Abstract
The threat of a new influenza pandemic has led to renewed interest in dose-sparing vaccination strategies such as intradermal immunization and the use of adjuvanted vaccines. In this study we compared the quality and kinetics of the serum antibody response elicited in mice after one or two immunizations with a split influenza A (H3N2) virus, using three different low-dose vaccination strategies. The mice were divided into four groups, receiving either a low-dose vaccine (3 μg hemagglutinin [HA]) intradermally or intramuscularly with or without aluminum adjuvant or the normal human vaccine dose (15 μg HA) intramuscularly. Sera were collected weekly after vaccination and tested in the hemagglutination inhibition, virus neutralization, and enzyme-linked immunosorbent assays. The antibody responses induced after intradermal or intramuscular low-dose vaccinations were similar and lower than those observed after the human vaccine dose. However, low-dose adjuvanted vaccine elicited a serum antibody response comparable to that elicited by the human dose, although the second immunization did not result in any increase in cross-reactive hemagglutination inhibition antibodies, and the peak serum antibody response was observed 1 week later than in the other vaccination groups. Our murine data suggest that the low-dose intradermal route does not show any obvious advantage over the low-dose intramuscular route in inducing a serum antibody response and that none of the low-dose vaccination strategies is as effective as intramuscular vaccination with the normal human dose. However, the low-dose aluminum-adjuvanted vaccine could present a feasible alternative in case of limited vaccine supply.
Influenza virus infects the respiratory tract in humans, with the clinical outcome ranging from symptomless infection to fulminant primary viral or secondary bacterial pneumonia. The continuous changes in the viral surface glycoproteins (hemagglutinin [HA] and neuraminidase) allow the virus to escape the host's acquired immunity. This leads to seasonal influenza outbreaks and epidemics, sometimes with high morbidity and excess mortality. Also, pandemics occur at unpredictable intervals due to major changes in the virus glycoproteins. Three influenza pandemics occurred during the last century, the Spanish influenza in 1918, the Asian influenza in 1957, and the Hong Kong influenza in 1968. In the last decade avian influenza viruses, such as the H5N1, H9N2, and H7N7 subtypes, have shown the ability to transmit directly from birds to humans (20, 25). Although no or only very limited human-to-human transmission has occurred, any of these subtypes has the potential to cause a new influenza pandemic. Vaccines are the most cost-effective intervention in terms of reducing the burden of disease and ultimately death in both pandemics and epidemics. Unfortunately, in a pandemic scenario, shortages in vaccine supplies will occur because of the substantial increase in worldwide vaccine demand and the limited manufacturing capacity. Dose-sparing vaccination strategies such as the use of adjuvant and new administration routes therefore need to be investigated.
Aluminum adjuvants have been widely used in vaccines for more than 60 years (18). Although other adjuvants have also proved to be effective (22), the aluminum adjuvant is the only nonproprietary alternative for influenza vaccine manufacturers. This adjuvant has shown the ability to enhance the immune response after influenza vaccination of immunologically naive subjects in several clinical trials (3, 8, 9, 17). Recently, intradermal (i.d.) administration of reduced vaccine doses has also been studied in three human clinical trials (1, 2, 16). In all studies one immunization with the reduced dose of influenza vaccine given i.d. met the licensing criteria of the European Union for annual influenza vaccines, and in two of the three studies the serum hemagglutination inhibition (HI) antibody response in the younger population (18 to 60 years) was similar to that found after intramuscular (i.m.) vaccination with the normal dose of vaccine (2, 16). However, subjects older than 60 years of age responded better to i.m. vaccination with the normal dose of vaccine (2). In these clinical trials, the reduced dose of vaccine was administered only i.d. and there was no comparison with a reduced dose given i.m. In addition, no comparison of i.d. versus i.m. adjuvanted vaccination has been performed, and for both aluminum-adjuvanted vaccines and i.d. vaccination less information is available on the kinetics of the immune response. The mouse is a commonly used preclinical model for studying the immunogenicity of influenza vaccines, using the same vaccine dose and administration routes as in humans. The aim of our study was therefore to compare the quality and kinetics of the serum antibody response (measured by enzyme-linked immunosorbent assay [ELISA] and HI, virus neutralization [VN], and cross-reactive HI antibody assays) elicited in mice after three different low-dose vaccination regimens and the normal human dose.
MATERIALS AND METHODS
Immunization and study design.
Six- to 8-week-old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, United Kingdom) and housed according to the Norwegian Regulation on Animal Experimentation. The animals were divided into four groups of 22 to 24 mice and vaccinated with one or two doses of A/Panama/2007/99 (H3N2) split virus vaccine (kindly provided by Sanofi Pasteur, Lyon, France). One group was vaccinated i.d. in the back skin (20 μl) with the low dose (3 μg) of HA, whereas the other three groups were vaccinated i.m. in the quadriceps muscles (50 μl per hind leg) with either the low dose, the low dose adjuvanted with 60 μg aluminum hydroxide [Al(OH)3], or the normal human dose of 15 μg HA. The aluminum hydroxide (Sanofi Pasteur) was mixed with the vaccine immediately before injection as described earlier (3). The mice immunized i.d. were anesthetized using isoflurane directly before vaccination. The injection site was observed during the i.d. administration, and the appearance of a skin bleb confirmed a correct deposition. In addition to the groups described above, four mice served as unvaccinated controls and a further four control mice were immunized i.m. with 60 μg aluminum hydroxide alone.
Blood samples were collected weekly after vaccination from 6 to 12 mice in each group (days 7, 14, and 21 after the first dose of vaccine and days 7 and 14 after the second dose). Groups of 10 to 12 mice were sacrificed 21 days after the first or second vaccination, and blood was collected by cardiac puncture. Sera were separated from the blood samples and stored at −80°C until used in the ELISA and HI and VN assays. Due to the limited serum volume of the blood samples, only blood from the cardiac puncture was analyzed for VN and cross-reactive HI antibodies.
HI assay.
The HI assay was carried out as described earlier (5) using eight HA units of influenza A/Panama/2007/99 (H3N2) virus and 0.7% turkey red blood cells (TRBC). To remove nonspecific inhibitors, all sera were pretreated with receptor-destroying enzyme, incubated overnight at 37°C, and subsequently heat inactivated at 56°C. Sera from day 21 after the first and second vaccinations were also examined for cross-reactivity using the earlier (A/Beijing/32/92 and A/Sydney/05/97) and later (A/New York/155/04) influenza A (H3N2) virus strains and 0.7% TRBC. All samples were analyzed on the same day, and HI titers were scored as the reciprocal of the highest serum dilution producing 50% inhibition of hemagglutination. Titers less than 20 were assigned a value of 10 for calculation purposes.
ELISA.
The influenza virus-specific serum immunoglobulin G (IgG), IgG1, and IgG2a antibodies were quantified using the ELISA as previously described (7, 11). Briefly, ELISA plates were coated with 1 μg/well of split influenza A/Panama/2007/99 (H3N2) virus or an appropriate dilution of capture goat anti-mouse IgG antibody overnight at 4°C. Serially diluted sera and immunoglobulin standards were then incubated for 2 hours at room temperature, followed by a 1-hour incubation with biotinylated goat anti-mouse IgG class or subclass antibodies and a 1-hour incubation with ExtrAvidin peroxidase. The antibody concentrations (μg/ml) were calculated by means of the IgG standard and linear regression of the log-transformed readings.
VN assay.
The VN assay was carried out as earlier described (6). Briefly, quadruplicates of the receptor-destroying enzyme-treated serum samples from the days of sacrifice (day 21 after the first and second vaccinations) were serially diluted twofold across 96-well U-bottom plates and incubated with 100 50% tissue culture infective doses of A/Panama/2007/99 (H3N2) influenza virus for 1 hour at room temperature. The serum-virus mixtures was added to MDCK cell monolayers prepared in 96-well tissue culture plates, incubated for 30 min at 35°C, and subsequently replaced with medium for 72 h. The presence of virus in the supernatant was tested by a hemagglutination assay using 0.7% TRBC, and the VN titers were expressed as the reciprocals of the dilutions required to neutralize 50% of the challenge dose of virus calculated by the method of Reed and Muench (23).
Statistical analyses.
Statistical analyses were performed using SPSS for windows (version 14.0.2, SPSS Inc., Chicago, IL). For the HI test, the 95% confidence interval of the geometric mean titer was calculated for each group based on log-transformed readings of the titers.
The ELISA data were analyzed using linear mixed models, whereas the VN results were analyzed using the two-sided Student t test. P values ≤0.05 were considered significant.
RESULTS
Ninety-six mice were vaccinated with one or two doses of influenza A (H3N2) split virus vaccine. The mice were divided into four different groups, of which three were given a low-dose vaccine (3 μg HA) i.d., i.m., or i.m. adjuvanted with 60 μg Al(OH)3 and the fourth group was immunized i.m. with the normal human vaccine dose (15 μg HA). In addition, there were four unvaccinated control mice and a further four control mice which were immunized i.m. with aluminum hydroxide alone. Sera were collected weekly after vaccination and used in various serological assays to examine the effect of vaccine formulation and route of immunization. No influenza virus-specific antibody response was observed in any of the control mice during the study, and data from these mice are therefore not presented.
HI antibodies.
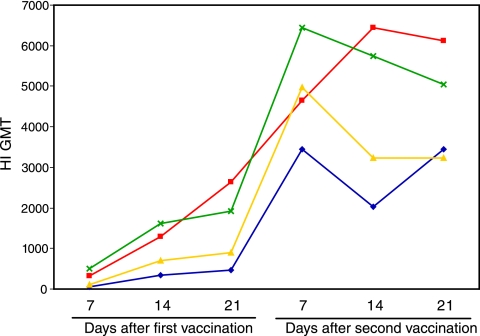
The HI assay is the standard method for detection of influenza virus-specific serum antibodies after vaccination. In humans an HI titer ≥40 is considered to be protective in at least 50% of the population (10). No such correlation is established in mice. However, we have previously observed that two doses of 15 μg split virus vaccine very effectively limited viral shedding in an upper respiratory tract murine challenge model and that higher prechallenge HI titers led to lower levels of viral shedding after challenge (12). In the current study, HI antibodies were initially detected at day 7 and increased up to day 21 after the first dose of vaccine in all four groups (Fig. 1). The second immunization significantly (P < 0.05) boosted the HI antibody response, with peak titers observed at day 7 for all the groups given the nonadjuvanted vaccines and at day 14 for the mice vaccinated with the adjuvanted low-dose vaccine. The animals vaccinated with the normal human dose or the adjuvanted low-dose vaccine had the highest titers, after both the first and second doses, whereas the mice immunized i.d. with the low-dose nonadjuvanted vaccine generally had the lowest HI antibody response.
FIG. 1.
The kinetics of the HI antibody response induced after vaccination with A/Panama/2007/99 (H3N2) vaccine. The HI titers are presented as the geometric mean titers (GMT) from mice vaccinated i.d. with 3 μg HA (blue), i.m. with 3 μg HA (yellow), i.m. with 3 μg HA adjuvanted with 60 μg aluminum hydroxide (red), or i.m. with 15 μg HA (green). The number of animals in each group is shown in Table 1.
VN antibodies.
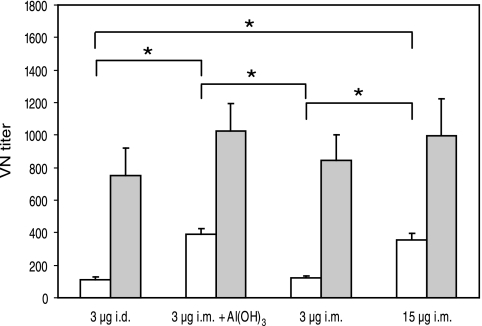
Sera from day 21 after the first and second vaccinations were examined for neutralizing antibodies by a VN assay (Fig. 2). All groups had VN antibodies after the first vaccination, but the titers were significantly higher (P < 0.05) after the second immunization. The mice immunized with the normal human dose or the low-dose adjuvanted vaccine had significantly higher VN titers (P < 0.05) than those given the nonadjuvanted low-dose vaccine i.d. or i.m. after the first immunization. In contrast, comparable VN titers were observed in all groups after the second dose of vaccine.
FIG. 2.
VN antibody titers elicited after vaccination with A/Panama/2007/99 (H3N2) vaccine. VN titers 3 weeks after the first (open bars) and second (filled bars) vaccinations are presented as mean titers ± standard errors of the means for all the vaccination groups (22 to 24 mice in each group after the first dose and 12 mice in each group after the second vaccination). An asterisk indicates a statistically significant difference (P ≤ 0.05) between the indicated paired groups.
ELISA antibodies.
Serum IgG and the subclasses IgG1 and IgG2a were measured in an ELISA to evaluate the profile of the immune response, as a dominance of IgG1 is believed to indicate a humoral immune response, whereas IgG2a is indicative of a cellular response. After the first dose of vaccine only low levels of influenza virus-specific IgG and the two subclasses were detected in all groups (Tables 1 and 2). Peak antibody responses were generally detected at day 14 after one dose of vaccine, except for IgG1, which peaked at day 21 in the groups vaccinated with the normal human dose and the low-dose adjuvanted vaccine. The second dose of vaccine significantly (P < 0.05) boosted the concentrations of IgG, IgG1, and IgG2a. The maximum antibody levels after the second immunization were detected at day 7 for all the vaccination groups, except for the mice immunized with the low-dose adjuvanted vaccine, which had a peak concentration at day 14. For all the groups, the second dose of vaccine elicited a greater increase in the IgG1 antibody concentration than in the IgG2a antibody concentration, which resulted in a lower IgG2a/IgG1 ratio compared to the ratio observed after the first vaccination.
TABLE 1.
Influenza virus-specific serum antibodies elicited after vaccination
| Antibody class or subclass | Vaccination group | Antibody concn (μg/ml) aftere:
|
|||||
|---|---|---|---|---|---|---|---|
| First vaccination
|
Second vaccination
|
||||||
| Day 7 (10-12) | Day 14 (12) | Day 21 (22-24) | Day 7 (6) | Day 14 (6) | Day 21f (12) | ||
| IgG | 3 μg i.d. | 7 ± 2 | 39 ± 4 | 28 ± 3 | 648 ± 228 | 307 ± 58 | 270 ± 54* |
| 3 μg i.m. + Al(OH)3 | 29 ± 5 | 153 ± 29 | 135 ± 11a | 554 ± 111 | 842 ± 243a,c | 415 ± 72a* | |
| 3 μg i.m. | 16 ± 4 | 74 ± 12 | 51 ± 6 | 646 ± 105 | 538 ± 99a | 326 ± 62* | |
| 15 μg i.m. | 22 ± 2 | 210 ± 21a | 160 ± 15a,c | 954 ± 93a,b,c | 656 ± 153a | 535 ± 72a,c* | |
| IgG1 | 3 μg i.d. | 1 ± 1 | 24 ± 4 | 21 ± 3 | 530 ± 199 | 277 ± 77 | 230 ± 41* |
| 3 μg i.m. + Al(OH)3 | 20 ± 5 | 119 ± 25 | 140 ± 13a | 477 ± 90 | 1,019 ± 358a,c,d | 369 ± 68* | |
| 3 μg i.m. | 4 ± 1 | 39 ± 7 | 34 ± 7 | 547 ± 177 | 389 ± 107 | 238 ± 57* | |
| 15 μg i.m. | 2 ± 0 | 82 ± 15 | 93 ± 19 | 520 ± 77 | 360 ± 80 | 248 ± 45* | |
| IgG2a | 3 μg i.d. | 2 ± 1 | 15 ± 2 | 12 ± 2 | 122 ± 46 | 93 ± 21 | 117 ± 46* |
| 3 μg i.m. + Al(OH)3 | 8 ± 2 | 38 ± 11 | 36 ± 4 | 111 ± 27 | 232 ± 62a | 101 ± 19* | |
| 3 μg i.m. | 6 ± 1 | 31 ± 5 | 28 ± 6 | 125 ± 18 | 199 ± 29a | 97 ± 14* | |
| 15 μg i.m. | 13 ± 2 | 116 ± 18a,b,c | 89 ± 9a,b,c | 400 ± 42a,b,c | 358 ± 79a,b,c | 246 ± 40a,b,c* | |
Significantly higher (P ≤ 0.05) than 3 μg i.d.
Significantly higher (P ≤ 0.05) than 3 μg i.m. plus Al(OH)3.
Significantly higher (P ≤ 0.05) than 3 μg i.m.
Significantly higher (P ≤ 0.05) than 15 μg i.m.
Antibody levels were measured on days 7, 14, and 21 after each vaccination. Values are means ± standard errors of the means. The number of mice in each group is given in parentheses after the day.
*, significant increase (P ≤ 0.05) in antibody concentration compared to day 21 after the first dose in each group.
TABLE 2.
IgG2a/IgG1 ratios for vaccination groups
| Vaccination group | Mean IgG2a/IgG1 ratio ± SEM after:
|
|||||
|---|---|---|---|---|---|---|
| First vaccination
|
Second vaccination
|
|||||
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | |
| 3 μg i.d. | 1.4 ± 0.6 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| 3 μg i.m. + Al(OH)3 | 0.8 ± 0.3 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 |
| 3 μg i.m. | 1.3 ± 0.3 | 0.9 ± 0.1 | 1.0 ± 0.2b | 0.5 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| 15 μg i.m. | 7.4 ± 1.7a,b,c | 2.1 ± 0.5a,b,c | 1.6 ± 0.2a,b | 0.8 ± 0.1 | 1.0 ± 0.2 | 1.1 ± 0.2 |
Significantly higher (P ≤ 0.05) than 3 μg i.d.
Significantly higher (P ≤ 0.05) than 3 μg i.m. + Al(OH)3.
Significantly higher (P ≤ 0.05) than 3 μg i.m.
The highest IgG response was observed in the mice vaccinated with the normal human dose or the low-dose adjuvanted vaccine. The latter formulation generally elicited the highest IgG1 level, whereas mice vaccinated with the normal human dose had the highest concentrations of IgG2a on all sampling days. Mice immunized i.m. or i.d. with the low-dose nonadjuvanted vaccines had significantly lower (P < 0.05) IgG and IgG2a antibody responses 3 weeks after one dose and two doses than those vaccinated with the normal human dose. No significant differences were found in the IgG1 response between these three groups. After the second immunization, the animals vaccinated with the low-dose nonadjuvanted vaccines also had a significantly lower (P < 0.05) IgG1 response (day 14) and IgG response (low dose i.d. at days 14 and 21, low dose i.m. at day 14) than those given the low-dose adjuvanted vaccine. No significant differences were found in the IgG2a response between the three groups vaccinated with the low-dose vaccines. The IgG2a/IgG1 ratio was highest for the mice vaccinated with the normal human dose of vaccine and lowest for those given the low-dose adjuvanted vaccine.
Cross-reactive antibodies against different influenza A (H3N2) virus strains.
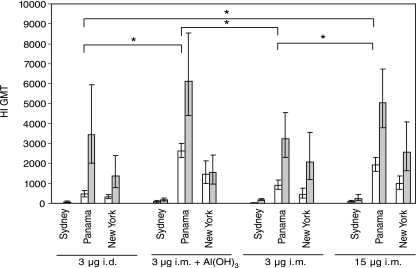
The HI assay was also used to examine differences between the various vaccine formulations and routes of administration in eliciting cross-reactive HI antibodies (Fig. 3). Sera from 21 days after the first and second immunizations were tested against two earlier H3N2 strains (A/Beijing/32/92 and A/Sydney/05/97) and one later H3N2 strain (A/New York/155/04). These viruses circulated in the years 1993 to 1994, 1998 to 2000, and 2005 to 2006, respectively, whereas our vaccine strain, A/Panama/2007/99, circulated in the years 2000 to 2004. In all groups the highest HI titers were observed in response to the vaccine strain, whereas the highest cross-reactive HI antibody titers were detected against A/New York/155/04. Only low titers against A/Sydney/05/97 were produced, and none of the groups had a detectable HI antibody response to the antigenically more distant A/Beijing/32/92 variant (data not shown). After the first dose of vaccine, the mice immunized with the normal human dose or the low-dose adjuvanted vaccine had the highest cross-reactive HI titers in response to both the A/New York/155/04 and the A/Sydney/05/97 strains. However, the second dose of vaccine resulted in an increase in cross-reactive antibodies in all groups except in the group immunized with the low-dose aluminum-adjuvanted vaccine, where no or only a small increase in the HI titers to the variant strains was observed.
FIG. 3.
Cross-reactive HI antibody titers induced after vaccination with A/Panama/2007/99 (H3N2) vaccine. Cross-reactive HI titers against the A/Sydney/05/97 (H3N2) and the A/New York/155/04 (H3N2) strains were measured in sera from all sacrificed mice (10 to 12 mice in each group) 3 weeks after the first (open bars) and second (filled bars) vaccinations. The HI titers against the vaccine strain A/Panama/2007/99 (H3N2) are also presented for reference purposes (22 to 24 mice in each group after the first dose and 12 mice in each group after the second vaccination). Results are presented as geometric mean titers (GMT), and the error bars represent the 95% confidence intervals. An asterisk indicates a statistically significant difference (P ≤ 0.05) between the indicated paired groups for the homologous A/Panama vaccine strain.
DISCUSSION
Influenza vaccination is the most cost-effective intervention in terms of reducing the human and economic consequences during influenza epidemics and pandemics. Unfortunately, a shortage in vaccine supply will occur in case of a pandemic, and consequently dose-sparing vaccination strategies such as the use of adjuvant or alternative administration routes need to be investigated. In this study we have therefore compared the immune responses in mice after three low-dose vaccination strategies (low-dose i.d. vaccination, and low-dose i.m. vaccination with or without aluminum adjuvant) with the immune response elicited after immunization with the normal human dose of a monovalent influenza vaccine.
Effect of aluminum adjuvant.
Aluminum adjuvants are by far the most commonly utilized adjuvants in human vaccines and are frequently used in childhood vaccination programs in many countries (19). Recent human trials have shown their ability to enhance the immune response after influenza vaccination (3, 8, 9). In our study, the mice vaccinated with the low-dose aluminum-adjuvanted vaccine had significantly higher HI titers after both vaccine doses and significantly higher VN titers after the first vaccination than those vaccinated with the low-dose nonadjuvanted vaccines (i.m. or i.d.). The aluminum-adjuvanted vaccine was as effective as the normal human dose in enhancing HI, VN, and IgG1 antibody responses, although the peak antibody concentrations in the aluminum-adjuvanted group generally were detected 1 week later after the second dose than in all the other groups. This later response could be due to the gradual release of antigen from the aluminum adjuvant at the injection site (18, 19) and could potentially present a problem in a pandemic scenario, when the time interval between vaccination and immune protection is particularly important. Possibly, priming with an aluminum-adjuvanted vaccine and boosting with a nonadjuvanted vaccine would elicit an earlier secondary response as well as retain the positive effect of aluminum adjuvant on the immune response, although this may not be practically feasible. It is generally accepted that aluminum adjuvants drive the immune response towards a humoral response (4, 19), characterized by higher IgG1 concentrations than after vaccination with nonadjuvanted vaccines (4, 24). Similarly, we found that the low-dose adjuvanted vaccine elicited higher IgG1 concentrations after the second dose than the nonadjuvanted alternatives (i.m. and i.d.) but still elicited IgG2a concentrations comparable to those elicited by the low-dose nonadjuvanted vaccines after both the first and second vaccine doses.
Influenza vaccines that offer broader cross-reactive immunity are desirable as they confer protection against antigenically drifted strains. We therefore also examined the cross-reactive HI antibody response against two earlier human vaccine strains (A/Beijing/32/92 and A/Sydney/05/97) and one later human vaccine strain (A/New York/155/04) of influenza A (H3N2) virus. The use of aluminum adjuvant has been shown to augment cross-reactive serum neutralizing antibodies in mice (21), and in our study the adjuvanted low dose and the normal human dose of vaccine elicited the highest HI antibody titers against the variant strains after the first immunization. However, the second dose of vaccine significantly boosted the cross-reactive HI antibody responses in all groups except in the adjuvanted-vaccine group, where no increase was observed. This absence of booster effect after the second vaccination was unexpected but important, considering the need for a two-dose vaccine-schedule for pandemic influenza vaccine candidates (3, 9, 17). Therefore, if a pandemic virus differs antigenically from the vaccine strain, an aluminum-adjuvanted vaccine may offer less protection. However, the induction of cross-reactive HI antibodies and the kinetics of the antibody response after aluminum adjuvanted vaccination need to be further examined in humans.
Effect of route of administration.
The heightened concern that an influenza pandemic is imminent has spurred a renewed interest in dose-sparing vaccination strategies, such as the use of i.d. vaccination. This administration route delivers antigen directly into the skin, an anatomical space that contains large numbers of specialized antigen-presenting cells and thus has the potential for better antigen presentation than i.m. injection (16). Three recent studies involving humans have compared the HI antibody responses after i.d. vaccination with a reduced dose of vaccine and i.m. administration of the normal human dose (1, 2, 16). They all found that one i.d. immunization with a reduced dose of influenza vaccine met the licensing criteria of the European Union for annual influenza vaccines (Note for guidance on harmonisation of requirements for influenza vaccines [CPMP/BWP/214/96], Committee for Proprietary Medicinal Products, March 1997 [posting date], http://www.emea.eu.int/pdfs/human/bwp/021496en.pdf). i.d. administration of a reduced vaccine dose was as immunogenic as i.m. vaccination with the normal human dose in two of the studies (2, 16), whereas the third study found that i.m. vaccination with the normal human dose elicited the highest antibody response (1). In our study, we found that the antibody response and IgG subclass profile after low-dose i.d. vaccination were comparable to the immune response after low-dose i.m. vaccination and lower than the immune response after i.m. vaccination with the normal human dose. This contrasted with the findings of two of the human studies (2, 16) and can be due to several factors. Specifically, the human studies did not compare the i.d. and the i.m. routes using the same amount of antigen. An earlier study by Treanor et al. found that one-half of a normal dose of i.m. influenza vaccine was almost as immunogenic as a normal dose of i.m. influenza vaccine in healthy young adults (26). This suggests that the low-dose i.d. vaccination route may not be better in inducing a serum antibody response than the low-dose i.m. vaccination. Furthermore, in the human i.d. studies, the participants were immunologically primed adults vaccinated i.d. with a trivalent influenza vaccine. In contrast, our study was performed with unprimed, immunologically naive mice. It would therefore be interesting to investigate the immune response in humans after intradermal vaccination with a novel vaccine strain to which the population is immunologically naive (e.g., vaccination with an avian virus).
Effect of vaccine antigen dose.
When we compared the high- and low-dose nonadjuvanted vaccines, we observed that the high-dose vaccine elicited higher serum IgG and HI responses than the low-dose vaccine. This has also been shown after vaccination with split and subunit vaccines in humans (14, 15). In our study we also found that the IgG2a antibody response was significantly higher after the normal human dose than after low-dose vaccination while similar concentrations of IgG1 were detected. It has been suggested that IgG1 antibodies play a major role in the neutralization of viral particles both in vitro and in vivo, while the IgG2a antibodies assist in the clearance of influenza virus from the infected host (13). We found no significant differences in the VN titers between the low- and high-dose groups after the second immunization, reflecting the finding of similar IgG1 levels in the high- and low-dose i.m. groups.
Conclusions.
In this study we have investigated the use of dose-sparing vaccination strategies in a mouse model using low-dose i.d., i.m., or i.m. adjuvanted split virus vaccine formulations. i.d. vaccination is a difficult procedure, which requires specially trained health personal, and we found that this route had no advantage over the commonly used i.m. route in inducing a serum antibody response. Of the three low-dose vaccination strategies, the aluminum-adjuvanted vaccine was the most immunogenic, with antibody levels similar to that observed after the normal human dose of vaccine. However, in this group the second immunization did not result in any increase in cross-reactive HI antibodies, and the peak serum antibody response was observed 1 week later than in the other vaccination groups. Therefore, our murine data suggest that the different low-dose vaccines investigated are not as effective as the normal human dose in eliciting a rapid and desirable serum antibody response. However, in case of a limited vaccine supply, the use of a low-dose aluminum-adjuvanted vaccine may be a feasible alternative.
Acknowledgments
This study was supported by the Norwegian Ministry of Health and Care Services and the European Union (Flupan QLK2-CT-2001-01786).
We thank Nina Harkestad for excellent technical assistance, the staff at the animal house at the University of Bergen for their help and care of the mice, and Geir Egil Eide and Stein Atle Lie for help with the statistics. We also thank Fred Vogel (Sanofi Pasteur) for useful discussion and for supplying the vaccine and the aluminum adjuvant.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. [DOI] [PubMed] [Google Scholar]
- 2.Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351:2286-2294. [DOI] [PubMed] [Google Scholar]
- 3.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657-1664. [DOI] [PubMed] [Google Scholar]
- 4.Brewer, J. M. 2006. (How) do aluminium adjuvants work? Immunol. Lett. 102:10-15. [DOI] [PubMed] [Google Scholar]
- 5.Cox, R. J., K. A. Brokstad, M. A. Zuckerman, J. M. Wood, L. R. Haaheim, and J. S. Oxford. 1994. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 12:993-999. [DOI] [PubMed] [Google Scholar]
- 6.Cox, R. J., E. Mykkeltvedt, J. Robertson, and L. R. Haaheim. 2002. Non-lethal viral challenge of influenza haemagglutinin and nucleoprotein DNA vaccinated mice results in reduced viral replication. Scand. J. Immunol. 55:14-23. [DOI] [PubMed] [Google Scholar]
- 7.Hauge, S., A. S. Madhun, R. J. Cox, K. A. Brokstad, and L. R. Haaheim. 2007. A comparison of the humoral and cellular immune responses at different immunological sites after split influenza virus vaccination of mice. Scand. J. Immunol. 65:14-21. [DOI] [PubMed] [Google Scholar]
- 8.Hehme, N., H. Engelmann, W. Kuenzel, E. Neumeier, and R. Saenger. 2004. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 103:163-171. [DOI] [PubMed] [Google Scholar]
- 9.Hehme, N., H. Engelmann, W. Kunzel, E. Neumeier, and R. Sanger. 2002. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med. Microbiol. Immunol. 191:203-208. [DOI] [PubMed] [Google Scholar]
- 10.Hobson, D., R. L. Curry, A. S. Beare, and A. Ward-Gardner. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 70:767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovden, A. O., R. J. Cox, and L. R. Haaheim. 2005. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand. J. Immunol. 62:36-44. [DOI] [PubMed] [Google Scholar]
- 12.Hovden, A. O., R. J. Cox, A. Madhun, and L. R. Haaheim. 2005. Two doses of parenterally administered split influenza virus vaccine elicited high serum IgG concentrations which effectively limited viral shedding upon challenge in mice. Scand. J. Immunol. 62:342-352. [DOI] [PubMed] [Google Scholar]
- 13.Huber, V. C., R. M. McKeon, M. N. Brackin, L. A. Miller, R. Keating, S. A. Brown, N. Makarova, D. R. Perez, G. H. Macdonald, and J. A. McCullers. 2006. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 13:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keitel, W. A., T. R. Cate, R. L. Atmar, C. S. Turner, D. Nino, C. M. Dukes, H. R. Six, and R. B. Couch. 1996. Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin. Diagn. Lab. Immunol. 3:507-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keitel, W. A., R. B. Couch, T. R. Cate, K. R. Hess, B. Baxter, J. M. Quarles, R. L. Atmar, and H. R. Six. 1994. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J. Clin. Microbiol. 32:2468-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 17.Lin, J., J. Zhang, X. Dong, H. Fang, J. Chen, N. Su, Q. Gao, Z. Zhang, Y. Liu, Z. Wang, M. Yang, R. Sun, C. Li, S. Lin, M. Ji, Y. Liu, X. Wang, J. Wood, Z. Feng, Y. Wang, and W. Yin. 2006. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368:991-997. [DOI] [PubMed] [Google Scholar]
- 18.Lindblad, E. B. 2004. Aluminium adjuvants—in retrospect and prospect. Vaccine 22:3658-3668. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad, E. B. 2004. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 82:497-505. [DOI] [PubMed] [Google Scholar]
- 20.Lipatov, A. S., E. A. Govorkova, R. J. Webby, H. Ozaki, M. Peiris, Y. Guan, L. Poon, and R. G. Webster. 2004. Influenza: emergence and control. J. Virol. 78:8951-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, X., L. E. Edwards, J. A. Desheva, D. C. Nguyen, A. Rekstin, I. Stephenson, K. Szretter, N. J. Cox, L. G. Rudenko, A. Klimov, and J. M. Katz. 2006. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 24:6588-6593. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937-1943. [DOI] [PubMed] [Google Scholar]
- 23.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 24.Sjolander, A., K. Lovgren Bengtsson, M. Johansson, and B. Morein. 1996. Kinetics, localization and isotype profile of antibody responses to immune stimulating complexes (iscoms) containing human influenza virus envelope glycoproteins. Scand. J. Immunol. 43:164-172. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson, I., K. G. Nicholson, J. M. Wood, M. C. Zambon, and J. M. Katz. 2004. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 4:499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treanor, J., W. Keitel, R. Belshe, J. Campbell, G. Schiff, K. Zangwill, M. Wolff, A. Klimov, R. Levandowski, and L. Lambert. 2002. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine 20:1099-1105. [DOI] [PubMed] [Google Scholar]