Abstract
The performance of the SD Bioline rapid antigen test kit for influenza virus detection was evaluated with 295 respiratory specimens during the influenza season. The overall sensitivity and specificity of the SD Bioline test were 61.9% and 96.8% for the influenza A virus antigen and 54.5% and 100% for the influenza B virus antigen, respectively. The results were consistent with peak influenza activities.
Influenza viruses are responsible for epidemics resulting in morbidity and excess mortality among the very young, the elderly, and those with chronic illness (10, 11). The rapid and accurate detection of influenza viruses is essential for appropriate antiviral therapy early in the illness and contributes to the reduction of medical costs and the lengths of hospital stays (5).
Recently developed rapid influenza tests have had a positive impact on medical management by decreasing the need for ancillary tests and the use of antibiotics in children evaluated for an influenza-like illness in an emergency department (4, 12). Furthermore, they have increasingly become a useful tool for the surveillance of influenza activity (7, 13, 16).
Several rapid influenza antigen detection kits using enzyme, optical, or chromatographic immunoassays provide a result in 15 to 30 min, with reported sensitivities ranging from 62% to 85% and specificities ranging from 79.5% to 99% compared to viral cultures (1, 14).
A newly developed SD Bioline rapid influenza test (Standard Diagnostics, Inc., Suwon, Korea) is a lateral-flow immunoassay that uses influenza virus-specific monoclonal antibodies on a strip for the qualitative detection of influenza A and B virus antigens, separately. However, the performance of the test has not been determined to date. In this study, we compared the performance of the SD Bioline test with that of the QuickVue test (Quidel Corp., San Diego, CA), a nontyping lateral-flow immunoassay (3) which is currently the only rapid influenza test available in Korea, and further evaluated its clinical usefulness during the influenza season.
The study population was comprised of 295 Korean children and adults who were admitted to or visited the outpatient clinics at Korea University Anam Hospital with symptoms of acute respiratory tract illness during the 2005-to-2006 influenza season. Demographic and clinical data were collected using case report forms. Written informed consent to participate in the study was obtained from patients or their parents. The study protocol was approved by the hospital ethics committee (approval no. AN0560).
Three anterior nasal or throat swab specimens per adult patient were collected. One swab, for virus culture, was transferred in standard refrigerated HH medium (Hanks' balanced salt solution with 0.88% sodium bicarbonate, 0.2% bovine serum albumin [Sigma], 30 mM HEPES buffer, and 50 μg/ml gentamicin [9]), and the other two swabs were used for rapid influenza virus antigen testing. Nasopharyngeal aspirates and two throat swabs collected from each pediatric patient were used for virus culture and the influenza virus antigen tests, respectively. The specimen for culture was immediately placed in 2 ml of HH medium after the collection, transported to the virus laboratory on ice, and kept at −20°C until the culture was performed. Virus culture was performed using the cryopreserved R-Mix cultures (Diagnostic Hybrids, Inc., Athens, OH), as previously described (2, 8).
The QuickVue and SD Bioline tests were run in parallel. Four physicians (two pediatric and two infectious disease doctors) performed the tests, as described in the package inserts, at bedside or at the outpatient clinic throughout the study period. Briefly, both tests involved the extraction of influenza virus antigens from the patients' specimens. Each swab specimen was placed in a tube containing an extraction agent provided in each test kit. After the disruption of the viral particles, a test strip from each test kit was placed in the extraction tube and allowed to react with the influenza virus-specific monoclonal antibodies for 10 to 30 min. In the SD Bioline test, a pink-to-purple test line in the A or B region with the presence of a purple control line indicates a positive result for influenza virus A or B, respectively. With the QuickVue test, a pink-to-red test line in the presence of a blue control line indicates a positive result (3).
Data are represented as medians (ranges), unless otherwise indicated. The variables or frequencies were compared between the two groups using the Student t test or the chi-square test. Viral cultures positive for influenza virus A or B were considered true positives. All data analyses were performed using SPSS version 12.0 (SPSS, Inc., Chicago, IL).
The clinical characteristics of the study population are shown in Table 1. Among the 295 patients tested, overall detection rates for the virus culture, the QuickVue test, and the SD Bioline test were 25.4%, 15.9%, and 17.6%, respectively. Although the sensitivities and the specificities of the SD Bioline test were somewhat higher than those of the QuickVue test, none of these differences was significant (Table 2). Both tests were simple and easy to perform at bedside, but the SD Bioline test showed somewhat slow color development compared to the QuickVue test.
TABLE 1.
Characteristics of the study population
| Characteristic | Value for:
|
|
|---|---|---|
| Children | Adults | |
| No. of patients (no. of males/no. of females) | 174 (94/80) | 121 (56/65) |
| Age, yr (range)a | 4.8 (0.3-15.0) | 48.0 (18.5-81.9) |
| No. (%) of patients hospitalized | 112 (64.6) | 62 (51.2) |
| Duration of hospitalization, days (range)a | 5 (2-11) | 12 (2-205) |
| Duration of fever, days (range)a | 2 (1-21) | 3 (1-36) |
| Interval between fever onset and testing, days (range)a | 2 (1-13) | 4 (1-27) |
Differences were considered statistically significant at a P of <0.05.
TABLE 2.
Performance of two rapid tests for the detection of influenza viruses
| Rapid test and virus detected | No. of specimens that were:
|
Sensitivity (%) | Specificity (%) | Predictive value (%)
|
||||
|---|---|---|---|---|---|---|---|---|
| Rapid test positive, culture positive | Rapid test positive, culture negative | Rapid test negative, culture positive | Rapid test negative, culture negative | Positive | Negative | |||
| SD Bioline test | ||||||||
| Influenza A virus | 26 | 8 | 16 | 245 | 61.9 | 96.8 | 76.5 | 93.1 |
| Influenza B virus | 18 | 0 | 15 | 262 | 54.5 | 100 | 94.6 | 94.9 |
| QuickVue test | 35 | 12 | 40 | 208 | 46.7 | 94.5 | 74.5 | 83.9 |
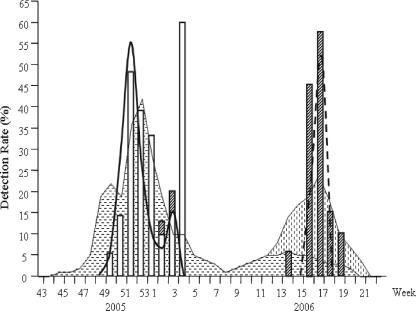
There were two outbreaks of local influenza activity reported by the Korean Influenza Surveillance Scheme (KISS), Korean Center for Disease Control and Prevention. The first outbreak (the 52nd week of 2005) was due to influenza virus type A, and the second outbreak (the 17th week of 2006) was due mainly to the type B virus (Fig. 1). The sensitivities of the QuickVue test and the SD Bioline test rose to 67.6% and 70.3% during the first outbreak and 37.9% and 62.1% during the second outbreak, respectively. However, the SD Bioline test showed an unacceptably low positive predictive value when the virus prevalence was low, suggesting its limitation as a screening program outside the influenza season.
FIG. 1.
Detection of influenza viruses by culture and by the SD Bioline rapid antigen test in conjunction with an epidemic curve with two peaks of influenza activity based on the report from the KISS for the 2005-to-2006 season., influenza A reported by the KISS;
influenza A reported by the KISS; , influenza B reported by the KISS; □, influenza A virus detected by culture;
, influenza B reported by the KISS; □, influenza A virus detected by culture;  , influenza B virus detected by culture; —, influenza A virus detected by the SD Bioline test; ----, influenza B virus detected by the SD Bioline test.
, influenza B virus detected by culture; —, influenza A virus detected by the SD Bioline test; ----, influenza B virus detected by the SD Bioline test.
In this study, the SD Bioline test showed excellent specificity for the detection of the influenza A and B viruses and fair-to-good sensitivities, compared to those of other studies of rapid influenza tests (1, 14). Differences in the specimen collection methods and the age of the study population are the likely explanation. It has been reported that nasopharyngeal aspirates are superior to nasal or throat swabs for the detection of influenza viruses by rapid antigen testing (6). Anterior nasal or throat swabs were easier and faster to collect and were mostly preferred by the adult patients, which might contribute to the lower sensitivities in this study. In addition, lower quantities of virus shedding have been reported for older patients than for children (15).
In conclusion, the SD Bioline rapid influenza test is useful for detecting influenza virus type A and type B. Within the recognized limitations, it might be a part of influenza surveillance programs for the influenza season.
This study was financially supported in part by Standard Diagnostics, Inc., Suwon, Korea, and in part by City of Seoul grant no. 10920.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Agoritsas, K., K. Mack, B. K. Bonsu, D. Goodman, D. Salamon, and M. J. Marcon. 2006. Evaluation of the Quidel QuickVue test for detection of influenza A and B viruses in the pediatric emergency medicine setting by use of three specimen collection methods. J. Clin. Microbiol. 44:2638-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenfanger, J., C. Drake, T. Mueller, T. Troutt, J. O'Brien, and K. Guttman. 2001. R-Mix cells are faster, at least as sensitive and marginally more costly than conventional cell lines for the detection of respiratory viruses. J. Clin. Virol. 22:101-110. [DOI] [PubMed] [Google Scholar]
- 3.Bellei, N., D. Benfica, A. H. Perosa, R. Carlucci, M. Barros, and C. Granato. 2003. Evaluation of a rapid test (QuickVue) compared with the shell vial assay for detection of influenza virus clearance after antiviral treatment. J. Virol. Methods 109:85-88. [DOI] [PubMed] [Google Scholar]
- 4.Bonner, A. B., K. W. Monroe, L. I. Talley, A. E. Klasner, and D. W. Kimberlin. 2003. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 112:363-367. [DOI] [PubMed] [Google Scholar]
- 5.Cazacu, A. C., J. Greer, M. Taherivand, and G. J. Demmler. 2003. Comparison of lateral-flow immunoassay and enzyme immunoassay with viral culture for rapid detection of influenza virus in nasal wash specimens from children. J. Clin. Microbiol. 41:2132-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, K. H., N. Maldeis, W. Pope, A. Yup, A. Ozinskas, J. Gill, W. H. Seto, K. F. Shortridge, and J. S. M. Peiris. 2002. Evaluation of the Directigen FluA+B test for rapid diagnosis of influenza virus type A and B infections. J. Clin. Microbiol. 40:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effler, P. V., M. Ieong, T. Tom, and M. Nakata. 2002. Enhancing public health surveillance for influenza virus by incorporating newly available rapid diagnostic tests. Emerg. Infect. Dis. 8:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong, C. K. Y., M. K. Lee, and B. P. Griffith. 2000. Evaluation of R-Mix FreshCells in shell vials for detection of respiratory viruses. J. Clin. Microbiol. 38:4660-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen, C., and F. B. Johnson. 1994. Comparison of various transport media for viability maintenance of herpes simplex virus, respiratory syncytial virus, and adenovirus. Diagn. Microbiol. Infect. Dis. 19:137-142. [DOI] [PubMed] [Google Scholar]
- 10.McBean, A. M., J. D. Babish, and J. L. Warren. 1993. The impact and cost of influenza in the elderly. Arch. Intern. Med. 153:2105-2111. [PubMed] [Google Scholar]
- 11.Neuzil, K. M., Y. Zhu, M. R. Griffin, K. M. Edwards, J. M. Thompson, S. J. Tollefson, and P. F. Wright. 2002. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J. Infect. Dis. 185:147-152. [DOI] [PubMed] [Google Scholar]
- 12.Noyola, D. E., and G. J. Demmler. 2000. Effect of rapid diagnosis on management of influenza A infections. Pediatr. Infect. Dis. J. 19:303-307. [DOI] [PubMed] [Google Scholar]
- 13.Pregliasco, F., S. Puzelli, C. Mensi, G. Anselmi, R. Marinello, M. L. Tanzi, C. Affinito, M. C. Zambon, and I. Donatelli. 2004. Influenza virological surveillance in children: the use of the QuickVue rapid diagnostic test. J. Med. Virol. 73:269-273. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez, W. J., R. H. Schwartz, and M. M. Thorne. 2002. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr. Infect. Dis. J. 21:193-196. [DOI] [PubMed] [Google Scholar]
- 15.Steininger, C., M. Kundi, S. W. Aberle, J. H. Aberle, and T. Popow-Kraupp. 2002. Effectiveness of reverse transcription-PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J. Clin. Microbiol. 40:2051-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunderli, W., Y. Thomas, D. A. Muller, M. Dick, and L. Kaiser. 2002. Rapid antigen testing for the surveillance of influenza epidemics. Clin. Microbiol. Infect. 9:295-300. [DOI] [PubMed] [Google Scholar]