Abstract
Five commercially available enzyme-linked immunosorbent assays (ELISAs), one in-house ELISA, and two hemagglutination assays were evaluated to determine their diagnostic accuracy for Chagas' disease in two studies. In study 1, ELISA kits showed 100% sensitivity, but specificities ranged from 82.84% to 100% when leishmaniasis cases were included and from 95.57% to 100% when leishmaniasis cases were excluded. Kits using recombinant antigens or synthetic peptides are more specific than those using crude extracts from Trypanosoma cruzi epimastigote forms. Kits evaluated in Panama, in study 2, showed 75% to 100% sensitivity and 97.12% to 100% specificity. These data were obtained by using a Western blot assay with T. cruzi trypomastigote excreted-secreted antigens as a reference test to confirm T. cruzi infection.
Chagas' disease is caused by the protozoan Trypanosoma cruzi, which is transmitted to humans by blood-sucking triatomine bugs, by blood transfusion, through the placenta, or by ingestion of contaminated food. It is estimated that 9 to 12 million people on the Latin American continent are infected with T. cruzi. The prevalence of Chagas' disease among blood donors from different countries in South America differs within each country and locality (8, 22). However, in recent decades, several million persons have emigrated to developed countries, and this may lead to an increase in the prevalence of the infection in such countries (3, 8, 22). Indeed, infection with T. cruzi transmitted by blood transfusion or organ transplantation has recently been described in the United States (1, 5, 8, 9, 19).
Serological diagnosis of Chagas' disease is frequently based on tests such as enzyme-linked immunoassays (EIAs), indirect immunofluorescence assays, and indirect hemagglutination assays (IHAs), which usually employ T. cruzi epimastigote forms as the antigen. Provided that good-quality kits are selected and correct laboratory practices followed, good sensitivity can be achieved with any of the tests. Sensitivities on the order of 95 to 99% can be obtained, and these can be increased to 100% by using more than one test (8, 10, 15). The use of recombinant antigens and/or synthetic peptides has been proposed (17, 21) to improve specificity and sensitivity, which is essential if false-positive or false-negative results are to be avoided.
Several reports show that results can be inconclusive or doubtful depending on the commercial diagnostic assay used for blood screening (5, 6, 7). The definition of inconclusive results differs with the commercial kit used, since reactions that are not clearly positive or negative are taken as inconclusive. Currently available kits are very effective at detecting blood donors presenting with high anti-T. cruzi antibody titers, but the results are often questionable when the kits are used for donors with low titers (7, 18). For the latter donors, it is not uncommon for a sample to be negative by one test when subjected to two or three tests (8). Some of these samples are known to be from genuine Chagas' disease patients, because they are confirmed by molecular biology methods (PCR) (7); other researchers have reported evidence that people infected with T. cruzi can have negative serology (16, 23).
Another factor that needs to be taken into consideration when one is using serological tests for Chagas' disease is cross-reactivity. Cross-reactivity between sera of patients infected with T. cruzi and sera of patients infected with Leishmania spp. in the serodiagnosis of Chagas' disease is well documented (2, 20). In some areas of endemicity in Central America and Brazil, where T. cruzi and the nonpathogenic protozoan Trypanosoma rangeli can be found infecting the same vectors and vertebrate hosts (12, 14), cross-reactivity has been the subject of discussion.
The aim of our study, which was divided into two separate studies (studies 1 and 2), was to compare the sensitivities and specificities of nine Chagas' disease assays for detection of anti-T. cruzi immunoglobulin G: six enzyme-linked immunosorbent assays (ELISAs), two IHAs, and one Western blot assay. Of these tests, the following seven are commercially available: three ELISAs manufactured with T. cruzi epimastigote antigens (ELISA Chagas III [BIOSChile-Ingenieria Genetica SA, Santiago, Chile], ELISAcruzi [bioMérieux Brasil SA], and Chagatek ELISA [Laboratório Lemos SRL, Buenos Aires, Argentina; distributed by bioMérieux Argentina]), two ELISAs prepared with recombinant T. cruzi antigens (Chagatest ELISA recombinant, version 3.0 [Chagatest Rec v3.0; Wiener Laboratories, Rosario, Argentina], and Pathozyme Chagas [Omega Diagnostics Ltd., Scotland, United Kingdom]), and two IHAs (HEMAcruzi [bioMérieux Brasil] and Imuno-HAI [Wama Diagnóstica, São Paulo, Brazil]). The following two tests were prepared at the Instituto de Medicina Tropical, São Paulo, Brazil (IMT): ELISA-IMT, which was prepared with whole extracts of T. cruzi Y strain epimastigotes, and a Western blot assay, prepared with T. cruzi trypomastigote excreted-secreted antigens (TESA blot), as previously described (20). The TESA blot was used as a reference test (20, 21, 23). All commercial kits were used according to the manufacturers' instructions, and the test results were analyzed in accordance with the technical information provided for each assay. The cutoffs were calculated as described in the respective sections of each manual. For ELISA-IMT, the cutoff was calculated as the mean optical density (OD) at 492 nm of the true-negative sera plus 3 standard deviations. The individual results were calculated as the ratio of the OD to the cutoff (see Fig. 1). A sample was considered positive if the ratio was equal to or greater than 1.0 and negative if the ratio was equal to or smaller than 0.99. Seropositivity rates for anti-T. cruzi antibodies in different tests, and their confidence intervals [CIs], were calculated using the mid-P 95% confidence interval (95% CI) using Epi Info (version 6.0) software (see Table 2). Samples that yielded discrepant results were tested at least twice on different days.
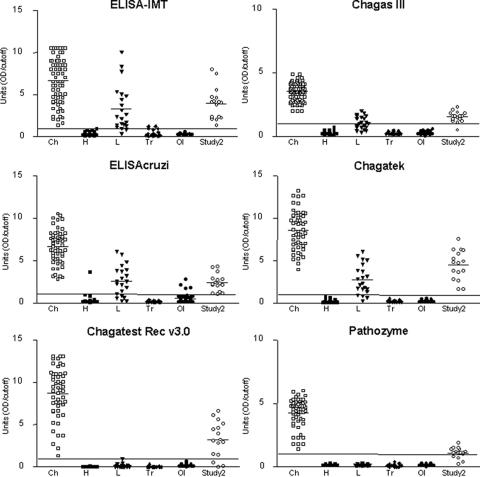
FIG. 1.
Distribution of individual results of five commercially available ELISAs (Chagas III, ELISAcruzi, Chagatek, Chagatest Rec v3.0, and Pathozyme) and one in-house ELISA (ELISA-IMT) for diagnosis of Chagas' disease. The first five groups of data in each graph are results, from study 1, for 53 Brazilian individuals with T. cruzi infections (Ch), 45 healthy individuals (H), 21 patients infected with Leishmania spp. (L), 23 individuals infected with Trypanosoma rangeli (Tr), and 45 patients with other infections (OI). The last group of data in each graph (Study 2) corresponds to 16 TESA-blot-positive Panamanian cases. The horizontal line within each data group is the arithmetic mean. Each data point was calculated as the ratio of the OD to the cutoff. The horizontal line at 1.0 unit represents the cutoff.
TABLE 2.
Sensitivities and specificities of seven assays to detect Trypanosoma cruzi antibodies
| Test | Study 1 (n = 187)
|
Study 2 (n = 120)
|
|||
|---|---|---|---|---|---|
| Sensitivitya (n = 53) | Specificitya,b
|
Sensitivity (n = 16) | Specificity (n = 104) | ||
| Including leishmaniasis cases (n = 134) | Excluding leishmaniasis cases (n = 113) | ||||
| TESA blot (IMT) | 100 (94.5-100) | 100 (97.8-100) | 100 (97.4-100) | 100 (82.9-100) | 100 (97.2-100) |
| ELISA-IMTc | 100 (94.5-100) | 85.07 (78.3-90.4) | 98.23 (94.3-99.7) | 100 (82.9-100) | 98.08 (93.8-99.7) |
| Chagas III (BIOSChile)c | 100 (94.5-100) | 90.30 (84.3-94.5) | 100 (97.4-100) | 93.75 (72.8-99.7) | 99.04 (95.4-100) |
| ELISAcruzi (bioMérieux Brasil)c | 100 (94.5-100) | 82.84 (75.7-88.5) | 95.57 (90.5-98.4) | 93.75 (72.8-99.7) | 97.12 (92.4-99.3) |
| Chagatek (bioMérieux Brasil)c | 100 (94.5-100) | 87.31 (80.9-92.2) | 100 (97.4-100) | 100 (82.9-100) | 98.08 (93.8-99.7) |
| Chagatest Rec v3.0 (Wiener)d | 100 (94.5-100) | 100 (97.8-100) | 100 (97.4-100) | 81.25 (57.0-95.0) | 100 (97.2-100) |
| Pathozyme Chagas (Omega)d | 100 (94.5-100) | 100 (97.8-100) | 100 (97.4-100) | 75 (50.1-91.5) | 100 (97.2-100) |
Both sensitivity and specificity are given as percentages, with 95% CIs in parentheses. Confidence intervals were calculated using the mid-P 95% confidence interval (95% CI) with Epi Info 6.0 software.
Determined with sera from non-Chagas' disease patients.
ELISA prepared with T. cruzi epimastigote antigens.
ELISA prepared with T. cruzi recombinant proteins.
The guidelines of the Standards for Reporting of Diagnostic Accuracy were applied in this study.
In study 1, all ELISAs and the TESA blot had a sensitivity of 100% (95% CI, 94.5 to 100%) when evaluated with 53 samples collected from Brazilian blood donors with T. cruzi infection (18 to 60 years old; 60% male, 40% female) who had previously been found positive by at least two commercial tests. This sensitivity was obtained for ELISAs manufactured with crude epimastigote antigens as well as with recombinant T. cruzi proteins (Tables 1 and 2; Fig. 1, Ch). Because the bioMérieux Brasil and Wama IHAs had low sensitivities (90.56% and 86.79%, respectively), they were excluded from subsequent analysis.
TABLE 1.
Numbers and percentages of positive cases obtained by six ELISAs for Chagas' disease diagnosis using the TESA blot as a reference test
| Status of patients | No. of cases | No. (%) of positive cases by:
|
||||||
|---|---|---|---|---|---|---|---|---|
| TESA blot (IMT)a | ELISA-IMTa,b | Chagas III (BIOSChile)b | ELISAcruzi (bioMérieux Brasil SA)b | Chagatek (bioMérieux Brasil SA)b | Chagatest Rec v3.0 (Wiener)c | Pathozyme Chagas (Omega)c | ||
| Study 1 | 187 | |||||||
| Chagas' disease patients | 53 | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 53 (100) |
| Subjects without Chagas' disease | 134 | |||||||
| Healthy | 45 | 0 | 0 | 0 | 1 (2.22) | 0 | 0 | 0 |
| VLd | 13 | 0 | 12 (92.31) | 12 (92.31) | 11 (84.60) | 11 (84.60) | 0 | 0 |
| CLe | 8 | 0 | 6 (75.00) | 1 (12.50) | 7 (87.50) | 6 (75.00) | 0 | 0 |
| T. rangeli | 23 | 0 | 2 (8.69) | 0 | 0 | 0 | 0 | 0 |
| Other infections | 45 | 0 | 0 | 0 | 4 (8.88) | 0 | 0 | 0 |
| Study 2 (Panamanians) | 120 | 16 (13.33) | 18 (15.00) | 16 (13.33) | 18 (15.00) | 18 (15.00) | 13 (10.83) | 12 (10.00) |
| TESA blot positive | 16 | 16 (100) | 16 (100) | 15 (93.75) | 15 (93.75) | 16 (100) | 13 (81.25) | 12 (75.00) |
| TESA blot negative | 104 | 0 | 2 (1.92) | 1 (0.96) | 3 (2.88) | 2 (1.92) | 0 | 0 |
Test prepared at the IMT.
ELISA prepared with T. cruzi epimastigote antigens.
ELISA prepared with T. cruzi recombinant proteins.
VL, visceral leishmaniasis.
CL, cutaneous leishmaniasis.
Specificity was evaluated with 134 samples collected from subjects without Chagas' disease, including (i) 45 healthy people (20 Brazilian blood donors and 25 Panamanians living in an area of endemicity), (ii) 21 patients infected with Leishmania spp. (13 with active visceral leishmaniasis, from an area in Brazil where Chagas' disease was not endemic, and 8 with mucocutaneous leishmaniasis, from Venezuela), (iii) 23 T. rangeli-infected Panamanians, and (iv) 45 patients with unrelated diseases, as defined by their respective clinical and laboratory diagnoses (5 with connective tissue diseases who were also positive for antinuclear antibodies, 5 with systemic lupus erythematosus, 5 positive for anti-streptolysin O, 5 with rheumatic fever, 5 with malaria, 5 with Toxoplasma gondii, 5 with Toxocara canis, 5 with Schistosoma mansoni, and 5 with Taenia solium) (Table 1). Patients infected with T. rangeli or Leishmania spp. were selected using epidemiological, clinical, and/or parasitological data (data not shown).
In study 1, the specificities (95% CIs) of EIAs prepared with T. cruzi epimastigote antigens, including results for Leishmania sp. cases, were as follows: for ELISA-IMT, 85.07% (78.3 to 90.4%); for Chagas III, 90.30% (84.3 to 94.5%); for ELISAcruzi, 82.84% (75.7 to 88.5%); and for Chagatek, 87.31% (80.9 to 92.2%) (Table 2; Fig. 1). A specificity of 100% (97.8 to 100%) was achieved with EIAs prepared with recombinant T. cruzi antigens (Chagatest Rec v3.0 and Pathozyme) (Table 2). When leishmaniasis cases (n = 21) were excluded, the specificities of the kits (95.57 to 100%) indicated improved diagnostic performance (Table 2). No cross-reactivity with the 23 T. rangeli cases was observed in the ELISAs or the TESA blot, except for the ELISA-IMT, which cross-reacted with two cases (8.69%). These two patients had low titers (Table 1; Fig. 1, Tr).
No cross-reactivity was observed with samples from T. rangeli or Leishmania sp. cases or from cases with other infections by using the TESA blot (Table 1). However, 2.24% (3/134) of the samples (1 from a healthy individual, 1 from an individual infected with Leishmania spp., and 1 from an individual infected with T. rangeli) reacted, resulting in a faint band of 170 kDa in the TESA blot; this band can be confused with the 150- to 160-kDa band that is evident with sera of T. cruzi-infected individuals (data not shown).
Individuals infected with T. rangeli appear not to produce T. cruzi- or T. rangeli-reactive antibodies; they failed to show reactivity in an ELISA prepared with T. rangeli extracts (data not shown). These results, which agree with the findings of other studies, show that human infection with T. rangeli does not cause the production of enough cross-reacting antibodies to interfere with the serodiagnosis of Chagas' disease (13, 14). However, this does not exclude the possibility that exposure to T. rangeli may elicit a particular humoral and/or cellular immune response that confers some degree of protection against subsequent infection with T. cruzi (11).
The next step, in study 2, was to validate these assays by a retrospective study with 120 samples from individuals, aged 18 to 60 years, living in an area of endemicity in Mendoza, Panama. According to an earlier study, 2.91% and 6.80% of the children in this area are infected with T. cruzi and T. rangeli, respectively (13). In study 2, Chagas III gave positive results for 13.33% (n = 16) of the samples. ELISA-IMT, ELISAcruzi, and Chagatek gave a positivity rate of 15% (n = 18). Positivity rates for Chagatest Rec v3.0 and Pathozyme were 10.83% (n = 13) and 10% (n = 12), respectively (Table 1). The sensitivity, calculated using the TESA blot as a reference test, was 100% (82.9 to 100.0%) for the ELISA-IMT and Chagatek tests. The same result was not observed for the other tests; Chagas III and ELISAcruzi had a sensitivity of 93.75% (72.8 to 99.7%), Chagatest Rec v3.0 had 81.25% (57.0 to 95.0%), and Pathozyme had 75% (50.1 to 91.5%) (Table 2; Fig. 1, Study 2). In this study, the specificities (95% CIs) of tests were also variable, since cross-reactivity was observed for ELISA-IMT and Chagatek in two cases (98.08% [93.8 to 99.7%]), for Chagas III in one case (99.04% [95.4 to 100%]), and for ELISAcruzi in three cases (97.12% [92.4 to 99.3%]) (Tables 1 and 2).
In summary, our data, and those from other laboratories, indicate that commercial kits that use recombinant antigens or synthetic peptides are a more specific alternative to those that use complex crude extracts, although they sometimes have variable sensitivities (18, 21). Confirmatory tests with higher specificities have already been proposed as reference standards (3, 4, 20, 21, 23), but unfortunately none of these are commercially available.
Acknowledgments
This study was supported by grants from LIM49-FMUSP and CNPq and was approved by the ICB Scientific Commission for Ethical Research, USP, Brazil. We are grateful to the following laboratories, which provided the commercial kits used in this study: bioMérieux Brasil, Wiener Laboratories, Abbott Laboratórios, Dade Behring, and Wama Diagnóstica.
We thank Monica de Paula Leal for invaluable technical help with the TESA blot assays.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). 2006. Chagas disease after organ transplantation—Los Angeles, California. Morb. Mortal. Wkly. Rep. 55:798-800. [PubMed] [Google Scholar]
- 2.Frank, F. M., M. M. Fernandez, N. J. Taranto, S. P. Cajal, R. A. Margni, E. Castro, V. Thomaz-Soccol, and E. L. Malchiodi. 2003. Characterization of human infection by Leishmania spp. in the Northwest of Argentina: immune response, double infection with Trypanosoma cruzi and species of Leishmania involved. Parasitology 126:31-39. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhoff, L. V., P. Paredes, A. Lomeli-Guerrero, M. Paredes-Espinoza, C. S. Ron-Guerrero, M. Delgado-Mejia, and J. G. Pena-Munoz. 2006. Transfusion-associated Chagas disease (American trypanosomiasis) in Mexico: implications for transfusion medicine in the United States. Transfusion 46:298-304. [DOI] [PubMed] [Google Scholar]
- 4.Leiby, D. A., S. Wendel, D. T. Takaoka, R. M. Fachini, L. C. Oliveira, and M. A. Tibbals. 2000. Serologic testing for Trypanosoma cruzi: comparison of radioimmunoprecipitation assay with commercially available indirect immunofluorescence assay, indirect hemagglutination assay, and enzyme-linked immunosorbent assay kits. J. Clin. Microbiol. 38:639-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiby, D. A., R. M. Herron, E. J. Read, B. A. Lenes, and R. J. Stumpf. 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42:549-555. [DOI] [PubMed] [Google Scholar]
- 6.Malan, A. K., E. Avelar, S. E. Litwin, H. R. Hill, and C. M. Litwin. 2006. Serological diagnosis of Trypanosoma cruzi: evaluation of three enzyme immunoassays and an indirect immunofluorescent assay. J. Med. Microbiol. 55:171-178. [DOI] [PubMed] [Google Scholar]
- 7.Marcon, G. E., P. D. Andrade, D. M. de Albuquerque, J. S. Wanderley, E. A. Almeida, M. E. Guariento, and S. C. B. Costa. 2002. Use of a nested polymerase chain reaction (N-PCR) to detect Trypanosoma cruzi in blood samples from chronic chagasic patients and patients with doubtful serologies. Diagn. Microbiol. Infect. Dis. 43:39-43. [DOI] [PubMed] [Google Scholar]
- 8.Moncayo, A., and M. I. Ortiz Yanine. 2006. An update on Chagas disease (human American trypanosomiasis). Ann. Trop. Med. Parasitol. 100:663-677. [DOI] [PubMed] [Google Scholar]
- 9.Nowicki, M. J., C. Chinchilla, L. Corado, L. Matsuoka, R. Selby, F. Steurer, T. Mone, R. Mendez, and S. Aswad. 2006. Prevalence of antibodies to Trypanosoma cruzi among solid organ donors in Southern California: a population at risk. Transplantation 81:477-479. [DOI] [PubMed] [Google Scholar]
- 10.Oelemann, W. M. R., M. G. M. Teixeira, G. C. Verissimo-da-Costa, J. Borges-Pereira, J. A. De Castro, J. R. Coura, and J. M. Peralta. 1998. Evaluation of three commercial enzyme-linked immunosorbent assays for diagnosis of Chagas' disease. J. Clin. Microbiol. 36:2423-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palau, M. T., A. J. Mejia, U. Vergara, and C. A. Zuniga. 2003. Action of Trypanosoma rangeli in infections with virulent Trypanosoma cruzi populations. Mem. Inst. Oswaldo Cruz 98:543-548. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez, L. E., E. Lages-Silva, F. Alvarenga-Franco, A. Matos, N. Vargas, O. Fernandes, and B. Zingales. 2002. High prevalence of Trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formerly-endemic area of Chagas disease in Southeast Brazil. Acta Trop. 84:189-198. [DOI] [PubMed] [Google Scholar]
- 13.Saldana, A., and O. E. Souza. 1996. Trypanosoma rangeli: epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J. Parasitol. 82:363-366. [PubMed] [Google Scholar]
- 14.Saldana, A., F. Samudio, A. Miranda, L. M. Herrera, S. P. Saavedra, L. Caceres, V. Bayard, and J. E. Calzada. 2005. Predominance of Trypanosoma rangeli infection in children from a Chagas disease endemic area in the west-shore of the Panama canal. Mem. Inst. Oswaldo Cruz 100:729-731. [DOI] [PubMed] [Google Scholar]
- 15.Salles, N. A., E. C. Sabino, M. G. Cliquet, J. Eluf-Neto, A. Mayer, C. Almeida-Neto, M. C. Mendonca, P. Dorliach-Llacer, D. F. Chamone, and A. Saéz-Alquézar. 1996. Risk of exposure to Chagas' disease among seroreactive Brazilian blood donors. Transfusion 36:969-973. [DOI] [PubMed] [Google Scholar]
- 16.Salomone, O. A., A. L. Basquiera, A. Sembaj, A. M. Aguerri, M. E. Reyes, M. Omelianuk, R. A. Fernandez, J. Enders, A. Palma, J. M. Barral, and R. J. Madoery. 2003. Trypanosoma cruzi in persons without serologic evidence of disease, Argentina. Emerg. Infect. Dis. 9:1558-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silveira, J. F., E. S. Umezawa, and A. O. Luquetti. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 18.Silveira-Lacerda, E. P., A. G. Silva, S. Favoreto, Jr., M. A. Souza, N. Kesper, A. Botelho-Filho, and E. S. Umezawa. 2004. Chagas disease: application of TESA-blot in inconclusive sera from a Brazilian blood bank. Vox Sang. 87:204-207. [DOI] [PubMed] [Google Scholar]
- 19.Tobler, L. H., P. Contestable, L. Pitina, H. Groth, S. Shaffer, G. R. Blackburn, H. Warren, S. R. Lee, and M. P. Busch. 2007. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion 7:90-96. [DOI] [PubMed] [Google Scholar]
- 20.Umezawa, E. S., M. S. Nascimento, N. Kesper, Jr., J. R. Coura, J. Borges-Pereira, A. C. Junqueira, and M. E. Camargo. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J. Clin. Microbiol. 34:2143-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umezawa, E. S., S. F. Bastos, J. R. Coura, M. J. Levin, A. Gonzalez, R. Rangel-Aldao, B. Zingales, A. O. Luquetti, and J. F. Silveira. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91-97. [DOI] [PubMed] [Google Scholar]
- 22.Wendel, S. 1998. Transfusion-transmitted Chagas disease. Curr. Opin. Hematol. 5:406-411. [DOI] [PubMed] [Google Scholar]
- 23.Zarate-Blades, C. R., M. S. Nascimento, J. F. da Silveira, and E. S. Umezawa. 2007. Diagnostic performance of tests based on Trypanosoma cruzi excreted-secreted antigens in an endemic area for Chagas disease in Bolivia. Diagn. Microbiol. Infect. Dis. 57:229-232. [DOI] [PubMed] [Google Scholar]