Abstract
Impaired BK virus (BKV)-specific immunity is a key risk factor of polyomavirus-associated nephropathy. We hypothesized that BKV agnoprotein might constitute an important immune target, as it is highly expressed after infection in vitro. We demonstrate abundant expression of BKV agnoprotein in vivo by immunostaining of kidney transplant (KT) biopsy specimens. Antibody responses to the recombinant affinity-purified BKV agnoprotein, large tumor (LT), and VP1 antigens in 146 sera from 38 KT patients and in 19 sera from 16 healthy donors (HD) were compared by enzyme immunoassay. In HD, low titers of anti-agnoprotein immunoglobulin G (IgG) were found in 15% of sera, compared to 41% for anti-LT antigen and 63% for anti-VP1. No anti-BKV IgM was detectable. In KT patients, anti-agnoprotein IgG and IgM were found in 8% and 3.6% of sera, compared to 63% and 18% for anti-LT IgG and IgM and 80% and 41% for anti-VP1 IgG and IgM, respectively. Anti-LT antigen and anti-VP1, but not anti-agnoprotein, activities increased during and after BKV viremia in KT patients. To investigate specific cellular immune responses, we compared levels of gamma interferon production in peripheral blood mononuclear cells (PBMC) of 10 HD and 30 KT patients by enzyme-linked immunospot assay. In HD, the median numbers of gamma interferon spot-forming units per million PBMC for the agnoprotein, LT antigen, and VP1 peptides were 1, 23, and 25, respectively, whereas the responses in KT patients were 2, 24, and 99, respectively. We conclude that BKV agnoprotein, though abundantly expressed in vivo, is poorly recognized immunologically.
The human polyomavirus BK virus (BKV) is the primary etiological agent of polyomavirus-associated nephropathy (PVAN), which causes irreversible graft loss in 1 to 10% of kidney transplant (KT) patients (15, 31). BKV was first discovered in 1970 in the urine of a KT patient with the initials B.K. who had ureteric stenosis and abundant decoy cell shedding (9). However, BKV asymptomatically infects 60 to 90% of the human population (23) and establishes a state of nonreplicative infection in the renourinary tract (13, 15). Intermittent low-level urinary replication with BKV loads of ≤10e6 per ml is detected in 5% of immunocompetent individuals, whereas high-level replication with BKV loads of ≥10e7 per ml is found in 20 to 60% of immunosuppressed patients (15). In KT patients, high-level urine BKV replication is found in 30%, which may be followed by BKV viremia in 13% and by histologically confirmed PVAN in 8% of patients (18). The risk factors for PVAN are not conclusively defined and likely involve complementing determinants of the triad of recipient, graft, and virus (17). Disruption of the balance between the BKV replication and host immune control is generally viewed as a key element of PVAN pathogenesis (5). In the absence of validated antivirals, reducing maintenance immunosuppression represents the primary treatment option for presumptive or definitive PVAN (3, 39).
BKV belongs to the genus Polyomavirus of the Polyomaviridae family, along with the related human polyomavirus JC virus (JCV) and simian virus 40 (SV40). The genomes are 70% homologous and consist of a circular double-stranded DNA of about 5,300 bp which can be divided into the noncoding control region (NCCR), containing the origin of replication and promoters of gene transcription, and the early and late gene regions (21, 37). The early genes encode two regulatory proteins called the small tumor and large tumor (LT) antigens. The late genes comprise genes encoding the viral capsid proteins VP1, VP2, and VP3, as well as a small open reading frame encoding a basic protein of 66 amino acids at the 5′ end of the VP1 mRNA. Studies of BKV infection of human endothelial cells and various cell lines demonstrated that BKV agnoprotein is abundantly expressed in the cytoplasm, with perinuclear accumulations (12, 34, 35). BKV agnoprotein, as well as the closely related JCV and SV40 agnoproteins, is expressed in a defined interval after early LT antigen expression, together with VP1 (24, 26, 28, 35). The function of BKV agnoprotein is not well defined, but data from BKV, JCV, and SV40 studies indicated roles in capsid assembly, virion egress, cell cycle regulation, viral replication, and gene expression. In vivo data on agnoprotein expression have been reported only for JCV replicating in brain tissue of cases with progressive multifocal leukoencephalopathy (29). Similar in vivo data are lacking for BKV agnoprotein, except for an isolated fatal human immunodeficiency virus (HIV)/AIDS case with meningoencephalitis, pneumonitis, nephritis, and disseminated BKV replication (2). In our ongoing study to identify relevant targets of the immune system controlling BKV replication in KT patients (1), we hypothesized that agnoprotein might represent an important antigen, given the conserved nature of the protein and the abundant expression pattern in vitro. We have therefore assessed the expression of BKV agnoprotein in vivo in kidney biopsy samples from KT patients and have compared both the humoral and cellular immune responses against this viral protein in KT patients and in healthy controls.
MATERIALS AND METHODS
Study participants.
Plasma and urine samples were obtained, with informed consent according to the protocol of the Ethics Committee of the Basel Cantons, from 16 healthy donors (HD) and 38 KT patients (Table 1). Samples from KT patients were divided into three groups according to the present and past levels of BKV viremia and/or viruria: group A comprised all samples from patients without high-level BKV replication (“no/low-level replication”); group B comprised all samples from patients with BKV viremia and/or viruria of >10e4 or >10e7 copies/ml, respectively (“high-level” replication); and group C comprised samples from those patients who had documented high-level BKV replication with definitive or presumptive PVAN (16) earlier but in whom this had decreased by >1.5 log10 by the time of sample drawing (past “high-level” replication).
TABLE 1.
Characteristics of study participants
| Characteristic | Value for:
|
Statistical comparison | ||||
|---|---|---|---|---|---|---|
| HD | KT patient groupa
|
|||||
| All | A | B | C | |||
| No. of subjects | 16 | 38b | 16 | 27 | 23 | |
| Age (yr) at drawing of first sample | ||||||
| Median | 39.4 | 52.8 | 48.0 | 52.3 | 53.9 | NSe (HD vs all) |
| Range | 27.4-59.0 | 17.2-71.1 | 18.1-58.2 | 17.2-71.1 | 25.7-69.1 | |
| No. (%) male | 8 (50.0) | 24 (63.2) | 10 (62.5) | 17 (63.0) | 10 (43.5) | NS (HD vs all) |
| Time (wk) posttransplantationc | ||||||
| Median | 30.3 | 9.4 | 25.5 | 120.3 | P = 0.003 (A vs B); P < 0.0001 (A vs C and B vs C) | |
| Range | 2.3-462.1 | 2.3-380.7 | 4.3-449.9 | 14.0-462.1 | ||
| BKV plasma load (log cp/ml) | ||||||
| Median | Not detectable | 4.0 | 3.0 | 4.7 | 3.0 | P < 0.0001 (A vs B and B vs C); NS (A vs C) |
| Range | 3.0d-7.7 | 3.0-3.7 | 3.0-7.7 | 3.0-3.8 | ||
A, no/low-level replication; B, high-level replication; C, past replication.
For some kidney transplant recipients, samples were drawn before the onset of, during, and after high-level BKV replication. Therefore, this number is smaller than the total for groups A, B, and C.
Values are for all the samples in the corresponding subject group.
Set limit of detection; also if no plasma sample was tested.
NS, not significant.
Quantitative PCR for BKV DNA detection.
Measurements of BKV viral load in plasma and urine were performed according to standard real-time PCR protocols. Briefly, DNA was isolated with the QIAamp DNA minikit (QIAGEN, Hilden, Germany) and quantified by real-time PCR (TaqMan/7700, Stratagene Mx4000, or Bio-Rad iCycler) as described previously (19).
For the detection of BKV in biopsy specimens, cells were scraped under sterile conditions, incubated at 95°C for 10 min, transferred to ice, and then treated with 5 μl of proteinase K (5 mg/ml) at 55°C for 3 to 5 h, followed by enzyme inactivation at 95°C for 10 to 15 min. Subsequently, the DNA was quantified by real-time PCR using an ABI Prism 7900 HT sequence detector (Applied Biosystems, Rotkreuz, Switzerland). The following primers were used for BKV LT antigen amplification: PYV.for (5′-TAGGTGCCAACCTATGGAACAGA-3′) and PYV.rev (5′-GAAAGTCTTTAGGGTCTTCTACC-3′). The BKV probe was 5′-FAM-CATTAAAGGAACTCCACCAGGACTCCCACTC-TAMRA-3′.
Infection and immunofluorescence microscopy.
Renal proximal tubule epithelial cells (Cambrex CC-2553) were infected with BKV (Dunlop) as described previously for human umbilical vein epithelial cells (10). At 2 days postinfection, cells were washed with phosphate-buffered saline (PBS) and fixed with methanol. Subsequently, cells were incubated at 37°C with primary and secondary antibodies for 30 min. The following antibodies were used: monoclonal SV40 T antibody (Pab416; Calbiochem, San Diego, CA) (dilution, 1:100) and rabbit antiserum directed against BKV agnoprotein (anti-BKV agnoprotein serum) (dilution, 1:800) (14, 35). The secondary antibodies were conjugated with AlexaFluor 568 or 488 (1:500; Molecular Probes, Inc., Eugene, OR). Images were collected using a Nikon TE2000 microscope equipped and processed with NIS Elements Basic Research software version 2.2 (Nikon Corporation).
Staining of kidney biopsy specimens for BKV protein expression.
Samples were screened for the presence of BKV agnoprotein and LT antigen by in situ immunohistochemical staining. Briefly, 3-μm tissue sections were dewaxed twice in xylene, followed by rehydration in a graded series of ethanol solutions. Nonenzymatic antigen retrieval was performed by heating the sections in 0.1 M citrate buffer (pH 6.0), first at 900 W for 3 min and then at 90 W for 30 min. After cooling in cold water for 15 min, the slides were washed in APK wash solution (Ventana Medical Systems Inc., Tucson, AZ). Endogenous peroxidase was quenched, and tissues were permeabilized by the incubation of sections in methanol containing 1.1% H2O2 at 37°C for 4 min and then washed in APK wash solution at room temperature (RT) for 5 min.
For immunostaining of agnoprotein, sections were incubated with the primary antibody at 37°C for 1 h, using the anti-BKV agnoprotein serum (14, 35) diluted 1:600. As a negative control, a set of parallel sections were incubated with rabbit preimmune serum. EnVision+ horseradish peroxidase (Dako, Glostrup, Denmark) was applied as a secondary antibody to the sections at RT for 30 min. The sections were developed with liquid diaminobenzidine+ substrate-chromogen (Dako) and counterstained with Shandon's instant hematoxylin (Shandon Inc., Pittsburgh, PA). Finally, the sections were mounted in Eukitt (Kindler GmbH, Freiburg, Germany) after dehydration in a graded series of ethanol solutions and in xylene.
For double immunostaining to detect LT antigen and agnoprotein simultaneously, sections were first incubated with the LT antigen-cross-reacting anti-SV40 LT antigen clone PAb416 (1:400 dilution) at 37°C for 30 min. Envision+ horseradish peroxidase and liquid diaminobenzidine+ substrate-chromogen were added as described above. Then, after being washed, the slides were incubated with the anti-BKV agnoprotein serum (1:600 dilution) as described above. Envision alkaline phosphatase (Dako) was added as a secondary antibody to the sections, with incubation at RT for 30 min. Finally, the sections were developed with Vector-red (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin, before being mounted in Eukitt.
Generation of recombinant baculoviruses expressing GST-BKV fusion proteins.
The sequence encoding the BKV agnoprotein was amplified by PCR from the urine of a KT patient who was shedding archetype NCCR BKV serotype 1. The following primers were used: Agno-for (5′-TTTTGGATCCACCATGGTTCTGCGCCAGCTG-3′) and Agno-rev (5′-TTTTGCGGCCGCTAGGAGTCTTTTACAGAGTCT-3′). The resulting amplicon was cloned into the pTRE2pur plasmid (Clontech, Mountain View, CA), yielding pTRE-agno, and verified by sequencing. The entire BKV agnoprotein gene was again amplified by PCR, using pTRE-agno as the template and the primers Agno-TEV-for (5′-TTGGA TCCCCGAAAACCTGTATTTTCAGGGCATGGTTCTGCGCCAGCTGTC- 3′) and Agno-rev. The resulting amplicon was cloned into the multiple cloning site of the pFastBacGST plasmid, digested with BamHI and NotI, cloned in frame with the gene coding for glutathione S-transferase (GST) (yielding pFastBacGST-BKVagno), and confirmed by restriction analysis and sequencing. The coding sequences of the entire BKV VP1 and the amino-terminal 15.6-kDa domain of BKV LT antigen (amino acids 1 to 133) were amplified using the primers BKV-LTD1-for (5′-GCGCGGATCCCCGAAAACCTGTATTTTCAGGGCATGGATAAGTT CTTAACAGGGAAGA-3′) and BKV-LTD1-rev (5′-TTTTCTCGAGTTACTTTCTTTTTTTTTTGGGTGGTGTTG-3′) for LT antigen and BKVP1-f (5′-GCGCGGATCCCCGAAAACCTGTATTTTCAGGGCATGGCCCCAACCGGCCCCA ACCAAAAGAAAAGGA-3′) and BKVP1-r (5′-TTTTCTCGAGTTAAAGCATTTTGGTTTGCA-3′) for VP1 antigen to generate comparably sized BKV LT domain 1 (LTD1), consisting of the amino-terminal 133 amino acids containing the sequences of the J domain, the Rb binding and nuclear localization sequences, and full-length VP1 amplicons that could be inserted in frame downstream of GST in pFastBacGST by using the restriction enzymes BamHI-XhoI and BamHI-HindIII, yielding pFastBacGST-BKV-LTD1 and pFastBacGST-BK-VP1 (S. Bodaghi and H. H. Hirsch, unpublished data).
Expression and purification of recombinant antigens GST-BKV agnoprotein, GST-BKV LTD1, and GST-BKV VP1.
Recombinant baculoviruses containing the coding sequence for the GST-BKV fusion proteins were generated using the Bac-to-Bac expression systems (Invitrogen Ltd., Carlsbad, CA) according to the manufacturer's instructions. Recombinant bacmids were isolated and used for transfection of Sf9 insect cells with the Cellfectin reagent. Recombinant baculovirus was recovered after 48 h and used for subsequent infection for Sf9 cells. Sf9 cell lysates were prepared between days 3 and 5 postinfection. GST fusion proteins (GST-BKV agnoprotein, GST-BKV VP1, and GST-BKV LTD1) and GST were purified by glutathione affinity chromatography using glutathione Sepharose 4 fast flow (GE Healthcare UK Ltd., Buckinghamshire, United Kingdom) according to the manufacturer's instructions. Protein expression and purity were assessed by polyacrylamide gel electrophoresis, Coomassie blue staining, Western blotting using the anti-GST antibody B-14 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or enzyme-linked immunosorbent assay using the anti-BKV agnoprotein serum (14), the cross-reacting SV40 LT Pab416, or the BKV VP1 antiserum (35).
Enzyme immunoassay (EIA) with GST-BKV fusion proteins.
Standard 96-well EIA plates with high-level coating properties were used for coating for 16 h overnight at 4°C. The wells were washed five times with 0.1% Tween 20, treated with blocking buffer (PBS [pH 7.4], 4.0% bovine serum albumin, 0.1% Tween 20) at RT for 2 h, and washed three times. The wells were incubated for 1 h at RT with 100 μl of diluted patient sera, washed five times, incubated with the secondary antibodies at RT for 1 h, and washed again five times before application of the secondary anti-human immunoglobulin G (IgG) or anti-human IgM antibodies (Sigma-Aldrich), washing, and detection using o-phenylenediamine hydrochloride (Sigma-Aldrich, St. Louis, MO) for 30 min at RT by adding 1 N sulfuric acid. Optical densities (ODs) were measured using an automated plate reader (Tecan Group Ltd., Männedorf, Switzerland) at 492 nm. Equivalents of 1.0 pmol of antigen/well, serum dilutions of 1:400, and dilutions of anti-IgG and -IgM antibodies of 1:10,000 were found to yield minimal background. Affinity-purified GST was run as a negative control and subtracted from the GST-BKV LTD1, GST-BKV VP1, and GST-BKV agnoprotein signals. For every sample, the OD was determined by subtraction of the GST background applied in parallel. The cutoff was defined as two standard deviations the GST background level taken as the negative control. Therefore, all OD values of <0.0400 were deemed to be negative. Values from 0.0400 to 0.0999 were classified as + (borderline positive), values of >0.1000 as 1+ (positive), and values of >1.0000 as 2+ (strongly positive). For each antigen, all samples tested for IgG were run on the same day, as were all samples tested for IgM. For confirmatory testing, we included three selected samples as references, which were negative, strongly positive, and borderline positive, indicating variation coefficients of <10%, which can cause minimal shifts in the groups.
Testing patient sera for agnoprotein antibodies on UTA-6 agnoprotein-expressing cells.
UTA-6 cells (8) were transfected with pTRE-agno in the absence of tetracycline. After 44 h, cells were transferred to wells of 10-well glass slides and incubated at 37°C in a CO2 atmosphere for 4 h. Cells were washed in PBS, fixed in cold 100% acetone for 5 min, and air dried. As a negative control, naive UTA-6 cells were treated in parallel. For immunofluorescence, cells were incubated with patient sera diluted in PBS-bovine serum albumin (1:40 or 1:160) at RT for 1 h, washed in PBS, incubated with fluorescein isothiocyanate-labeled anti-human IgG diluted 1:3,200 (bioMerieux, France) as before, washed, mounted with N-propyl gallate in glycerin, and viewed with a Nikon E800 epifluorescence microscope.
BKV overlapping peptide pools.
Overlapping peptide pools which covered the entire coding sequence of the BKV LT antigen (ppLT), VP1 (ppVP1), and agnoprotein (ppAgno) as defined by the sequence of the BKV Dunlop strain (GenBank accession no. V01108) were used for peripheral blood mononuclear cell (PBMC) stimulation. The pools consisted of 180 (LT antigen), 88 (VP1), and 16 (agnoprotein) peptides with 15 amino acids each and 11-amino-acid overlaps (Eurogentec, Geneva, Switzerland), dissolved in dimethyl sulfoxide.
ESA for IFN-γ.
The PBMCs for enzyme-linked immunospot assay (ESA) were obtained from 10 HD and 30 KT patients as described previously (1). PBMCs were isolated in CPT tubes (Becton-Dickinson, Allschwil, Switzerland). The cells were washed, counted, and stored frozen in fetal calf serum containing 10% dimethyl sulfoxide (Sigma-Aldrich). For ESA, PBMCs were diluted in RPMI 1640-5% human serum-1% Glutamax-1% PenStrep (R5AB) (Sigma-Aldrich). The BKV-specific cellular immune response was determined by measuring gamma interferon (IFN-γ) upon stimulation of PBMCs in an ESA. Ninety-six-well multiscreen filter plates (MSIPN; Millipore, Volketswil, Switzerland) were coated with 100 μl (10 μg/ml) mouse anti-human IFN-γ monoclonal antibody (1-D1K; Mabtech, Hamburg, Germany) and incubated overnight at 4°C. PBMCs were seeded at a concentration of 2.5 × 105/well (2.5 × 104/well for the positive control) and incubated overnight at 37°C in 5% CO2 with 2 μg/ml pooled BKV antigens (ppLT, ppVP1, and ppAgno), a negative control (R5AB), and 1 μg/ml Staphylococcus enterotoxin B (Sigma-Aldrich) as a positive control. ESAs were performed in triplicate. After incubation, the plates were washed with PBS with 0.05% Tween 20 (Sigma-Aldrich), incubated at RT for 2 h with 100 μl (1 μg/ml) detection biotinylated mouse anti-human IFN-γ monoclonal antibody (7-B6-1 biotin; Mabtech), washed again, and incubated at RT for 1 h with 100 μl streptavidin ALP (1 μg/ml; Mabtech). Spots were developed by treatment with 100 μl 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Calbiochem, Lucerne, Switzerland) per well for 10 min, washed 5 times with H2O from both sides, and dried in the dark. IFN-γ spots were counted by using an ESA reader (AID, Büron, Switzerland). The number of spots per well was determined by subtracting the negative control value.
Statistical methods.
Statistical analyses were performed using XLSTAT 2006 software (Addinsoft, New York, NY). Comparisons between immune responses to different antigens were performed using the Kruskal-Wallis test for nonparametric dependent samples, with the Bonferroni adjustment for pairwise comparisons. Comparisons between immune responses to a single antigen in the different subject groups were performed using the Friedman test for nonparametric independent samples, again with the Bonferroni adjustment for pairwise comparisons.
RESULTS
BKV agnoprotein is abundant in the cytoplasm of infected renal tubular cells.
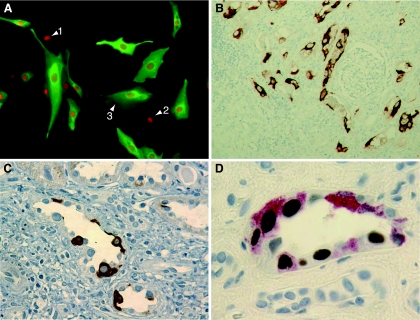
We investigated the patterns of expression of agnoprotein and LT antigen in renal primary proximal tubular epithelial cells at 2 days postinfection with BKV strain Dunlop (Fig. 1A). BKV agnoprotein was located predominantly in the cytoplasm of renal proximal tubular epithelial cells (green fluorescence), whereas LT antigen was detected exclusively in the nucleus (red fluorescence). The cells were positive either for LT antigen alone (Fig. 1A, cell 1), for LT antigen and low-level cytoplasmic agnoprotein (cell 2), or for high-level cytoplasmic agnoprotein (cell 3) but were never positive for agnoprotein without LT antigen. The data demonstrate that BKV agnoprotein is abundantly expressed in the cytoplasm of renal primary proximal tubular epithelial cells infected in vitro, as expected for the BKV late gene.
FIG. 1.
BKV agnoprotein expression in kidney tubular epithelial cells. (A) Indirect immunofluorescence of primary kidney proximal tubular epithelial cell culture infected with BKV strain Dunlop and stained at 48 h postinfection with rabbit anti-BKV agnoprotein serum (green) and anti-SV40 LT Pab416 (red). Arrows indicate cells with LT antigen (cell 1), LT antigen plus a little agnoprotein (cell 2), and LT antigen plus abundant agnoprotein (cell 3) (note that there are no cells with agnoprotein alone). (B) Immunohistochemistry of renal allograft biopsy specimen stained with anti-BKV agnoprotein serum (brown). Magnification, ×100. (C) Immunohistochemistry of renal allograft biopsy specimen stained with anti-BKV agnoprotein serum (brown). Magnification, ×400. (D) Immunohistochemistry of renal allograft biopsy specimen double stained with anti-BKV agnoprotein serum (red) and anti-SV40 LT Pab416 (brown). Magnification, ×1,000.
To investigate the expression of BKV agnoprotein in vivo, we examined 16 needle biopsy specimens from renal transplant recipients with BKV viruria. PCR analysis of DNA extracted from the paraffin-embedded specimens revealed the presence of BKV DNA sequences in 6 of the 16 samples (38%). Immunohistochemical analysis revealed agnoprotein expression in 4 of the 6 BKV PCR-positive biopsy specimens (67%) but in none of the BKV PCR-negative renal tissues (overall frequency, 25%). Agnoprotein-expressing cells frequently showed a focal distribution surrounded by neighboring negative areas which often contained inflammatory infiltrates as in PVAN B (Fig. 1B). Single immunostaining for BKV agnoprotein revealed predominant staining of the cytoplasm of renal tubular epithelial cells with unstained, typically enlarged nuclei (Fig. 1C). Double immunostaining for LT antigen and BKV agnoprotein (Fig. 1D) showed the exclusive nuclear staining of LT antigen and a range of intensities for agnoprotein in the cytoplasm of infected cells, similar to the picture obtained by in vitro infection of renal primary proximal tubular epithelial cells (Fig. 1A). Indeed, some cells were positive for LT antigen but negative for agnoprotein (Fig. 1D), while different levels of agnoprotein expression were observed in adjacent cells of the same tissue sample (Fig. 1D), all of which were positive for LT antigen as expected for different stages of the early and late phases of the BKV replication cycle.
BKV agnoprotein elicits only poor IgG and IgM responses compared to LT antigen and VP1.
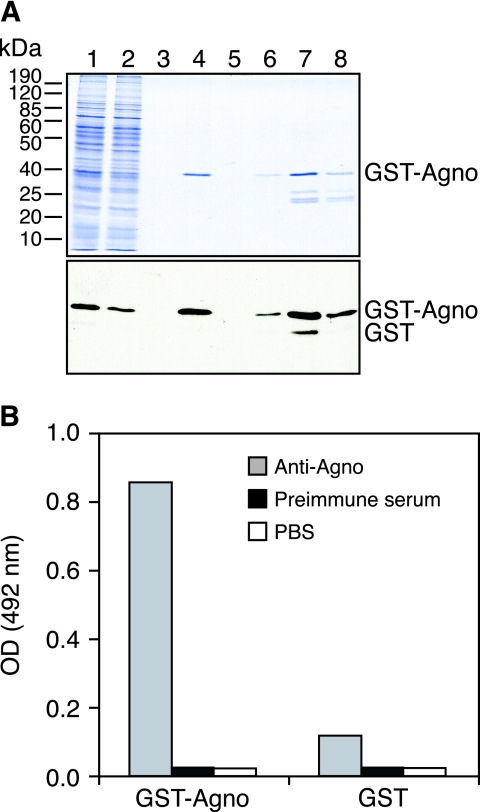
To investigate the antibody response against BKV agnoprotein by EIA, we generated GST fusion proteins with the full-length BKV agnoprotein, the amino-terminal 133 amino acids of LT antigen, and the full-length major capsid protein VP1 for high-level expression in insect cells and convenient purification via glutathione affinity chromatography. As shown for GST-BKV agnoprotein by polyacrylamide gel electrophoresis and Western blotting with anti-GST (Fig. 2A), GST fusion proteins were prominently expressed and highly enriched by single-step glutathione-Sepharose 4B, with some limited amounts of smaller proteins corresponding to endogenous GST and breakdown products. Using purified GST-BKV agnoprotein, GST-BKV LTD1, GST-BKV VP1, and GST alone, we adapted antigen coating conditions, serum dilutions, and dilutions of secondary antibodies in order to obtain optimal signal-to-noise ratios (Fig. 2B and data not shown).
FIG. 2.
GST-BKV agnoprotein purification from Sf9 cells and EIA. (A) Coomassie blue staining and detection of purified GST-agnoprotein by Western blotting by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lane 1, cell lysate; lane 2, flowthrough; lane 3, wash; lane 4, Sepharose beads after elution; lanes 5 to 8, elution fractions 1 to 4, respectively. GST-agnoprotein was detected with monoclonal anti-GST antibody (1:5,000) and goat anti-mouse antibody conjugated to horseradish peroxidase (1:10,000). (B) A 96-well enzyme-linked immunosorbent assay plate was coated with GST-agnoprotein or GST as a control in PBS, pH 7.4 (1 pmol/well). A polyclonal rabbit anti-BKV agnoprotein serum was used as the primary antibody at a dilution of 1:20,000. Rabbit preimmune serum and PBS (pH 7.4) were used as negative controls. All assays were performed in triplicate.
We tested 146 plasma samples from 38 KT patients as well as 19 samples from 16 HD for IgG and IgM activity. The characteristics of these subject groups are shown in Table 1. No significant differences were found concerning age or gender distribution. None of the HD had detectable viremia, and only one HD had detectable low-level viruria (6 × 103 copies/ml) in one of the samples (frequency, 1/19 [5%]). By contrast, KT patients had a median plasma BKV load of 104 copies/ml. Not unexpectedly, the median time of sampling of KT patients posttransplantation increased from group A (9 weeks) to group B (26 weeks) to group C (120 weeks; P < 0.001).
The results of the antibody responses found in the study participants (Table 2; Fig. 3) can be summarized as follows. Only 4 of 54 individuals had no detectable anti-BKV antibodies, yielding an overall BKV seropositivity rate of 92.6%, which is in the range of published results for other serological assays (5, 22). IgG responses were more frequent and with higher OD values in KT patients than in HD, particularly with ongoing or past high-level BKV replication, and were more pronounced against the BKV VP1 capsid protein than against the BKV LT antigen. Anti-VP1 IgG was detected in 12/19 (63%) HD and in 117/146 (80%) KT patients. Anti-LTD1 IgG were detected in 8/19 (42%) HD and in 91/146 (62%) KT patients. By contrast, IgG responses against BKV agnoprotein were found in only 3/19 (15%) HD and 11/146 (7.5%) KT patients. When borderline positive results were regarded as seronegative, only 1/165 samples (1%) had detectable anti-agnoprotein IgG, compared to 60/165 (41%) and 78/165 (53%) samples for anti-LTD1 IgG and anti-VP1 IgG, respectively. Finally, IgG responses were generally more frequently detected than IgM responses for any of the three antigens and had higher OD values. In sera from HD, no IgM responses against any of the three BKV antigens could be found. In sera from KT patients, IgM responses were most frequently found in group B patients with ongoing high-level replication (Table 2). Anti-agnoprotein IgM responses were rarely detected in 6/165 (3.6%) samples tested, 5 of which were weakly positive and 1 (0.6%) of which was positive.
TABLE 2.
Antibody activity against purified recombinant BKV proteins
| Group (no. of subjects) | Antibody (no. of samples tested) | Protein | No. (%) of samples that werea:
|
Median OD at 492 nm (interquartile range) | |||
|---|---|---|---|---|---|---|---|
| Negative | Borderline positive | Positive | Strongly positive | ||||
| HD (16) | IgG (19) | LTD1 | 11 (57.9) | 5 (26.3) | 3 (15.8) | 0 | 0.0322 (0.0128-0.0688) |
| VP1 | 7 (36.8) | 4 (21.1) | 7 (36.8) | 1 (5.3) | 0.0744 (0.0320-0.1380) | ||
| Agnoprotein | 16 (84.2) | 1 (5.3) | 2 (10.5) | 0 | 0.0120 (0.0050-0.0201) | ||
| IgM (19) | LTD1 | 19 (100) | 0 | 0 | 0 | 0.0088 (0.0043-0.0131) | |
| VP1 | 19 (100) | 0 | 0 | 0 | 0.0099 (0.0026-0.0133) | ||
| Agnoprotein | 19 (100) | 0 | 0 | 0 | 0.0046 (0.0003-0.0076) | ||
| KT patient groupsb | |||||||
| A (16) | IgG (25) | LTD1 | 11 (44.0) | 7 (28.0) | 6 (24.0) | 1 (4.0) | 0.0433 (0.0238-0.1018) |
| VP1 | 7 (28.0) | 12 (48.0) | 5 (20.0) | 1 (4.0) | 0.0676 (0.0319-0.0958) | ||
| Agnoprotein | 23 (92.0) | 2 (8.0) | 0 | 0 | 0.0006 (0.0000-0.0145) | ||
| IgM (25) | LTD1 | 20 (80.0) | 3 (12.0) | 2 (8.0) | 0 | 0.0146 (0.071-0.0316) | |
| VP1 | 19 (76.0) | 4 (16.0) | 2 (8.0) | 0 | 0.0214 (0.0103-0.0284) | ||
| Agnoprotein | 23 (92.0) | 2 (8.0) | 0 | 0 | 0.0002 (0.0000-0.0044) | ||
| B (27) | IgG (83) | LTD1 | 34 (41.0) | 18 (21.7) | 14 (16.9) | 17 (20.5) | 0.0499 (0.0193-0.5721) |
| VP1 | 14 (16.9) | 14 (16.9) | 55 (66.3) | 0 | 0.1708 (0.0633-0.2854) | ||
| Agnoprotein | 78 (94.0) | 4 (4.8) | 1 (1.2) | 0 | 0.0064 (0.0000-0.0147) | ||
| IgM (83) | LTD1 | 62 (74.7) | 15 (18.1) | 6 (7.2) | 0 | 0.0156 (0.0031-0.0389) | |
| VP1 | 37 (44.6) | 17 (20.5) | 29 (34.9) | 0 | 0.0446 (0.0226-0.1556) | ||
| Agnoprotein | 79 (95.2) | 3 (3.6) | 1 (1.2) | 0 | 0.0016 (0.0000-0.0063) | ||
| C (23) | IgG (38) | LTD1 | 10 (26.3) | 6 (15.8) | 15 (39.5) | 7 (18.4) | 0.1863 (0.0425-0.7489) |
| VP1 | 8 (21.1) | 13 (34.2) | 17 (44.7) | 0 | 0.0820 (0.0488-0.2115) | ||
| Agnoprotein | 34 (89.5) | 4 (10.5) | 0 | 0 | 0.0027 (0.0000-0.0103) | ||
| IgM (38) | LTD1 | 32 (84.2) | 3 (7.9) | 3 (7.9) | 0 | 0.0107 (0.0047-0.0212) | |
| VP1 | 23 (60.5) | 6 (15.8) | 9 (23.7) | 0 | 0.0250 (0.0095-0.0805) | ||
| Agnoprotein | 38 (100.0) | 0 | 0 | 0 | 0.0020 (0.0000-0.0062) | ||
| All (38c) | IgG (146) | LTD1 | 55 (37.7) | 31 (21.2) | 35 (24.0) | 25 (17.1) | 0.0578 (0.0215-0.5187) |
| VP1 | 29 (19.9) | 39 (26.7) | 77 (52.7) | 1 (0.7) | 0.1144 (0.0549-0.2222) | ||
| Agnoprotein | 135 (92.5) | 10 (6.8) | 1 (0.7) | 0 | 0.0042 (0.0000-0.0139) | ||
| IgM (146) | LTD1 | 114 (78.1) | 21 (14.4) | 11 (7.5) | 0 | 0.0142 (0.0041-0.0309) | |
| VP1 | 79 (54.1) | 27 (18.5) | 40 (27.4) | 0 | 0.0360 (0.0132-0.1038) | ||
| Agnoprotein | 140 (95.9) | 5 (3.4) | 1 (0.7) | 0 | 0.0016 (0.0000-0.0056) | ||
| All subjects (54) | IgG (165) | LTD1 | 66 (40.0) | 36 (21.8) | 38 (23.0) | 25 (15.2) | 0.0570 (0.0185-0.3585) |
| VP1 | 36 (21.8) | 43 (26.1) | 84 (50.9) | 2 (1.2) | 0.1093 (0.0541-0.2147) | ||
| Agnoprotein | 151 (91.5) | 11 (6.7) | 3 (1.8) | 0 | 0.0058 (0.0000-0.0148) | ||
| IgM (165) | LTD1 | 133 (80.6) | 21 (12.7) | 11 (6.7) | 0 | 0.0127 (0.0041-0.0265) | |
| VP1 | 98 (59.4) | 27 (16.4) | 40 (24.2) | 0 | 0.0277 (0.0103-0.0896) | ||
| Agnoprotein | 159 (96.4) | 5 (3.0) | 1 (0.6) | 0 | 0.0018 (0.0000-0.0066) | ||
Antibody activity was measured as the OD at 492 nm in an enzyme-linked immunosorbent assay at a serum dilution of 1:400 and was classified as follows: negative, <0.0400; borderline positive, 0.0400 to 0.0999; positive, 0.1000 to 0.9999; and strongly positive, >0.9999.
A, no/low-level replication; B, high-level replication; C, past replication.
For some KT patients, samples were drawn before the onset of, during, and after the resolution of PVAN. Therefore, this number is smaller than the total for groups A, B, and C.
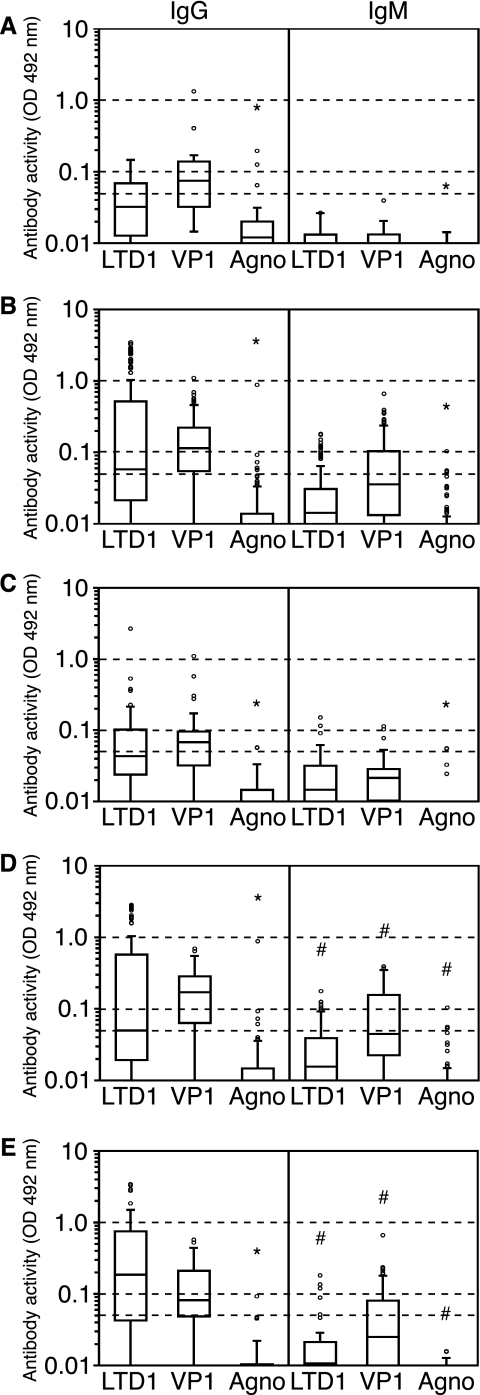
FIG. 3.
Antibody responses to BKV proteins in HD and KT patients. Box-and-whiskers plots, with the length of each box corresponding to the interquartile range and the upper and lower boundaries of the box representing the 75th and 25th percentiles, respectively, are shown. The line in the box indicates the median value. Dashed horizontal lines show the different cutoff levels for antibody activity. *, significant difference (P = 0.02) for anti-agnoprotein activity compared to activity against LTD1 or VP1; #, significant activity difference (P < 0.02) for all the antigen groups (Kruskal-Wallis test). (A) Anti-BKV antibody activity in healthy donors (19 samples from 16 participants). (B) Anti-BKV antibody activity in all renal transplant recipients (146 samples from 38 patients). (C) Anti-BKV antibody activity in renal transplant recipients without evidence of BKV replication or prior to the development of PVAN (25 samples from 16 patients). (D) Anti-BKV antibody activity in renal transplant recipients with ongoing PVAN (83 samples from 27 patients). (E) Anti-BKV antibody activity in renal transplant recipients after resolution of PVAN (38 samples from 23 patients).
Among KT patients, the percentage of positive samples and the median OD values for anti-LTD1 responses increased from group A (“no/low-level” replication) (56% and 0.0433) to group B (“high-level” replication) (59% and 0.0499) and group C (past “high-level” replication) (74% and 0.1863) (P = 0.01), with the strongest difference between groups A and C (P = 0.008). The anti-VP1 IgG response was increased in KT patients from group A (72%; 0.0676) to group B (83%; 0.1708) (P < 0.008) and was lower in group C (79%; 0.0820) but did not reach statistical significance. By contrast, anti-agnoprotein responses remained low in all groups, with no statistically significant changes (percentages of positive samples and median ODs: group A, 8% and 0.0006; group B, 6% and 0.0064; and group C, 10% and 0.0027). In all subgroups, the response to agnoprotein was significantly lower than that to any of the other antigens (P < 0.0001 for all comparisons). The anti-LTD1 response was significantly higher than the anti-VP1 response only in group C. To confirm the poor anti-agnoprotein IgG response by a different technique based on native agnoprotein, we tested 30 sera from KT patients by indirect immunofluorescence of agnoprotein-expressing UTA-6 cells. Only 1 of 30 patient sera (3.3%) was found to be reactive at a serum dilution of 1:40.
The IgM responses showed no clear tendency for anti-LTD1 responses, whereas anti-VP1 responses increased significantly during the active PVAN phase and subsided afterwards (percentages of positive samples for groups A, B, and C, 24%, 55%, and 39%, respectively; median OD values for groups A, B, and C, 0.0214, 0.0446, and 0.0250, respectively; P < 0.008 for the increase in group B, but the subsequent decrease in group C did not reach statistical significance). Again, anti-agnoprotein responses remained very low throughout this time and were significantly lower than responses to any of the other antigens.
BKV agnoprotein elicits poor cellular immune responses.
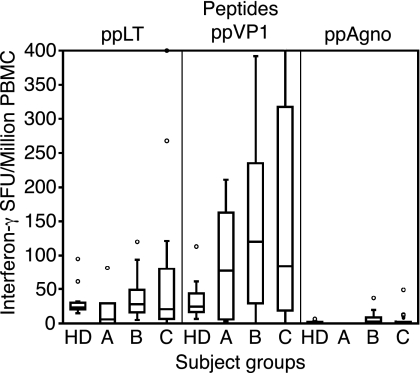
To test whether the BKV agnoprotein elicited cellular immune responses, we analyzed 10 PBMC samples from 10 HD and 48 PBMC samples from 30 KT recipients (Fig. 4). ESAs were performed to detect IFN-γ secretion as described previously (1), using Staphylococcus enterotoxin B as a positive control (median of 8,080 spot-forming units [SFU] per million PBMCs). In HD, the median number of IFN-γ SFU for LT antigen peptide stimulation was 23 (interquartile range, 20 to 30), that for VP1 peptides was 25 (17 to 44), and that for agnoprotein peptides was 1 (0 to 2). In KT patients, the median number of IFN-γ SFU for LT antigen peptides was 24 (interquartile range, 8 to 76), that for VP1 peptides was 99 (18 to 246), and that for agnoprotein peptides was 2 (0 to 5). Thus, the response to agnoprotein peptides was significantly lower, even after adjusting for protein length by dividing the number of SFU by the number of 15-mer peptides per protein pool (P < 0.05 by the Kruskal-Wallis test).
FIG. 4.
BKV agnopeptide-specific cellular immune response. IFN-γ responses after stimulation of PBMCs with BKV LT antigen, VP1, and agnoprotein peptides are shown. Results are for HD (n = 10), KT patients without high-level BKV replication (group A; n = 4), KT patients with high-level BKV replication (group B; n = 19), and KT patients after high-level replication (group C; n = 25). Agnoprotein responses were significantly lower than LT antigen and VP1 responses (P < 0.05 by the Kruskal-Wallis test).
Time courses of immune responses in selected patients.
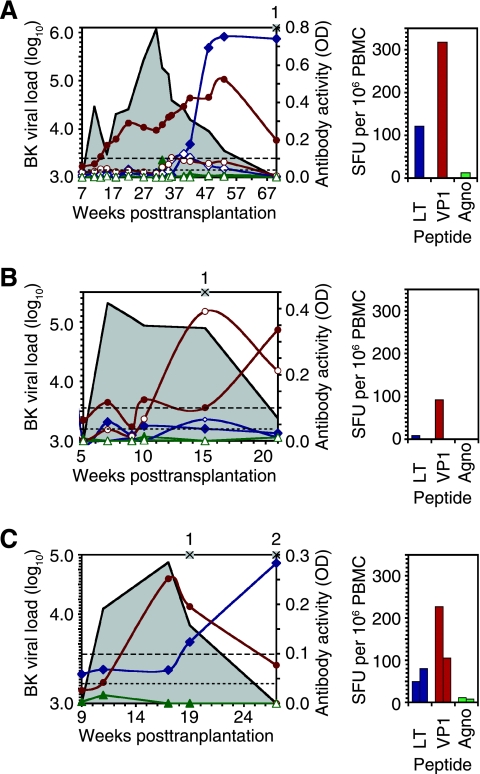
To explore the evolution of humoral and cellular immune responses to different BKV proteins over time, we identified three patients with sufficient sampling density to show different patterns of antibody activity changes before, during, and after high-level BKV replication and PVAN.
Patient 1 (Fig. 5A) is a 49-year-old female who developed definitive PVAN at 7 weeks posttransplantation and had a prolonged course of high-level BKV replication, defined as a plasma BKV load of >10e4/ml (16) over more than 35 weeks. The plasma BKV load peaked at 1.16 × 106 copies/ml at 27 weeks posttransplantation. Initially, she had only a weakly positive IgG anti-VP1 antibody response, which over the next weeks increased without any effect on BKV load. At about 34 weeks posttransplantation, increases in anti-VP1 IgM were noted, followed by anti-LT antigen IgM. The increase in the IgM antibody activities heralded a further increase in anti-VP1 IgG (peak at 54 weeks posttransplantation), followed by a steep rise of anti-LT antigen IgG from week 41 to a plateau at week 54 posttransplantation. Simultaneously with the latter changes in anti-BKV responses, the BKV load dropped to 1.36 × 105 at week 37 and decreased further below the threshold of detection at 71 weeks posttransplantation. ESA at 71 weeks posttransplantation showed strong anti-VP1 and anti-LT antigen responses (317 and 121 spots, respectively). The agnoprotein-specific antibody and cellular responses were very low and undetectable, respectively.
FIG. 5.
BKV-specific antibody titers and cellular immune response in KT patients with PVAN. Left panels show plasma BKV load (black line, log/ml, left y axis) and antibody activity (OD at 492 nm, right y axis) for anti-VP1 (brown lines) (IgG, solid circles; IgM, open circles), anti-LT antigen (blue lines) (IgG, solid diamonds; IgM, open diamonds), and anti-agnoprotein (green lines) (IgG, solid triangles; IgM, open triangles) as well as the time points (×) of ESAs. Right panels show IFN-γ ESAs after stimulation with LT antigen peptides (blue bars), VP1 peptides (brown bars), and agnoprotein peptides (green bars) at the time points indicated in the left panels. (A) Patient 1 (49-year-old female), with definitive PVAN diagnosed at 7 weeks posttransplant. (B) Patient 2 (54-year-old male), with definitive PVAN at 5 weeks posttransplant. (C) Patient 3 (53-year-old male), with definitive PVAN at 9 weeks posttransplant.
Patient 2 (Fig. 5B) is 54-year-old male who developed PVAN just 5 weeks posttransplantation and had a intermediate course of high-level BKV replication over 15 weeks. The BKV viral load peaked at 2.08 × 105 copies/ml at 7 weeks posttransplantation and then remained stable at 1 × 105 copies/ml until week 16, before it dropped to 2.39 × 103 copies/ml at week 22. Anti-VP1 IgG was detectable at low levels from the first sample on, but it started to increase only after week 15, after anti-VP1 IgM peaked. In contrast to the case for patient 1, anti-LTD1 responses remained low or undetectable throughout, as did the anti-agnoprotein antibodies. ESA was performed at week 16, when the BKV viral load was still high, and found a moderate IFN-γ response to VP1 peptides (49 spots), while the LT-specific response was still low (19 spots). Agnopeptide responses were negative.
Patient 3 (Fig. 5C) is a 53-year-old male who developed PVAN at 9 weeks after transplantation and had a relatively short course of high-level BKV replication over 8 weeks. His BKV viral load peaked at 7.90 × 104 copies/ml at 18 weeks posttransplantation and steadily declined thereafter. He was weakly positive for anti-VP1 antibodies, which increased in parallel with the BKV viral load rise and peaked at 18 weeks. Anti-LT IgG antibodies remained low until 20 weeks posttransplantation and started to increase at the same time that the BK viral load started to do so. No BKV-specific IgM was detected. The cellular immune response was assayed at week 20, revealing a strong VP1- and LT antigen-specific IFN-γ response with 227 and 49 SFU, respectively. At 28 weeks, LT antigen-specific IFN-γ responses had slightly increased, while the anti-VP1 response had dropped (80 and 105 SFU, respectively). At both time points, agnoprotein-specific IFN-γ responses were low (12 and 10 SFU, respectively).
DISCUSSION
In this study, we demonstrate that BKV agnoprotein is expressed in vivo in renal tubular epithelial cells of needle biopsy samples from KT patients with histologically defined PVAN. The cellular staining pattern in vivo is indistinguishable from the one found upon BKV infection of primary renal tubular epithelial cells in vitro (Fig. 1), where BKV agnoprotein is abundant in the cytoplasm and enriched in the perinuclear area, as reported previously (35). Of note, agnoprotein expression in vitro and in vivo was found exclusively in cells positive for the early viral gene product LT antigen. By contrast, LT antigen was detectable in the nuclei of cells in which agnoprotein ranged from undetectable to maximal cytoplasmic staining, as expected for sequential progression from viral early to late gene expression. The asynchronous side-by-side quality of different stages of the polyomaviral replication cycle in vivo is typically seen in tissue culture after spread and secondary infection of susceptible cells in vitro. This interpretation is in line with ultrastructural studies of PVAN demonstrating the uptake of polyomavirus particles (7). Alternatively, asynchronous replication could result from differential reactivation of latent BKV infection and progression from early to late gene expression in response to qualitative or quantitative differences in (micro)environmental costimuli. Since agnoprotein is an abundant late, but nonstructural, viral protein of the lytic BKV infection cycle, staining of renal biopsy specimens may be an interesting marker of viral cytopathic damage to renal tubular epithelia. Further investigations are certainly warranted at this point.
Despite the abundant expression of BKV agnoprotein in vivo, we did not find a corresponding level of humoral and cellular immune responses. Anti-agnoprotein IgG was detected in only 15% and 7.5% of HD and KT patients, respectively, which was significantly lower than the 63% and 80% seropositivity for VP1 and the 42% and 62% seropositivity for LTD1, respectively. In adult populations similar to our study groups, BKV seroprevalence rates of between 60 and 90% have been reported using assays which detect antibody responses directed against the major capsid protein VP1 by EIA or by inhibiting its interaction with receptor-like glycosylated surface structures of type O erythrocytes, i.e., by hemagglutination inhibition (18, 23, 25, 32). The higher VP1-type response may partly be due to the fact that the host immune system is more readily and even systemically (re)exposed to viral surface proteins which typically contain neutralizing epitopes (11). Accordingly, standard serological assays for other viruses such as herpesviruses or parvoviruses are commonly based on glycoproteins of enveloped viruses or capsid proteins of nonenveloped viruses, respectively. In keeping with this notion, anti-LT antigen responses were lower than anti-VP1 responses but still significantly higher than anti-agnoprotein responses. A technical problem seems unlikely to explain this difference, since the applied antigen was well detectable by the rabbit anti-BKV agnoprotein serum. To rule out effects of a potentially nonnative conformation of agnoprotein epitopes in the GST fusion protein, we investigated anti-agnoprotein IgG by indirect immunofluorescence. In line with the EIA results, only 1/30 KT patient sera (3.3%) was reactive using agnoprotein-expressing UTA-6 cells. The smaller size of the agnoprotein and a potentially lower antigenicity could be contributing factors, but the LT antigen used was truncated after the amino-terminal 133 amino acids (LTD1), which should reduce the impact of size differences on detection. When we corrected the response rate for the antigen size, anti-agnoprotein responses were still lower than those against VP1 and far lower than those against LTD1.
The anti-BKV response pattern in nonimmunosuppressed individuals was similar to that in KT patients with no/low-level BKV replication (group A). In particular, we found no IgM responses to any of the three BKV antigens in HD sera. By contrast, KT patients with ongoing high-level replication (group B) had the highest IgG responses to VP1 and also the most prominent IgM responses. Interestingly, the median anti-LTD1 responses in group B KT patients were also increased relative to those in HD or group A KT patients, but those in KT patients with past high-level BKV replication (group C) were significantly higher (but with a slight decline in anti-VP1 IgG). These data indicate that anti-LT antigen IgG responses increase later than anti-VP1 responses, coinciding with emerging immune control of BKV replication. This is highlighted by the time courses demonstrating that anti-LT antigen responses evolve significantly later than anti-VP1 responses, at a time of declining plasma BKV load. In fact, testing before week 39 in patient 1 and before week 17 in patient 3 detected only anti-VP1 responses. Thus, the time courses underline the absence of anti-agnoprotein responses and reduce the likelihood that the time points of the cross-sectional sampling for anti-agnoprotein responses were merely suboptimal.
Our serological studies are further complemented by the poor IFN-γ responses elicited by agnoprotein peptide pools (median, 2 SFU; range, 0 to 5) compared to LT antigen (median, 24; range, 8 to 76) or VP1 (median, 99; range, 18 to 246) peptide pools, even after correction for peptide pool size. Although specific IFN-γ-producing CD8+ T cells have been detected for other viruses, e.g., cytomegalovirus (38), we cannot exclude the possibility that the use of overlapping 15-mers may be suboptimal for the detection of agnoprotein-specific major histocompatibility complex class I-restricted CD8+ T-cell responses. On the other hand, we would expect a significant agnoprotein-specific major histocompatibility complex class II-restricted CD4+ T-cell response in the fairly sensitive ESA format. Taken together, humoral and cellular immune responses to the abundant BKV agnoprotein are rare and likely reflect its poor immunogenicity. Moreover, anti-agnoprotein immune responses seem to have no apparent importance in overcoming BKV viremia and PVAN.
Only a few studies have examined the presence of antibodies against the major regulatory protein LT antigen. Antibodies to BKV LT antigen have been detected by indirect immunofluorescence in 1.15% of 952 neoplasia patients, 4.40% of 113 renal transplant recipients, and 0.80% of healthy controls (6). In another study, antibodies to LT antigen were found in 29.1% of 103 BKV-infected renal transplant recipients and were absent in BKV-negative patients (27). These results show that not only virions but also remnants of infected cells are processed by antigen-presenting cells (20). It remains to be seen if anti-LT antibody titers can be used as a surrogate for BKV replication control.
The low immunogenicity of BKV agnoprotein is in contrast to the HIV protein Tat, consisting of 86 to 101 amino acids. Anti-TAT antibodies have been found in only 10 to 14% of HIV type 1 (HIV-1)-positive patients (4) and were predictive of nonprogression both in HIV-1 (33)- and HIV-2 (36)-positive patients. Consequently, HIV Tat has been considered for use as a candidate anti-HIV vaccine (30). At this point, we can only speculate about the underlying mechanism of the poor immunogenicity of agnoprotein, which might involve host as well as viral factors. Since abundant agnoprotein expression is tightly restricted to switching to late gene expression, it is tempting to hypothesize that efficient immune recognition of agnoprotein would significantly curtail the replication and spread of polyomavirus in its host population. Our results indicate that agnoprotein may not be a sensitive tool for monitoring the immune response in KT patients, but its potential as a candidate anti-BKV vaccine cannot be conclusively ruled out.
Acknowledgments
We thank Jacqueline Samaridis and Claudia Mistl for technical support in performing the enzyme-linked immunosorbent assays and ESAs.
This work was supported in part by grants from the Swiss National Fund (3200B0-110040/1) and the Stiftung Forschung Infektionskrankheiten Basel (SFI 6/2005) to H.H.H.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Binggeli, S., A. Egli, M. Dickenmann, I. Binet, J. Steiger, and H. H. Hirsch. 2006. BKV replication and cellular immune responses in renal transplant recipients. Am. J. Transplant. 6:2218-2219. [DOI] [PubMed] [Google Scholar]
- 2.Bratt, G., A. L. Hammarin, M. Grandien, B. G. Hedquist, I. Nennesmo, B. Sundelin, and S. Seregard. 1999. BK virus as the cause of meningoencephalitis, retinitis and nephritis in a patient with AIDS. AIDS 13:1071-1075. [DOI] [PubMed] [Google Scholar]
- 3.Brennan, D. C., I. Agha, D. L. Bohl, M. A. Schnitzler, K. L. Hardinger, M. Lockwood, S. Torrence, R. Schuessler, T. Roby, M. Gaudreault-Keener, and G. A. Storch. 2005. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 5:582-594. [DOI] [PubMed] [Google Scholar]
- 4.Butto, S., V. Fiorelli, A. Tripiciano, M. J. Ruiz-Alvarez, A. Scoglio, F. Ensoli, M. Ciccozzi, B. Collacchi, M. Sabbatucci, A. Cafaro, C. A. Guzman, A. Borsetti, A. Caputo, E. Vardas, M. Colvin, M. Lukwiya, G. Rezza, and B. Ensoli. 2003. Sequence conservation and antibody cross-recognition of clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J. Infect. Dis. 188:1171-1180. [DOI] [PubMed] [Google Scholar]
- 5.Comoli, P., S. Binggeli, F. Ginevri, and H. H. Hirsch. 2006. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl. Infect. Dis. 8:86-94. [DOI] [PubMed] [Google Scholar]
- 6.Corallini, A., G. Barbanti-Brodano, M. Portolani, P. G. Balboni, M. P. Grossi, L. Possati, C. Honorati, M. La Placa, A. Mazzoni, A. Caputo, U. Veronesi, S. Orefice, and G. Cardinali. 1976. Antibodies to BK virus structural and tumor antigens in human sera from normal persons and from patients with various diseases, including neoplasia. Infect. Immun. 13:1684-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drachenberg, C. B., J. C. Papadimitriou, R. Wali, C. L. Cubitt, and E. Ramos. 2003. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am. J. Transplant. 3:1383-1392. [DOI] [PubMed] [Google Scholar]
- 8.Englert, C., X. Hou, S. Maheswaran, P. Bennett, C. Ngwu, G. G. Re, A. J. Garvin, M. R. Rosner, and D. A. Haber. 1995. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 14:4662-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner, S. D., A. M. Field, D. V. Coleman, and B. Hulme. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet i:1253-1257. [DOI] [PubMed] [Google Scholar]
- 10.Grinde, B., M. Gayorfar, and C. H. Rinaldo. 2007. Impact of a polyomavirus (BKV) infection on mRNA expression in human endothelial cells. Virus Res. 123:86-94. [DOI] [PubMed] [Google Scholar]
- 11.Hangartner, L., R. M. Zinkernagel, and H. Hengartner. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6:231-243. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen Rinaldo, C., H. Hansen, and T. Traavik. 2005. Human endothelial cells allow passage of an archetypal BK virus (BKV) strain—a tool for cultivation and functional studies of natural BKV strains. Arch. Virol. 150:1449-1458. [DOI] [PubMed] [Google Scholar]
- 13.Heritage, J., P. M. Chesters, and D. J. McCance. 1981. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J. Med. Virol. 8:143-150. [DOI] [PubMed] [Google Scholar]
- 14.Hey, A. W., J. I. Johnsen, B. Johansen, and T. Traavik. 1994. A two fusion partner system for raising antibodies against small immunogens expressed in bacteria. J. Immunol. Methods 173:149-156. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch, H. H. 2005. BK virus: opportunity makes a pathogen. Clin. Infect. Dis. 41:354-360. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, H. H., C. B. Drachenberg, J. Steiger, and E. Ramos. 2006. Polyomavirus-associated nephropathy in renal transplantation: critical issues of screening and management. Adv. Exp. Med. Biol. 577:160-173. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, H. H., W. Knowles, M. Dickenmann, J. Passweg, T. Klimkait, M. J. Mihatsch, and J. Steiger. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 347:488-496. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, H. H., M. Mohaupt, and T. Klimkait. 2001. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J. Infect. Dis. 184:1494-1495. (Author reply, 184:1495-1496.) [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 21.Imperiale, M. J. 2001. The human polyomaviruses: an overview, p. 53-71. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses. Wiley-Liss, Hoboken, NJ.
- 22.Knowles, W. A. 2006. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv. Exp. Med. Biol. 577:19-45. [DOI] [PubMed] [Google Scholar]
- 23.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115-123. [DOI] [PubMed] [Google Scholar]
- 24.Low, J., H. D. Humes, M. Szczypka, and M. Imperiale. 2004. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology 323:182-188. [DOI] [PubMed] [Google Scholar]
- 25.Lundstig, A., and J. Dillner. 2006. Serological diagnosis of human polyomavirus infection. Adv. Exp. Med. Biol. 577:96-101. [DOI] [PubMed] [Google Scholar]
- 26.Nomura, S., G. Khoury, and G. Jay. 1983. Subcellular localization of the simian virus 40 agnoprotein. J. Virol. 45:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noss, G. 1987. Human polyoma virus type BK infection and T antibody response in renal transplant recipients. Zentbl. Bakteriol. Mikrobiol. Hyg. A 266:567-574. [DOI] [PubMed] [Google Scholar]
- 28.Okada, Y., S. Endo, H. Takahashi, H. Sawa, T. Umemura, and K. Nagashima. 2001. Distribution and function of JCV agnoprotein. J. Neurovirol. 7:302-306. [DOI] [PubMed] [Google Scholar]
- 29.Okada, Y., H. Sawa, S. Endo, Y. Orba, T. Umemura, H. Nishihara, A. C. Stan, S. Tanaka, H. Takahashi, and K. Nagashima. 2002. Expression of JC virus agnoprotein in progressive multifocal leukoencephalopathy brain. Acta Neuropathol. (Berlin) 104:130-136. [DOI] [PubMed] [Google Scholar]
- 30.Patel, J., D. Galey, J. Jones, P. Ray, J. G. Woodward, A. Nath, and R. J. Mumper. 2006. HIV-1 Tat-coated nanoparticles result in enhanced humoral immune responses and neutralizing antibodies compared to alum adjuvant. Vaccine 24:3564-3573. [DOI] [PubMed] [Google Scholar]
- 31.Ramos, E., C. B. Drachenberg, M. Portocarrero, R. Wali, D. K. Klassen, J. C. Fink, A. Farney, H. Hirsch, J. C. Papadimitriou, C. B. Cangro, M. R. Weir, and S. T. Bartlett. 2002. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin. Transpl. 2002:143-153. [PubMed] [Google Scholar]
- 32.Randhawa, P. S., G. Gupta, A. Vats, R. Shapiro, and R. P. Viscidi. 2006. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin. Vaccine Immunol. 13:1057-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezza, G., V. Fiorelli, M. Dorrucci, M. Ciccozzi, A. Tripiciano, A. Scoglio, B. Collacchi, M. Ruiz-Alvarez, C. Giannetto, A. Caputo, L. Tomasoni, F. Castelli, M. Sciandra, A. Sinicco, F. Ensoli, S. Butto, and B. Ensoli. 2005. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: findings in a cohort of HIV-1 seroconverters. J. Infect. Dis. 191:1321-1324. [DOI] [PubMed] [Google Scholar]
- 34.Rinaldo, C. H., M. R. Myhre, H. Alstad, O. Nilssen, and T. Traavik. 2003. Human polyomavirus BK (BKV) transiently transforms and persistently infects cultured osteosarcoma cells. Virus Res. 93:181-187. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldo, C. H., T. Traavik, and A. Hey. 1998. The agnogene of the human polyomavirus BK is expressed. J. Virol. 72:6233-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez, S. K., A. D. Sarr, O. Olorunnipa, S. J. Popper, A. Gueye-Ndiaye, I. Traore, M. C. Dia, S. Mboup, and P. J. Kanki. 2006. The absence of anti-Tat antibodies is associated with risk of disease progression in HIV-2 infection. J. Infect. Dis. 194:760-763. [DOI] [PubMed] [Google Scholar]
- 37.Seif, I., G. Khoury, and R. Dhar. 1979. The genome of human papovavirus BKV. Cell 18:963-977. [DOI] [PubMed] [Google Scholar]
- 38.Sester, M., U. Sester, B. C. Gartner, M. Girndt, A. Meyerhans, and H. Kohler. 2002. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J. Am. Soc. Nephrol. 13:2577-2584. [DOI] [PubMed] [Google Scholar]
- 39.Trofe, J., H. H. Hirsch, and E. Ramos. 2006. Polyomavirus-associated nephropathy: update of clinical management in kidney transplant patients. Transpl. Infect. Dis. 8:76-85. [DOI] [PubMed] [Google Scholar]