Abstract
To determine the systemic cytokine pattern induced by vaccination with human papillomavirus (HPV) L1 virus-like particles (VLP), we analyzed 22 different cytokines in culture supernatants of L1 VLP-stimulated peripheral blood mononuclear cells from vaccine (n = 19) and placebo (n = 7) recipients at months 0 and 2 after vaccination, using a multiplex cytokine bead array. In vaccine recipients, incubation with L1 VLP in vitro led to a statistically significant increase in production of Th1 (granulocyte-macrophage colony-stimulating factor, interleukin-2 [IL-2], gamma interferon; P < 0.0007) and Th2 (IL-4, IL-5, IL-10, IL-13; P < 0.0017) cytokines and the chemokine IP-10 (P = 0.0021) at month 2 after immunization, compared to levels seen prior to vaccination. These responses were not seen in placebo recipients. Cytokine and neutralizing antibody responses to vaccination followed the same pattern, with the highest antibody responses seen for subjects with higher cytokine responses. Cytokine profiling studies using samples from efficacy trials may provide important information about discriminators of long-term protection against HPV.
Noninfectious L1 human papillomavirus (HPV) virus-like particles (VLP) are the lead candidate vaccines to prevent HPV and associated disease (13, 14). A quadrivalent HPV VLP has recently been licensed. Clinical trials have shown a strong induction of humoral responses after vaccination (1, 7, 10, 15, 22) and near-complete protection against infection with homologous HPV types (9, 18, 26). Neutralizing antibodies are believed to be the main effector of protection.
In previous studies, we and others demonstrated that vaccination with HPV L1 VLP is also associated with an increase in T-cell proliferation and cytokine production (4, 5, 21). Recently, we used an 11-plex cytokine system that revealed that HPV type 16 (HPV16) L1 VLP vaccination induced a wide spectrum of cytokines in whole blood and peripheral blood mononuclear cells (PBMCs) (20, 21). Cytokine responses are important for both the induction and maintenance of humoral responses (28, 29) and might play a role in the efficacy of HPV VLP vaccines. Therefore, characterization of cytokine/chemokine patterns produced upon vaccination can contribute to the identification of biomarkers of vaccine response.
Here, we have further characterized the systemic cytokine and chemokine profiles induced by L1 VLP vaccination using the multiplex technology (22-plex) and evaluated their correlation with serum HPV16-neutralizing antibody titers. We also performed a cluster analysis to identify cytokine patterns of response to the vaccine.
MATERIALS AND METHODS
Study design.
A phase II clinical trial of the HPV16 L1 VLP vaccine without adjuvant (Novavax, Rockville, MD) was conducted with a total of 220 healthy, adult, female volunteers 18 to 25 years of age, as previously described (10, 21). Briefly, subjects were enrolled at The Johns Hopkins University Center for Immunization Research (Baltimore). Participants received three intramuscular doses of either 50 μg of HPV16 L1 VLP vaccine or a placebo (0.5 ml of saline). Blood was collected before the initial vaccination (at month 0) and 1 month after each of the subsequent vaccinations (months 2 and 7). The Johns Hopkins University Institutional Review Board approved the protocol for this study.
Twenty-six subjects were randomly selected (19 vaccine recipients [mean age ± standard deviation, 20.4 ± 2.1 years; median age, 21.9 years; race, 3 black and 16 white] and 7 placebo recipients [mean age, 21.9 ± 1.9; race, 1 black and 6 white]) among those with enough cryopreserved cells available. Month 0 and month 2 samples were selected for this study. Cytokine responses to L1 VLP at month 2 (1 month following the second dose of vaccine) are in general close to peak levels (21).
Cytokine induction assay.
Cryopreserved PBMCs were thawed and cultured (2.0 × 106 cells/ml) for 3 days as previously described (21). The following conditions were used in vitro: media; HPV16 L1 VLP (2.5 μg/ml); influenza A virus (Flu, H3N2, 1:100; ATCC); and Sf9/baculovirus insect cell lysate (Bac, 0.1 μg/ml; Novavax). Flu was used as a positive control. Media and the baculovirus cell lysate were used as negative controls. Cell-free supernatants were harvested, aliquoted, and frozen at −80°C.
Cytokine determinations.
Culture supernatants were tested in duplicate for 22 cytokine/chemokines (interleukin-1α [IL-1α], IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage colony-stimulating factor [GM-CSF], monocyte chemoattractant protein-1 [MCP-1], Eotaxin, macrophage inflammatory protein-1α [MIP-1α], IFN-γ-inducible 10-kDa protein [IP-10], and RANTES [Linco Research, Inc.]). Data acquisition and analysis were performed using Bioplex manager software version 2.0 (Bioplex; Bio-Rad). The lower levels of detection for all the cytokines were 1.6 pg/ml. Specimens with levels below the detection limit were assigned a value of one-half of this limit (20). All IL-7 and IL-12 levels were below detection level and were excluded from analysis. Cytokines were grouped into Th1 type (G-CSF, GM-CSF, IL-2, IL-15, IL-17, IFN-γ, TNF-α), Th2 type (IL-4, IL-5, IL-6, IL-10, IL-13), and inflammatory/chemokine type (IL-1α, IL-1β, IL-8, MIP-1α, MCP-1, IP-10, Eotaxin, RANTES).
Neutralization assay.
HPV16-neutralizing antibody titers were determined using a secreted alkaline phosphatase reporter gene pseudovirus-based neutralization assay performed as described previously (19). Neutralizing titers were defined as the reciprocal of the highest dilution that caused at least 50% reduction in secreted alkaline phosphatase activity. Undetectable antibody levels were considered 0.
At month 0, only one participant (a placebo recipient) had detectable neutralizing antibodies, and she maintained detectable levels throughout the study. All other subjects were negative. At month 2, all vaccine recipients had detectable antibody levels (mean, 1,050; median, 664). Two out of the seven placebo recipients had detectable anti-HPV16 antibody titers at month 2 (month 2 titers, 93 and 209, respectively), presumably due to natural infection.
Statistical analysis.
Wilcoxon signed-rank test was used to determine significance in changes of cytokine expression after stimulation with L1 VLP before and after vaccination. In addition, Bonferroni's correction for multiple comparisons was applied in the analysis of vaccine-induced changes in cytokine levels.
Cytokines were clustered (R version 2.2.0) to visualize their expression patterns using Euclidean distance metric and average linkage. Samples were clustered according to the log difference in response pre- and postvaccination. Only cytokines with an unadjusted P value of <0.05 (comparing pre- and postvaccination specimens among vaccine recipients) were included (GM-CSF, IL-1α, IL-17, IL-8, TNF-α, IL-6, IL-13, IL-5, IL-4, IFN-γ, IL-10, IL-2, IP-10, and MIP-1α).
RESULTS
Cytokine profile induced by L1 VLP upon vaccination.
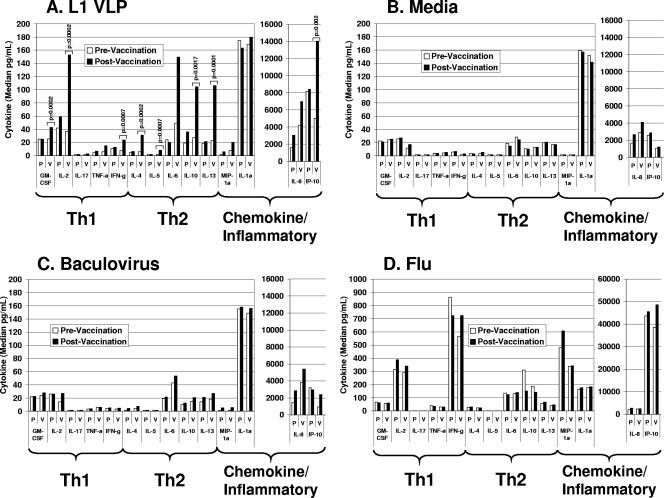
Twenty-two different cytokines were evaluated in supernatants from VLP-treated PBMCs before and after vaccination in vaccine and placebo recipients. A total of eight cytokines were significantly upregulated in L1 VLP cultures following vaccination compared to levels in cultures taken before vaccination (Fig. 1A). These include Th1-type cytokines (GM-CSF, median n-fold increase, 1.7-fold, P = 0.0002; IL-2, 4.2-fold, P = 0.0002; IFN-γ, 3.1-fold, P = 0.0007), Th2-type cytokines (IL-4, 5.2-fold, P = 0.0002; IL-5, 4.95-fold, P = 0.0007; IL-10, 3.8-fold, P = 0.0017; IL-13, 4.7-fold, P = 0.0001), and the chemokine IP-10 (2.8-fold, P = 0.0021). An increase in IL-1α (1.1-fold), IL-6 (3.0-fold), IL-8 (1.7-fold), IL-17 (1.5-fold), TNF-α (2.5-fold), and MIP-1α (2.6-fold) was observed but did not reach statistical significance after adjusting for multiple comparisons.
FIG. 1.
Cytokine profiles in supernatants of treated PBMCs collected before and after vaccination among L1 VLP (V; n = 19) and placebo (P; n = 7) recipients. Supernatants were tested for cytokine content using multiplex cytokine analysis as described in the text. Results are presented as median cytokine levels in picograms per milliliter. Only cytokines that exhibited a statistically significant increase in response to L1 VLP before adjusting for multiple comparisons are shown. Only significant P values are indicated. A nonparametric Wilcoxon test was used to determine statistical significance. (A) Cells from vaccine and placebo recipients were treated with HPV16 L1 VLP (2.5 μg/ml) for 72 h. P values shown were obtained from the comparisons between responses to L1 VLP from pre- and postvaccination samples and adjusted for multiple comparisons. A P value of <0.0025 was considered significant for the primary hypothesis. (B) Cells from vaccine and placebo recipients were left untreated (media) for 72 h. (C) Cells from vaccine and placebo recipients were incubated with baculovirus extract (0.1 μg/ml) for 72 h. (D) Cells from vaccine and placebo recipients were incubated with flu (1:100) for 72 h.
No significant changes in cytokine levels were observed in untreated (Fig. 1B) or control (Fig. 1C and D) cultures (baculovirus or flu cultures) when prevaccination (month 0) and postvaccination (month 2) samples were compared for vaccine or placebo recipients. In addition, no significant changes in cytokine levels were observed in L1 VLP-stimulated cultures from placebo recipients when month 0 and month 2 samples were compared (Fig. 1A).
Identification of patterns of cytokine response to vaccination.
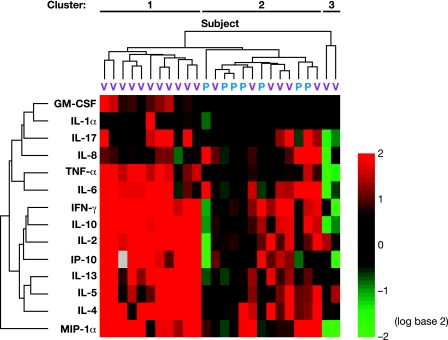
Cluster analysis was performed to determine the patterns of response induced by vaccination in vaccine recipients compared to the placebo group (Fig. 2). Subjects clustered into three groups, when their pre- and postvaccination levels of response to L1 VLP were compared. Two of these groups include only vaccine recipients, and one group contains all placebo recipients plus six vaccine recipients.
FIG. 2.
Two-dimensional cluster analysis of cytokine/chemokine profiles produced in response to incubation with HPV16 L1 VLP. The top dendrogram represents the similarity between cytokine profiles of different subjects, and the left dendrogram represents the similarity between measured cytokines/chemokines. Differences in relative levels of gene expression are indicated in color, where red indicates upregulation and green indicates downregulation relative to the corresponding gene expression in prevaccination samples. Each row represents the values for a cytokine, and columns correspond to patients (blue label, placebo; pink label, vaccine recipient).
The first group includes 11 vaccine recipients and is characterized by a considerable increase for most cytokines. The n-fold increase for the majority of cytokines in this group (10 out of 14; namely, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-α, IFN-γ, IP-10, and MIP-1α) was higher than fourfold. The remaining four cytokines (GM-CSF, IL-1α, IL-17, and IL-8) showed a weaker increment in response postvaccination, but the responses observed were higher than those observed for the other two groups identified.
The second group was composed of six vaccine recipients, presenting a heterogeneous cytokine expression pattern, with weaker cytokine responses than the first group. A twofold increase or higher was observed in at least four out of six vaccine recipients for IL-4, IL-5, IL-6, IL-8, IFN-γ, and IP-10 and in three out of six vaccinees for IL-2, IL-10, IL-13, and IL-17. Flu-induced responses for these cytokines were not significantly different between the vaccine recipients within these two clusters (data not shown).
Finally, the third group contains two vaccine recipients for whom a decrease in cytokine expression was detected for most of the cytokines following vaccination.
Comparison of neutralizing antibody titers with cytokine levels.
Antibody titers from individuals from each cluster were also evaluated (Table 1). Neutralizing anti-HPV16 titers were not detectable at the first visit for vaccine recipients (month 0) and increased after vaccination, with peak titers at month 7. Although the number of individuals in each cluster is small and differences in neutralizing antibody titers did not reach statistical significance, vaccine recipients in cluster 1 had higher titers than those in cluster 2 (for cluster 1, the month 2 mean was 1,426 and the median was 928; for cluster 2, the month 2 mean was 595 and the median was 548; P = 0.23). In general, the level of overall cytokine response to VLP tends to mirror the neutralizing antibody titer pattern at month 2. However, no significant correlations between individual cytokine levels and neutralizing antibody titers were detected.
TABLE 1.
Neutralizing antibody titers in vaccine and placebo recipients by cluster group
| Group | Cluster | Month | Anti-HPV16 titer
|
No. of recipients | ||
|---|---|---|---|---|---|---|
| Mean | SD | Median | ||||
| Vaccine | 1 | 0 | 0 | 0 | 0 | 11 |
| 1 | 332 | 499 | 192 | 11 | ||
| 2 | 1,426 | 1,428 | 928 | 11 | ||
| 7 | 8,891 | 8,947 | 5,180 | 9 | ||
| 12 | 4,864 | 6,117 | 1,791 | 9 | ||
| 2 | 0 | 0 | 0 | 0 | 6 | |
| 1 | 198 | 396 | 49 | 6 | ||
| 2 | 595 | 409 | 548 | 6 | ||
| 7 | 952 | 1,338 | 250 | 3 | ||
| 12 | 263 | 157 | 200 | 3 | ||
| 3 | 0 | 0 | 0 | 0 | 2 | |
| 1 | 69 | 97 | 69 | 2 | ||
| 2 | 346 | 34 | 346 | 2 | ||
| 7 | 1,889 | 590 | 1,889 | 2 | ||
| 12 | 694 | 564 | 694 | 2 | ||
| Placebo | 2 | 0 | 12 | 32 | 0 | 7 |
| 1 | 19 | 36 | 0 | 7 | ||
| 2 | 43 | 81 | 0 | 7 | ||
| 7 | 45 | 81 | 0 | 7 | ||
| 12 | 51 | 112 | 0 | 7 | ||
Cytokine production induced by HPV16 L1 VLP prior to vaccination.
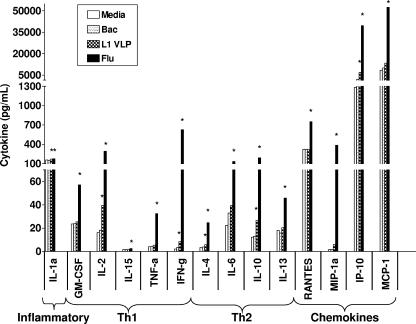
Cytokine levels obtained after in vitro treatment of PBMCs with L1 VLP were compared to media cultures in all 26 subjects (Fig. 3). L1 VLP induced a statistically significant increase in the level of inflammatory cytokines or chemokines (IL-1α, IP-10) and in Th1-type (IL-2, IFN-γ) and Th2-type (IL-4, IL-10) cytokines. The relative increase in cytokine expression ranged from 1.1-fold for IL-1α to a 5.4-fold increase in the expression of IP-10. IFN-γ expression increased 4.0-fold, and the induction observed for IL-2, IL-4, and IL-10 was 2.4-fold, 1.7-fold, and 2.2-fold, respectively. No significant changes were observed when baculovirus-treated cultures were compared to unstimulated cells.
FIG. 3.
Cytokine profiles in supernatants of treated PBMCs collected before vaccination (n = 26). Cells were left untreated (media) or incubated with HPV16 L1 VLP, baculovirus extract, or flu (1:50) for 72 h. Supernatants were tested for cytokine content using multiplex cytokine analysis as described in the text. Results are presented as median cytokine levels in picograms per milliliter. P values were obtained from comparisons between media and stimulated cultures. A nonparametric Wilcoxon test was used to determine statistical significance. A P value of <0.05 was considered significant and is indicated with an asterisk (*). Only cytokines that exhibited a statistically significant increase in response to any stimulus are shown.
A wider spectrum of cytokines was induced by flu with significantly increased levels of 14 out of the 22 cytokines, compared to media cultures. The relative increase in cytokine levels following flu stimulation ranged from 1.13-fold for IL-1α to 294-fold for IFN-γ. Significant increases were detected in inflammatory cytokines/chemokines (IL-1α, median n-fold increase, 1.13-fold; MIP-1α, 241-fold; IP-10, 31-fold; RANTES, 2.3-fold; MCP-1, 6.2-fold), Th1-type cytokines (GM-CSF, 2.4-fold; IL-2, 18.1-fold; IL-15, 1.5-fold; IFN-γ, 294-fold; TNF-α, 7.9-fold), and Th2-type cytokines (IL-4, 7.4-fold; IL-6, 5.9-fold; IL-10, 15.4-fold; IL-13, 2.5-fold).
DISCUSSION
To extend our previous studies on cytokine profiles induced by L1 VLP vaccination, we used a 22-multiplex cytokine bead assay to better characterize the cytokine pattern elicited in PBMCs from vaccine recipients in response to L1 VLP and compared this profile to that obtained from placebo recipients or from prevaccination samples. The 22-multiplex system includes important cytokines and chemokines, which have not been previously investigated in the context of vaccination with L1 VLP. A subset of these cytokines may represent discriminators of high and low antibody responses to vaccination and duration of protection.
The results from this study confirm earlier observations of induction of Th1 and Th2 cytokines by vaccination (4, 5, 20, 21). Here, we report for the first time induction of IL-13 and IP-10 following L1 VLP vaccination.
Prior to vaccination, L1 VLP induced a statistically significant increase in the production of Th1-type (IL-2, IFN-γ), Th2-type (IL-4, IL-10), and inflammatory (IL-1α, IP-10) cytokines/chemokines in PBMCs. However, the magnitude of induction for IL-1α, IL-2, IL-4, and IL-10 was lower than 2.4-fold compared to media cultures.
The pattern of cytokine expression observed in this study is in agreement with results demonstrating the ability L1 VLP to induce potent B cell responses and dendritic cell activation (2, 6, 8, 16, 17, 20, 27). Alternatively, cytokine responses to L1 VLP observed prior to vaccination and in some of the placebo recipients during the study could be due to natural HPV infection that resulted in priming of the PBMCs to the L1 HPV antigens.
It is well documented that responses to viruses and subunit vaccines comprise a broad spectrum of soluble immune mediators (3, 11, 12, 23-25). A wider spectrum of cytokines was induced by influenza virus (flu) than by the L1 VLP. This marked difference in stimulation potency might reflect the fact that the VLP is a recombinant purified protein, while flu is a live virus. In addition, the strong response to flu could be explained by recent infections and priming/boosting with the virus or vaccine prior to entry in our study.
The cytokines induced by vaccination did not appear to cluster based on their functional Th1-Th2 category. However, cluster analysis of vaccine recipients revealed a heterogeneous cytokine response to vaccination. Three clusters with different intensities of expression and spectrums of response were found, including a very small group with a decrease in cytokine production. The potential role of these responses in the outcome of vaccination and the duration of protection against infection is unknown, but the results raise the possibility that different response patterns could be associated with distinct outcomes. Although we cannot interpret the decrease in cytokine production observed in the third cluster, a possible explanation could be the variability and noise in media background responses combined with a lack of cellular immune response to vaccination. The observed variability in cytokine production was not likely due to compromised function of samples, because samples showed robust responses to our positive control (flu).
Cytokine responses and neutralizing antibody titers produced after vaccination at month 2 followed a similar pattern. However, differences in antibody levels between the clusters were not significant, likely due to the small sample size and the high variability in the neutralization titers induced by vaccination.
Results obtained in this study may not be directly extrapolated to the cytokine patterns that will be obtained after vaccination with the commercially available L1 VLP vaccine, because participants in our study were immunized with VLP without an adjuvant. In addition, the L1 VLP used in our study are monovalent and produced in a recombinant baculovirus system.
In conclusion, we have identified a panel of cytokines and chemokines significantly and heterogeneously induced by HPV16 L1 VLP following the vaccination of healthy young women. Comparison of cytokine profiles elicited in response to L1 VLP developed after natural infection or after vaccination will be of interest in future studies. In addition, further analyses of cytokine profiles in samples from individuals from efficacy studies will be necessary to identify the cytokine profiles associated with long-term protection against HPV infection.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (N01-CO-12400).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Brown, D. R., K. H. Fife, C. M. Wheeler, L. A. Koutsky, L. M. Lupinacci, R. Railkar, G. Suhr, E. Barr, A. Dicello, W. Li, J. F. Smith, A. Tadesse, and K. U. Jansen. 2004. Early assessment of the efficacy of a human papillomavirus type 16 L1 virus-like particle vaccine. Vaccine 22:2936-2942. [DOI] [PubMed] [Google Scholar]
- 2.Da Silva, D. M., M. P. Velders, J. D. Nieland, J. T. Schiller, B. J. Nickoloff, and W. M. Kast. 2001. Physical interaction of human papillomavirus virus-like particles with immune cells. Int. Immunol. 13:633-641. [DOI] [PubMed] [Google Scholar]
- 3.De Rosa, S. C., F. X. Lu, J. Yu, S. P. Perfetto, J. Falloon, S. Moser, T. G. Evans, R. Koup, C. J. Miller, and M. Roederer. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173:5372-5380. [DOI] [PubMed] [Google Scholar]
- 4.Emeny, R. T., C. M. Wheeler, K. U. Jansen, W. C. Hunt, T. M. Fu, J. F. Smith, S. MacMullen, M. T. Esser, and X. Paliard. 2002. Priming of human papillomavirus type 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine. J. Virol. 76:7832-7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, T. G., W. Bonnez, R. C. Rose, S. Koenig, L. Demeter, J. A. Suzich, D. O'Brien, M. Campbell, W. I. White, J. Balsley, and R. C. Reichman. 2001. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J. Infect. Dis. 183:1485-1493. [DOI] [PubMed] [Google Scholar]
- 6.Fausch, S. C., D. M. Da Silva, and W. M. Kast. 2003. Differential uptake and cross-presentation of human papillomavirus virus-like particles by dendritic cells and Langerhans cells. Cancer Res. 63:3478-3482. [PubMed] [Google Scholar]
- 7.Fife, K. H., C. M. Wheeler, L. A. Koutsky, E. Barr, D. R. Brown, M. A. Schiff, N. B. Kiviat, K. U. Jansen, H. Barber, J. F. Smith, A. Tadesse, K. Giacoletti, P. R. Smith, G. Suhr, and D. A. Johnson. 2004. Dose-ranging studies of the safety and immunogenicity of human papillomavirus type 11 and type 16 virus-like particle candidate vaccines in young healthy women. Vaccine 22:2943-2952. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Pineres, A. J., A. Hildesheim, M. Trivett, M. Williams, L. Wu, V. N. Kewalramani, and L. A. Pinto. 2006. Role of DC-SIGN in the activation of dendritic cells by HPV-16 L1 virus-like particle vaccine. Eur. J. Immunol. 36:437-445. [DOI] [PubMed] [Google Scholar]
- 9.Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-1255. [DOI] [PubMed] [Google Scholar]
- 10.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 11.Julkunen, I., T. Sareneva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikainen. 2001. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann, A., R. Salentin, R. G. Meyer, D. Bussfeld, C. Pauligk, H. Fesq, P. Hofmann, M. Nain, D. Gemsa, and H. Sprenger. 2001. Defense against influenza A virus infection: essential role of the chemokine system. Immunobiology 204:603-613. [DOI] [PubMed] [Google Scholar]
- 13.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 16.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 17.Lenz, P., C. D. Thompson, P. M. Day, S. M. Bacot, D. R. Lowy, and J. T. Schiller. 2003. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin. Immunol. 106:231-237. [DOI] [PubMed] [Google Scholar]
- 18.Mao, C., L. A. Koutsky, K. A. Ault, C. M. Wheeler, D. R. Brown, D. J. Wiley, F. B. Alvarez, O. M. Bautista, K. U. Jansen, and E. Barr. 2006. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet. Gynecol. 107:18-27. [DOI] [PubMed] [Google Scholar]
- 19.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216. [DOI] [PubMed] [Google Scholar]
- 20.Pinto, L. A., P. E. Castle, R. B. Roden, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, M. Williams, W. Kopp, I. H. Frazer, J. A. Berzofsky, and A. Hildesheim. 2005. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine 23:3555-3564. [DOI] [PubMed] [Google Scholar]
- 21.Pinto, L. A., J. Edwards, P. E. Castle, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, W. Kopp, J. W. Adelsberger, M. W. Baseler, J. A. Berzofsky, and A. Hildesheim. 2003. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J. Infect. Dis. 188:327-338. [DOI] [PubMed] [Google Scholar]
- 22.Poland, G. A., R. M. Jacobson, L. A. Koutsky, G. M. Tamms, R. Railkar, J. F. Smith, J. T. Bryan, P. F. Cavanaugh, Jr., K. U. Jansen, and E. Barr. 2005. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2-year randomized controlled clinical trial. Mayo Clin. Proc. 80:601-610. [DOI] [PubMed] [Google Scholar]
- 23.Powers, D. C., J. E. McElhaney, O. A. Florendo, Jr., M. C. Manning, C. M. Upshaw, D. W. Bentley, and B. E. Wilkinson. 1997. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J. Infect. Dis. 175:342-351. [DOI] [PubMed] [Google Scholar]
- 24.Querec, T., S. Bennouna, S. Alkan, Y. Laouar, K. Gorden, R. Flavell, S. Akira, R. Ahmed, and B. Pulendran. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronni, T., T. Sareneva, J. Pirhonen, and I. Julkunen. 1995. Activation of IFN-alpha, IFN-gamma, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J. Immunol. 154:2764-2774. [PubMed] [Google Scholar]
- 26.Tomson, T. T., R. B. Roden, and T. C. Wu. 2004. Human papillomavirus vaccines for the prevention and treatment of cervical cancer. Curr. Opin. Investig. Drugs 5:1247-1261. [PubMed] [Google Scholar]
- 27.Yang, R., F. M. Murillo, M. J. Delannoy, R. L. Blosser, W. H. Yutzy, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2005. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol. 174:7912-7919. [DOI] [PubMed] [Google Scholar]
- 28.Yao, Q., R. Zhang, L. Guo, M. Li, and C. Chen. 2004. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J. Immunol. 173:1951-1958. [DOI] [PubMed] [Google Scholar]
- 29.Zinkernagel, R. M., M. F. Bachmann, T. M. Kundig, S. Oehen, H. Pirchet, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14:333-367. [DOI] [PubMed] [Google Scholar]