schulze, P. Riese, C. Link, M. Morr, and C. A. Guzman. 2007. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 25:1464-1469. [DOI] [PubMed] [Google Scholar]
- 10.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 11.Guy, B., and N. Burdin. 2005. New adjuvants for parenteral and mucosal vaccines. Therapie 60:235-241. [DOI] [PubMed] [Google Scholar]
- 12.Hermans, I. F., J. D. Silk, J. Yang, M. J. Palmowski, U. Gileadi, C. McCarthy, M. Salio, F. Ronchese, and V. Cerundolo. 2004. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J. Immunol. Methods 285:25-40. [DOI] [PubMed] [Google Scholar]
- 13.Hickman, J. W., D. F. Tifrea, and C. S. Harwood. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA 102:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu, C.-Y., D. Dennis, and R. A. Jones. 1985. Synthesis and physical characterization of bis 3′5′ cyclic dinucleotides (-NpNp-): RNA polymerase inhibitors. Nucleosides Nucleotides 4(3):377-389. [Google Scholar]
- 15.Inngjerdingen, M., B. Damaj, and A. A. Maghazachi. 2001. Expression and regulation of chemokine receptors in human natural killer cells. Blood 97:367-375. [DOI] [PubMed] [Google Scholar]
- 16.Inngjerdingen, M., B. Damaj, and A. A. Maghazachi. 2000. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J. Immunol. 164:4048-4054. [DOI] [PubMed] [Google Scholar]
- 17.Karaolis, D. K., K. Cheng, M. Lipsky, A. Elnabawi, J. Catalano, M. Hyodo, Y. Hayakawa, and J. P. Raufman. 2005. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem. Biophys. Res. Commun. 329:40-45. [DOI] [PubMed] [Google Scholar]
- 18.Karaolis, D. K., T. K. Means, D. Yang, M. Takahashi, T. Yoshimura, E. Muraille, D. Philpott, J. T. Schroeder, M. Hyodo, Y. Hayakawa, B. G. Talbot, E. Brouillette, and F. Malouin. 2007. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 178:2171-2181. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi, M., M. Adachi, N. Oda, F. Kokubu, and S. K. Huang. 2004. IL-17 cytokine family. J. Allergy Clin. Immunol. 114:1265-1273, 1274. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, M., F. Kokubu, M. Odaka, S. Watanabe, S. Suzuki, K. Ieki, S. Matsukura, M. Kurokawa, M. Adachi, and S. K. Huang. 2004. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. J. Allergy Clin. Immunol. 114:444-450. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, M. N., J. K. Kolls, K. Happel, J. D. Schwartzman, P. Schwarzenberger, C. Combe, M. Moretto, and I. A. Khan. 2005. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 73:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21:467-476. [DOI] [PubMed] [Google Scholar]
- 23.Lavelle, E. C. 2005. Generation of improved mucosal vaccines by induction of innate immunity. Cell. Mol. Life Sci. 62:2750-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizotte-Waniewski, M., W. Tawe, D. B. Guiliano, W. Lu, J. Liu, S. A. Williams, and S. Lustigman. 2000. Identification of potential vaccine and drug target candidates by expressed sequence tag analysis and immunoscreening of Onchocerca volvulus larval cDNA libraries. Infect. Immun. 68:3491-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther, S. A., and J. G. Cyster. 2001. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2:102-107. [DOI] [PubMed] [Google Scholar]
- 26.Matloubian, M., A. David, S. Engel, J. E. Ryan, and J. G. Cyster. 2000. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 1:298-304. [DOI] [PubMed] [Google Scholar]
- 27.Maurer, M., and E. von Stebut. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882-1886. [DOI] [PubMed] [Google Scholar]
- 28.Medina, E., and C. A. Guzman. 2000. Modulation of immune responses following antigen administration by mucosal route. FEMS Immunol. Med. Microbiol. 27:305-311. [DOI] [PubMed] [Google Scholar]
- 29.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 30.Reading, N. C., and V. Sperandio. 2006. Quorum sensing: the many languages of bacteria. FEMS Microbiol. Lett. 254:1-11. [DOI] [PubMed] [Google Scholar]
- 31.Ross, P., R. Mayer, and M. Benziman. 1991. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55:35-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross, P., R. Mayer, H. Weinhouse, D. Amikam, Y. Huggirat, M. Benziman, E. de Vroom, A. Fidder, P. de Paus, L. A. Sliedregt, et al. 1990. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J. Biol. Chem. 265:18933-18943. [PubMed] [Google Scholar]
- 33.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simm, R., J. D. Fetherston, A. Kader, U. Romling, and R. D. Perry. 2005. Phenotypic convergence mediated by GGDEF-domain-containing proteins. J. Bacteriol. 187:6816-6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simm, R., M. Morr, A. Kader, M. Nimtz, and U. Romling. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123-1134. [DOI] [PubMed] [Google Scholar]
- 36.Stanic, A. K., A. D. De Silva, J. J. Park, V. Sriram, S. Ichikawa, Y. Hirabyashi, K. Hayakawa, L. Van Kaer, R. R. Brutkiewicz, and S. Joyce. 2003. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency. Proc. Natl. Acad. Sci. USA 100:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevceva, L., and M. G. Ferrari. 2005. Mucosal adjuvants. Curr. Pharm. Des. 11:801-811. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan, V. J., J. A. Mikszta, P. Laurent, J. Huang, and B. Ford. 2006. Noninvasive delivery technologies: respiratory delivery of vaccines. Expert Opin. Drug Deliv. 3:87-95. [DOI] [PubMed] [Google Scholar]
- 39.Teicher, B. A. 2000. Molecular targets and cancer therapeutics: discovery, development and clinical validation. Drug Resist. Updat. 3:67-73. [DOI] [PubMed] [Google Scholar]
- 40.Tischler, A. D., and A. Camilli. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tischler, A. D., and A. Camilli. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witowski, J., K. Ksiazek, and A. Jorres. 2004. Interleukin-17: a mediator of inflammatory responses. Cell. Mol. Life Sci. 61:567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zareba, G. 2006. A new combination vaccine for measles, mumps, rubella and varicella. Drugs Today (Barcelona) 42:321-329. [DOI] [PubMed] [Google Scholar]
- 44.Zeng, F., and R. Jones. 1996. Synthesis of cyclic dinucleotides by an H-phosphonate method in solution. Nucleosides Nucleotides 15:1679-1686. [Google Scholar]
- 45.Zou, G. M., and Y. K. Tam. 2002. Cytokines in the generation and maturation of dendritic cells: recent advances. Eur. Cytok. Netw. 13:186-199. [PubMed] [Google Scholar]