Abstract
Trypanosoma cruzi infection causes Chagas' disease, a chronic inflammatory disease. The specific inflammatory responses that cause Chagas' disease remain unclear, but data argue that parasites that persist in the host stimulate chronic self-damaging immune responses. Because T. cruzi appears to stimulate self-damaging responses, the enthusiasm to develop vaccines that boost antiparasite responses that might increase self-damaging responses has been limited. We previously demonstrated that immunization with a T. cruzi trans-sialidase protein or adoptive transfer of trans-sialidase-specific T-cell clones decreased parasitemia, morbidity, and mortality. Here we report that immunization or adoptive transfer with the protein or clones, before or during T. cruzi infection, boosts the anti-T. cruzi immune response without exacerbating acute or chronic tissue inflammation. These results argue that prophylactic and therapeutic immunotherapy for Chagas' disease can be developed safely.
Trypanosoma cruzi, the causative agent of Chagas' disease, infects approximately 18 million individuals in Latin America (4, 23). Most individuals survive the acute infection, but 30% of those infected develop Chagas' disease, a chronic inflammatory disease that commonly results in heart failure or severe gastrointestinal disease (23). To decrease the incidence of Chagas' disease, programs to eliminate insect vector transmission of T. cruzi have been implemented successfully in areas where domiciliary insect species exist, but in areas where sylvatic insect species exist, other approaches are required. Currently, there are no vaccines, and existing drug therapies (with benznidazole or nifurtimox) are poorly efficacious. Clearly, there is a need for additional treatments or prevention of T. cruzi infection.
The etiology of the chronic inflammatory pathology of Chagas' disease remains unclear, but for many years it has been argued that parasite-triggered autoimmune responses contribute to the disease (13). Alternatively, it has been argued that immune responses that control the persistent parasite cause the inflammatory damage (1). Because the chronic immune pathology appears to be caused by autoimmune responses or antiparasite responses, efforts to develop anti-T. cruzi vaccines have been limited, as it is feared that a vaccine will exacerbate the self-damaging inflammatory responses. Despite these concerns, several T. cruzi proteins have been used as immunogens in mice to augment the acute immune response and to better control parasitemia and improve survival (8-10, 15-17, 20-22). Furthermore, a therapeutic vaccine administered to mice during acute or chronic infection has been shown to augment the anti-T. cruzi immune response and to decrease tissue inflammation (5, 24). These reports argue that safe and effective vaccines for prevention and treatment of Chagas' disease can be developed.
We previously demonstrated that immunization of mice with a recombinant protein that carries a fragment of the SA85-1.1 protein, a protein of the T. cruzi trans-sialidase superfamily, or a single peptide epitope of this protein (called epitope 1) can limit parasitemia and improve survival (16). Similar results were obtained following adoptive transfer of Th1 clones that specifically respond to epitope 1 (16). It is possible that these treatments, though able to limit parasitemia and improve survival, triggered self-damaging responses (autoimmune or bystander) that worsened chronic tissue inflammation. Although T. cruzi-infected mice do not develop the cardiac or gastrointestinal disease that becomes evident in people, infected mice develop widespread tissue inflammation. In this study, we investigated how augmentation of the anti-trans-sialidase immune response, through immunization or adoptive transfer, affects acute and chronic tissue inflammation. Our data indicate that these treatments, given prophylactically before infection or therapeutically after acute infection, augment the anti-T. cruzi immune response without exacerbating tissue inflammation and further argue that safe and effective vaccines can be developed for Chagas' disease.
MATERIALS AND METHODS
T. cruzi.
A recently derived clone of CL strain subclone 3 was used (3, 19). Trypomastigotes were obtained from culture supernatants of infected 3T3 cells grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated calf serum and 50,000 U penicillin-streptomycin (all from BioWhittaker, Walkersville, MD).
Infection of mice and serum collection.
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were infected intraperitoneally with trypomastigotes. An inoculum of 1 × 105 trypomastigotes was used unless otherwise indicated in the text or figure legends. Mice were bled by venesection of the tail, the blood was allowed to clot at 4°C overnight, and then sera were isolated and stored at −20°C.
Immunization with SA85-1.1 protein.
Mice were immunized by subcutaneous injection of 40 μg recombinant SA85-1.1 protein or ovalbumin (OVA) diluted in 200 μl complete Freund's adjuvant (Sigma, St. Louis, MO). Mice were injected again 14 and 28 days later with 40 μg of the same protein diluted in 200 μl incomplete Freund's adjuvant (Sigma). Immunization occurred either before infection, with the final boost 14 days before trypomastigote inoculation, or during infection, with the first immunization occurring 28 days after trypomastigote inoculation.
Adoptive transfer of SA85-1.1-specific T-cell clones.
The isolation of SA85-1.1 epitope 1-specific CD4+ Th1-cell clones has been described previously (12). The 0C3.4 T-cell clone was maintained by stimulation every 2 weeks with recombinant SA85-1.1 and supplemental interleukin-2 (Chiron Therapeutics, Emeryville, CA). T cells were grown in RPMI 1640 (BioWhittaker) supplemented with 5% heat-inactivated fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 50,000 U of penicillin-streptomycin, 10 mM HEPES, and 50 μM β-mercaptoethanol. T-cell clones were rested for 2 weeks prior to adoptive transfer. T cells (1 × 107) in 200 μl Dulbecco's modified Eagle's medium were transferred into mice by intravenous injection.
Analysis of parasitemia.
Parasitemia was monitored by venesection of the tail. Two microliters of blood was diluted in 18 μl of 1.66% ammonium chloride in phosphate-buffered saline (PBS), and the trypomastigotes were counted on a hemacytometer (11). For statistical analysis, Student's t test was used to compare the total parasitemia of each mouse within one treatment group with the total parasitemia of each mouse in another treatment group.
Analysis of antibody responses.
End-point titers for individual mouse sera were determined using the previously described anti-T. cruzi enzyme-linked immunosorbent assay (ELISA) or anti-SA85-1 protein ELISA (6). Briefly, ELISA plates (Nunc, Rochester, NY) were coated by adding 50 μl/well of PBS containing either 5 × 106 heat-killed trypomastigotes or 5 μg/ml recombinant SA85-1 protein. After overnight incubation at 4°C, the plates were washed with PBS-Tween, blocked with 1% bovine serum albumin (BSA)-PBS for 1 h at 37°C, and washed, and serum samples diluted with 1% BSA-PBS were added. Individual serum samples from each treatment group were diluted threefold, beginning at a 1:100 dilution. In addition, for each experiment, the sera of five naïve, uninfected mice were diluted threefold, beginning at a 1:100 dilution. Plates were incubated at room temperature for 3 h and then washed, and either biotinylated anti-immunoglobulin G (anti-IgG; Pharmingen, San Diego, CA), biotinylated anti-IgG2a (R19-15; Pharmingen), or biotinylated anti-IgG1 (A85-1; Pharmingen) (1 μg/ml in 1% BSA-PBS) antibodies were added. The plates were incubated for 1 h at room temperature and washed three times, streptavidin-horseradish peroxidase (Genzyme, Cambridge, MA) was added for 1 h at room temperature and the plates were washed four times, 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)-H2O2 (ABTS-H2O2; Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added, and the plates were analyzed at 405 nm. At each dilution, the optical densities at 405 nm (OD405) for each mouse in the treatment groups and for the five naïve, uninfected mice were calculated. An end-point titer for each mouse in the treatment groups was determined as the highest dilution with an OD405 that remained twofold above the mean OD405 of the five naïve, uninfected mice at the same dilution. The individual mouse titers were used to calculate the mean titer for each treatment group. To determine statistical significance, Student's t test analyses were performed to compare the antibody responses of the different treatment groups.
Histology and inflammatory scores.
Skeletal muscle inflammatory scores were determined by quantifying the amount of blue (dark)-staining nuclei present in skeletal muscle tissue following hematoxylin and eosin (H&E) staining. Normal skeletal muscle contains few nuclei and has a low background of dark-staining nuclei, which permits sensitive detection of increased inflammatory cells in the skeletal muscles. To perform these analyses, quadriceps muscles were fixed in formalin (Sigma, St. Louis, MO), sectioned, and stained with H&E (Sigma). Five random 10× images of the left and right quadriceps muscles (10 images per mouse) (Eclipse E200 microscope and Coolpix 4500 camera; Nikon, Tokyo, Japan) were captured by blinded investigators. Blinded investigators overlaid the printed images with a grid of 49 evenly dispersed points and determined the percentage of points intersected by nuclei to provide an inflammatory score, or alternatively, images were analyzed using Image-Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD) to determine the percentage of each section that was occupied by nuclei.
Statistics.
P values were determined using Student's t test or single-factor analysis of variance (Microsoft Excel; Microsoft Corporation, Redmond, WA), as indicated.
RESULTS
Adoptive transfer of an SA85-1.1-specific Th1 clone before infection reduces parasitemia without increasing chronic inflammation.
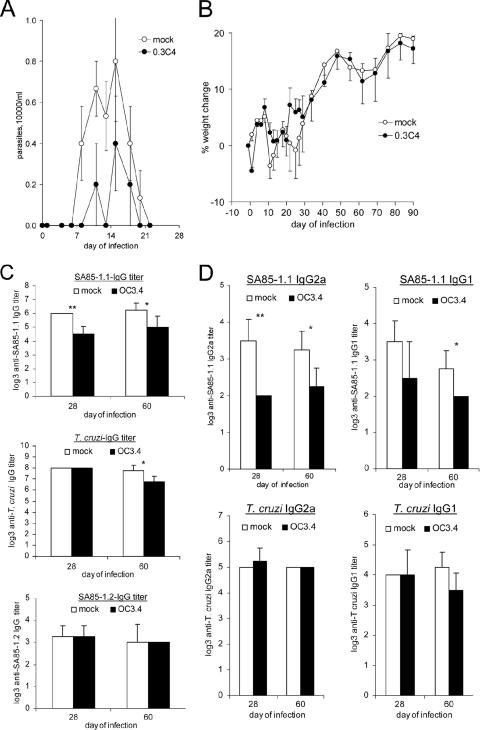
In a previous study, we demonstrated that adoptive transfer, before T. cruzi infection, of a major histocompatibility complex class II-restricted Th1 clone (clone 0C3.4) reduced parasitemia and increased survival (16). It is possible that this immune intervention worsened the T. cruzi-induced chronic inflammation. To investigate this possibility, mice were given clone 0C3.4 by adoptive transfer 1 day before they were infected with a sublethal dose of T. cruzi that permitted analyses of both acute and chronic inflammation. Observed parasitemia was lower in the 0.3C4 recipients (P = 0.017) (Fig. 1A). Growth, as assessed by weight gain, was similar for the 0.3C4 recipients and the control mice, suggesting that augmenting the antiparasite immune response can lower parasitemia without increasing morbidity (Fig. 1B).
FIG. 1.
Parasitemia, weights, and antibody titers during T. cruzi infection following adoptive transfer of the SA85-1.1-specific Th1 clone 0C3.4. Groups of C57BL/6 mice received 1 × 107 0C3.4 cells or medium, and 1 day later, both groups were inoculated with 2 × 105 trypomastigotes and (A) parasitemia and (B) weight were monitored. (C) On days 28 and 60 of infection, sera were collected and IgG end-point titers to SA85-1.1 protein, heat-inactivated trypomastigotes, and SA85-1.2 protein were determined. (D) Sera obtained on day 28 and day 60 of infection were analyzed for IgG1 and IgG2a end-point titers to the SA85-1.1 protein and heat-inactivated trypomastigotes. All results are expressed as means and standard errors (SE) for five mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We expected that transfer of the SA85-1.1-specific Th1 clone would enhance the antiparasite immune response, including the antiparasite antibody response. To our surprise, 0C3.4 transfer resulted in a reduction in the end-point titer of the IgG response to the SA85-1.1 protein on day 28 and day 60 of the infection (Fig. 1C). Transfer did not alter the anti-T. cruzi IgG end-point titer to heat-inactivated trypomastigotes on day 28 of the infection, but a mild reduction of this response was noted on day 60 of the infection (Fig. 1C). Adoptive transfer of 0C3.4 cells does not appear to alter the lower-titer IgG response to the SA85-1.2 protein, a trans-sialidase protein that carries a variant of epitope 1 (Fig. 1C). Further analysis of the antibody responses to SA85-1.1 on day 28 and day 60 of the infection argue that the 0C3.4 transfer decreased both the IgG2a and IgG1 responses (Fig. 1D). These data indicate that the transfer of 0C3.4 diminished parasitemia and the antibody response to selective T. cruzi antigens.
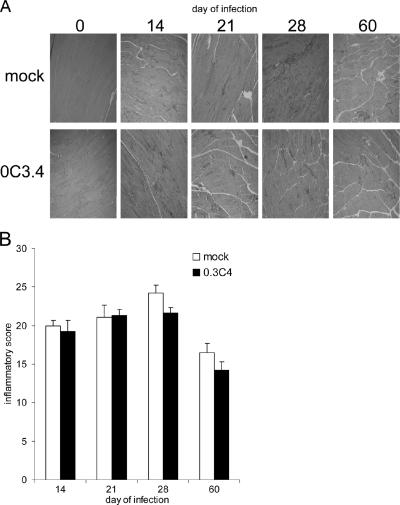
To determine if the transfer of the 0C3.4 cells worsened tissue inflammation, histochemical analyses of skeletal muscles were performed at different times of the infection. Skeletal muscle histology was investigated throughout this study because (i) the T. cruzi CL strain is known to invade and generate significant inflammation of all muscle types (skeletal, smooth, and cardiac); (ii) our previous unpublished analyses argue that during T. cruzi infection, cardiac and skeletal muscle inflammatory infiltrates develop with similar kinetics and cellular compositions; and (iii) the paucity of nuclei within normal uninfected skeletal muscle facilitates quantifying the inflammation by enumerating nuclei within the skeletal muscle (2, 14). Our studies indicate that skeletal muscles from uninfected mice obtained after mock or 0.3C4 adoptive transfer display similar amounts of staining nuclei (inflammation) as skeletal muscles from uninfected mice that have not undergone any manipulation (data not shown). In contrast, significantly more inflammation was present in both the mock-adoptively transferred and 0.3C4-adoptively transferred mice on days 14, 21, 28, and 60 of the infection (Fig. 2A and B). These studies indicated that transfer of 0C3.4 cells did not worsen tissue inflammation at any time during the infection (Fig. 2A and B). Together, these data argue that transfer of SA85-1.1-specific Th1 clones before T. cruzi infection reduces parasitemia and alters the immune response but does not exacerbate tissue inflammation.
FIG. 2.
Transfer of the 0C3.4 clone before T. cruzi infection does not exacerbate tissue inflammation. C57BL/6 mice received 1 × 107 0C3.4 cells or medium, and 1 day later, they were inoculated with 2 × 105 trypomastigotes. On the indicated days of the infection, skeletal muscles were obtained, H&E stained, and analyzed for inflammation. (A) Representative images. (B) Mean-plus-SE inflammatory scores for four muscles per group. In panel A, day 0 sections are uninfected tissue and the dark-staining areas of the muscle represent areas of inflammatory cell infiltrates. P values were as follows: day 14, 0.647; day 21, 0.984; day 28, 0.082; and day 60, 0.182.
Transfer of SA85-1.1-specific cells during T. cruzi infection transiently enhances the antibody response but does not worsen muscle inflammation.
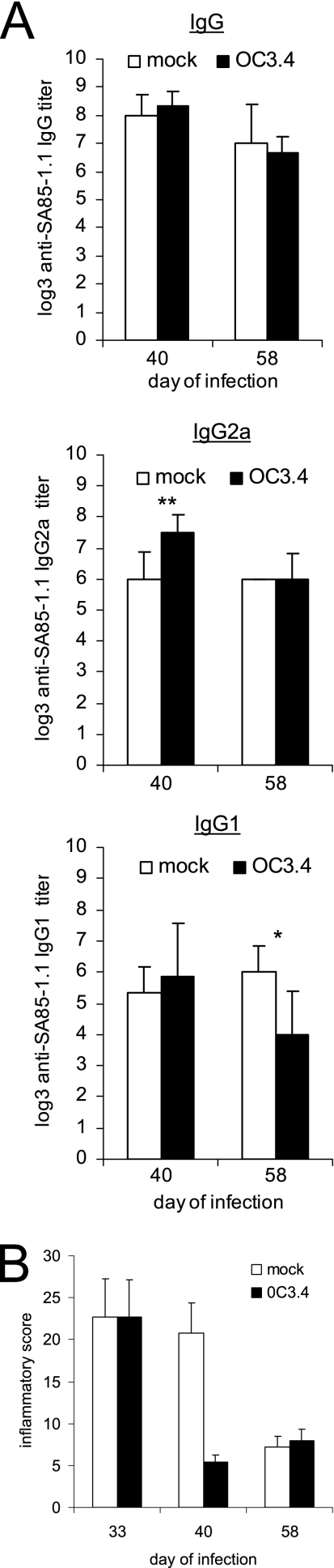
We also wanted to investigate if transfer of SA85-1.1-specific Th1 cells during infection would affect the immune response or worsen tissue inflammation. Thus, mice were given a sublethal inoculum of trypomastigotes, and on day 28 of the infection, after parasitemia had resolved, they were given 0C3.4 cells by adoptive transfer. In contrast to the reduction of anti-SA85-1.1 antibody titers observed when 0.3C4 cells were transferred before the infection, transfer during the infection enhanced the anti-SA85-1.1 IgG2a response that was titrated 12 days later (on day 40 of infection) (Fig. 3A). This enhancement of the IgG2a end-point titer was not present on day 58 of infection (30 days after transfer), while the IgG1 end-point titer was diminished (Fig. 3A). Thus, 0C3.4 clones transferred during infection transiently enhanced the Th1-driven anti-SA85-1.1 antibody responses and might have worsened tissue inflammation. Histological analysis of muscles indicated that 0.3C4-recipient mice had less muscle inflammation than medium-treated mice on day 40 of the infection and a similar amount of muscle inflammation on day 58 of the infection (Fig. 3B). Together, these data argue that increasing the number of SA85-1.1-specific Th1 cells during infection can augment the anti-T. cruzi immune response without worsening muscle inflammation.
FIG. 3.
Transfer of 0C3.4 cells during T. cruzi infection transiently affects the anti-SA85-1.1 antibody response but does not exacerbate tissue inflammation. Two groups of mice were inoculated with 1 × 105 trypomastigotes, and 28 days later, they received either 1 × 107 0C3.4 cells or medium. (A) On days 40 and 58 of infection, sera were collected, and IgG, IgG2a, and IgG1 end-point titers to the SA85-1.1 protein were determined (data are mean titers and SE for four or more mice per group). *, P < 0.05; **, P < 0.01. (B) Also on days 40 and 58 of infection, skeletal muscles were obtained and H&E stained, inflammatory scores were determined, and results were expressed as means and SE for four muscles per group.
Immunization with the SA85-1.1 protein before T. cruzi infection does not exacerbate chronic inflammation.
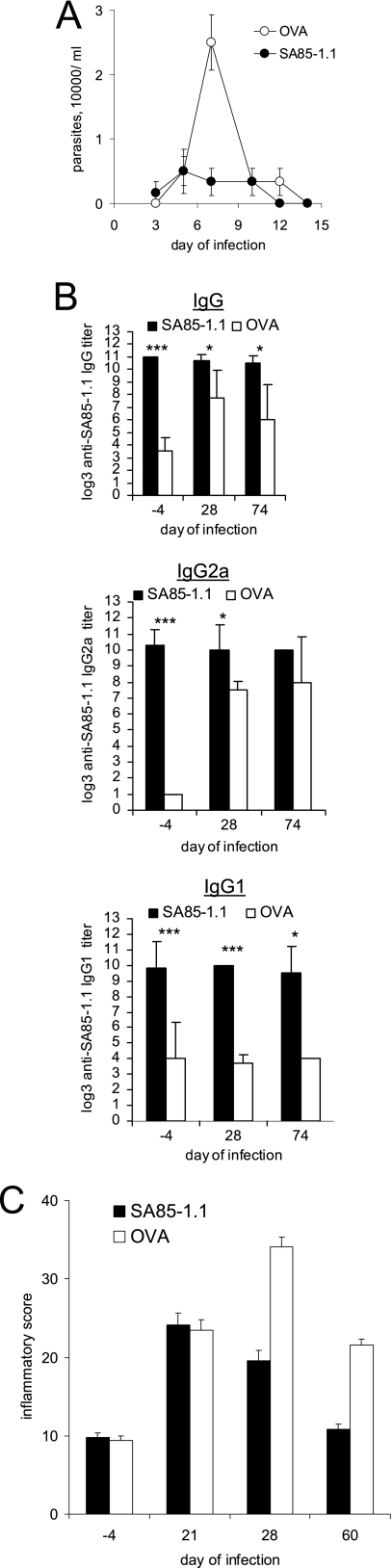
We previously demonstrated that immunization with the SA85-1.1 protein before a lethal inoculum of T. cruzi reduces parasitemia and increases survival (16). To determine if SA85-1.1 immunization affects acute or chronic tissue inflammation, groups of mice were immunized with either the SA85-1.1 protein or a control protein (OVA), and 14 days later, both groups were inoculated with a sublethal dose of trypomastigotes. The observed parasitemia was lower in the SA85-1.1-immunized group (P = 0.023) (Fig. 4A). Ten days after the final immunizing boost (4 days before T. cruzi infection) and on days 28 and 74 of the infection, the mice were analyzed for SA85-1.1 antibodies. The mice immunized with SA85-1.1 had much higher end-point titers of anti-SA85-1.1 IgG, IgG2a, and IgG1 4 days before the infection than did mice immunized with OVA (Fig. 4B). Furthermore, on day 28 of the infection, the mice immunized with SA85-1.1 had significantly higher end-point titers of both SA85-1.1 IgG2a and SA85-1.1 IgG1, in contrast to mice immunized with OVA, who also developed high titers of SA85-1.1 IgG2a during the infection but did not develop high titers of SA85-1.1 IgG1 (Fig. 4A). These data demonstrate that immunization with SA85-1.1 promotes both Th1- and Th2-driven anti-SA85-1.1 antibody responses that persist throughout infection.
FIG. 4.
Immunization with SA85-1.1 protein before T. cruzi infection decreases parasitemia and increases SA85-1.1 antibody titers but does not exacerbate tissue inflammation. Groups of mice were immunized with SA85-1.1 or OVA. Fourteen days after the immunization, mice were inoculated with 1 × 105 trypomastigotes. (A) Parasitemia was monitored (data are means and standard errors of the means for five mice per group). (B) Sera were collected 4 days before the infection and on days 28 and 74 of the infection, and anti-SA85-1.1 IgG, IgG2a, and IgG1 end-point titers were determined. Results are expressed as means and SE for at least four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) On the indicated days of infection, skeletal muscles were obtained and H&E stained, and inflammatory scores were derived. Results are expressed as means and SE for four muscles per group.
In addition, immunization with the SA85-1.1 protein, but not OVA, before T. cruzi infection generated detectable IgG antibody to heat-inactivated trypomastigotes (data not shown). On day 28 and day 74 of the infection, however, mice immunized with either SA85-1.1 or OVA had developed detectable IgG and IgG2a antibodies to heat-inactivated trypomastigotes (data not shown). These data argue that immunization with SA85-1.1 before infection has little effect on the antibody response to heat-inactivated trypomastigotes that develops during T. cruzi infection.
To determine if SA85-1.1 immunization before T. cruzi infection exacerbated tissue inflammation, we performed skeletal muscle histological analysis at different times of the infection. These studies argue that immunization did not worsen tissue inflammation (Fig. 4C). In fact, on some days, the SA85-1.1-immunized mice appeared to have less tissue inflammation (Fig. 4C). Taken together, the data argue that immunization with the SA85-1.1 protein affects the antiparasite immune response without exacerbating tissue inflammation.
Immunization with the SA85-1.1 protein during T. cruzi infection affects the immune response but does not exacerbate muscle inflammation.
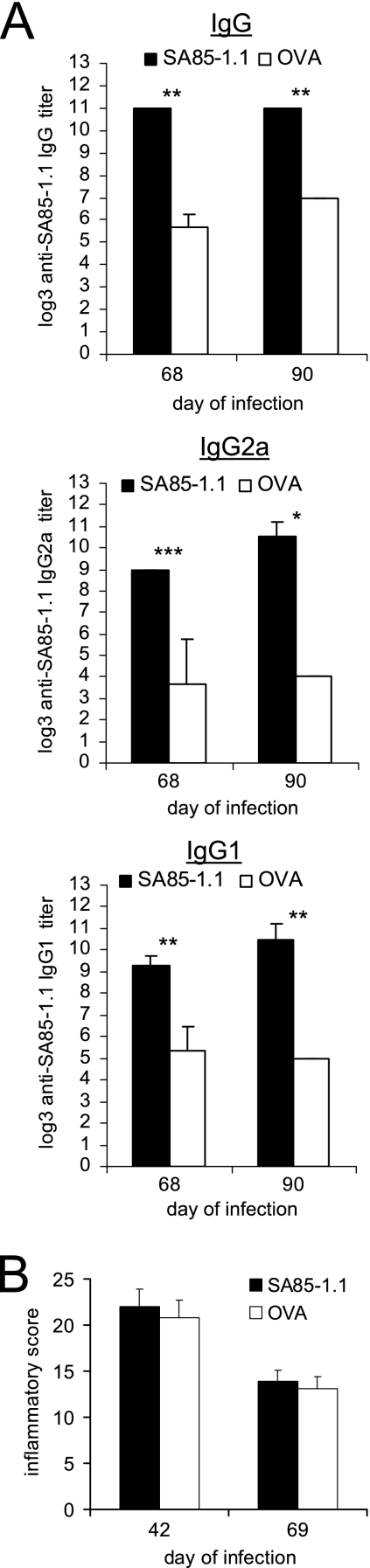
If persistent parasites stimulate the pathological immune responses that cause Chagas' disease, then vaccination of individuals infected with T. cruzi could decrease the parasite burden and thereby decrease the chronic inflammatory pathology. To examine this possibility, mice were inoculated with a sublethal dose of T. cruzi and on day 28 of the infection, after parasitemia had resolved, immunized with the SA85-1.1 protein or OVA. The immunization was boosted 14 and 28 days later (days 42 and 56 of infection), and on days 68 and 90 of the infection, antibody end-point titers to the SA85-1.1 protein and heat-inactivated trypomastigotes were determined. The immunization increased the anti-SA85-1.1 IgG, IgG2a, and IgG1 end-point titers on day 68 of infection (Fig. 5A). The end-point titers to heat-inactivated trypomastigotes were not affected by the immunization (data not shown). Following this immunization, unlike the transient increase in the antibody titers that occurred when 0C3.4 cells were transferred during infection, the anti-SA85-1.1 IgG, IgG2a, and IgG1 titers remained elevated for a prolonged period of time (until day 90 of infection [>60 days after immunization]) (Fig. 5A). These data demonstrate that immunization with the SA85-1.1 protein during T. cruzi infection stimulates and augments a persistent IgG2a and IgG1 antibody response.
FIG. 5.
Immunization with SA85-1.1 protein during T. cruzi infection enhances anti-SA85-1.1 antibody responses but does not alter tissue inflammation. C57BL/6 mice were inoculated with 1 × 105 trypomastigotes, immunized with SA85-1.1 or OVA in complete Freund's adjuvant on day 28 of the infection, and 14 days and 28 days later, immunized with the same proteins again in incomplete Freund's adjuvant. (A) Sera were collected 40 and 62 days after the initial immunization (on days 68 and 90 of infection), and SA85-1.1 IgG, IgG2a, and IgG1 end-point titers were determined. Results are expressed as means and SE for at least four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Skeletal muscles were collected on day 42 and day 69 of infection, sectioned, and H&E stained, and inflammatory scores were determined. Results are expressed as means and SE for four muscles per group.
Again, to determine if augmenting the antiparasite immune response during infection would exacerbate tissue inflammation, histological analyses of muscles were performed, and the data indicate that SA85-1.1 protein immunization does not exacerbate tissue inflammation (Fig. 5B and data not shown). Together, all the data argue that enhancing the antiparasite immune response by adoptive transfer or immunization, before T. cruzi infection or during chronic T. cruzi infection, does not exacerbate tissue inflammation. These results argue that increasing the anti-T. cruzi immune response before or during the infection is safe and might be used to prevent or treat Chagas' disease.
DISCUSSION
Chagas' disease has three phases, namely, acute, indeterminate, and chronic. During the acute phase, T. cruzi replicates, parasitemia is observed, and flu-like symptoms occur. The indeterminate phase occurs without signs and symptoms of the infection. The chronic phase affects 30% of those infected, begins 10 to 20 years after the initial infection, and manifests as severe cardiomyopathy or gastrointestinal dysfunction (23). Most individuals are diagnosed with T. cruzi infection when they present with chronic Chagas' disease. Treatment options for chronic Chagas' disease are limited: only prolonged treatment with benznidazole (an antiparasitic drug) has demonstrated some efficacy in preventing the development of cardiac disease. Benznidazole treatment, however, has little effect in many infected individuals, frequently causes adverse reactions, and may be susceptible to the development of drug resistance (7, 18). Better treatments or preventative vaccines for T. cruzi infection are needed.
The pathogenesis of chronic Chagas' disease has been controversial. Significant evidence indicates that inflammatory responses directed at the parasite contribute to the tissue pathology, but for many years it has been argued that autoimmune responses cause or contribute to the chronic pathology (1). The possibility that vaccines against T. cruzi could contribute to pathological autoimmune or bystander responses has dampened enthusiasm for their development. Our previous studies using experimental mouse infection demonstrated that transfer of SA85-1.1-specific Th1 clones (clone 0C3.4 or 1C11) or immunization with the SA85-1.1 protein or a synthetic peptide carrying the epitope recognized by the Th1 clone 0.3C4 or 1C11 reduced parasitemia and prolonged survival (16). These data permitted us to investigate if these antiparasitic treatments worsened chronic tissue inflammation. In this study, we examined the effects of adoptive transfer of the Th1 clone 0.3C4 or immunization with the SA85-1.1 protein. Both adoptive transfer and immunization, performed before infection or after the acute infection, affected the anti-T. cruzi immune response without increasing acute or chronic tissue inflammation.
All of the adoptive transfer and immunization protocols affected the anti-SA85-1.1 antibody responses, but in different ways. For example, adoptive transfer of the 0.3C4 clone before T. cruzi infection decreased the anti-SA85-1.1 antibody response, whereas immunization with the SA85-1.1 protein before T. cruzi infection increased the anti-SA85-1.1 response. We also observed that T. cruzi infection stimulated a more robust anti-SA85-1.1 IgG2a than anti-SA85-1.1 IgG1 response, whereas immunization with the SA85-1.1 protein stimulated similarly robust anti-SA85-1.1 IgG2a and IgG1 responses.
Effective drug therapy for T. cruzi is lacking. An alternative approach for therapy is a therapeutic vaccine, and the effectiveness of a therapeutic vaccine in mice has been demonstrated by Dumonteil et al. (5, 24). To further explore the ability of immunotherapy to affect the T. cruzi-induced immune response, we subjected C57BL/6 mice infected with the T. cruzi CL strain to adoptive transfer or immunized them with the SA85-1.1 protein after the acute phase had resolved. Following the 0.3C4 adoptive transfer, the SA85-1.1 IgG2a response was increased. Similarly, immunization with SA85-1.1 augmented the SA85-1.1 IgG, IgG2a, and IgG1 responses. The adoptive transfer or immunization did not worsen tissue inflammation. We attempted to determine if the adoptive transfer or immunization diminished the number of parasites present in the muscle tissue by using real-time PCR or immunohistochemistry; however, these methods were insufficiently sensitive to quantitate the parasites present in the muscles of chronically infected mice (unpublished observations). Nonetheless, these data indicate that immunotherapy during T. cruzi infection can alter the immune response without exacerbating tissue inflammation.
In these studies, the CL strain and C57BL/6 mice were used because the protective antigen was derived from the CL strain and the T-cell clone was derived from C57BL/6 mice. In future studies, it will be important to determine if the SA85-1.1 protein, when used as an immunogen, can provide protection against other strains of T. cruzi without exacerbating tissue inflammation. Furthermore, during T. cruzi infection, other strains of mice are known to develop more intense acute and chronic inflammation than that in C57BL/6 mice, and it will be important to determine if SA85-1 protein immunization can provide protection without exacerbating muscle inflammation in a variety of mouse strains as well. These studies will provide important safety data to support the investigation of T. cruzi vaccines in human clinical trials. In addition, if a T. cruzi vaccine advances to use in people, it is possible that the vaccine will be administered during acute infection. Although other experimental mouse studies have demonstrated that a protective vaccine administered during acute infection does not exacerbate inflammation, it will important for other investigators to provide similar preclinical safety data using other vaccine candidates (5, 24).
The immune responses that cause chronic Chagas' disease remain unclear (4). It is possible that T-cell responses that control the parasite also contribute to the immune responses that cause chronic inflammatory damage. The studies presented here argue that CD4 Th responses generated by adoptive transfer of Th1 clones or immunization with the SA85-1.1 protein help to control the parasite but do not contribute to the chronic inflammation. These results also argue that selected T. cruzi antigens, such as the SA85-1.1 protein, could be used in prophylactic or therapeutic vaccines. In conclusion, our results indicate that adoptive transfer or immunization before or during T. cruzi infection can alter the antiparasite immune response. Furthermore, these interventions do not exacerbate T. cruzi-induced tissue inflammation. The data argue that prophylactic or therapeutic vaccines for Chagas' disease could be developed safely.
Acknowledgments
We thank Raj Kapur, Karen Krause, Lori Lager, and Sally Norton at Children's Hospital and Regional Medical Center, Seattle, WA, for advice and assistance with histological analyses.
NIH grant AI48777 supported this work.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Buckner, F. S., and W. C. Van Voorhis. 2000. Immune response to Trypanosoma cruzi: control of infection and pathogenesis of Chagas' disease, p. 569-591. Lippincott-Raven Press, Philadelphia, PA.
- 2.Buckner, F. S., A. J. Wilson, and W. C. Van Voorhis. 1999. Detection of live Trypanosoma cruzi in tissues of infected mice by using histochemical stain for beta-galactosidase. Infect. Immun. 67:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano, M. I., A. Gruber, M. Vazquez, et al. 1995. Molecular karyotype of clone CL Brener chosen for the Trypanosoma cruzi genome project. Mol. Biochem. Parasitol. 71:273-278. [DOI] [PubMed] [Google Scholar]
- 4.Dias, J. C., A. C. Silveira, and C. J. Schofield. 2002. The impact of Chagas disease control in Latin America: a review. Mem. Inst. Oswaldo Cruz 97:603-612. [DOI] [PubMed] [Google Scholar]
- 5.Dumonteil, E., J. Escobedo-Ortegon, N. Reyes-Rodriguez, A. Arjona-Torres, and M. J. Ramirez-Sierra. 2004. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect. Immun. 72:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 8.Frank, F. M., P. B. Petray, S. I. Cazorla, M. C. Munoz, R. S. Corral, and E. L. Malchiodi. 2003. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 22:77-86. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoff, R. 1974. A method for counting and concentrating living Trypanosoma cruzi in blood lysed with ammonium chloride. J. Parasitol. 60:527-528. [PubMed] [Google Scholar]
- 12.Kahn, S. J., and M. Wleklinski. 1997. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J. Immunol. 159:4444-4451. [PubMed] [Google Scholar]
- 13.Kierszenbaum, F. 2005. Where do we stand on the autoimmunity hypothesis of Chagas disease? Trends Parasitol. 21:513-516. [DOI] [PubMed] [Google Scholar]
- 14.Lenzi, H. L., D. N. Oliveira, M. T. Lima, and C. R. Gattass. 1996. Trypanosoma cruzi: paninfectivity of CL strain during murine acute infection. Exp. Parasitol. 84:16-27. [DOI] [PubMed] [Google Scholar]
- 15.Luhrs, K. A., D. L. Fouts, and J. E. Manning. 2003. Immunization with recombinant paraflagellar rod protein induces protective immunity against Trypanosoma cruzi infection. Vaccine 21:3058-3069. [DOI] [PubMed] [Google Scholar]
- 16.Millar, A. E., M. Wleklinski-Lee, and S. J. Kahn. 1999. The surface protein superfamily of Trypanosoma cruzi stimulates a polarized Th1 response that becomes anergic. J. Immunol. 162:6092-6099. [PubMed] [Google Scholar]
- 17.Miyahira, Y., Y. Takashima, S. Kobayashi, et al. 2005. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect. Immun. 73:7356-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murta, S. M., R. T. Gazzinelli, Z. Brener, and A. J. Romanha. 1998. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 93:203-214. [DOI] [PubMed] [Google Scholar]
- 19.Plata, F., F. Garcia-Pons, and H. Eisen. 1984. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur. J. Immunol. 14:392-399. [DOI] [PubMed] [Google Scholar]
- 20.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasconcelos, J. R., M. I. Hiyane, C. R. Marinho, et al. 2004. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain after vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 15:878-886. [DOI] [PubMed] [Google Scholar]
- 23.WHO. 2002. Control of Chagas disease, p. 1-109. WHO Technical Report Series 905. World Health Organization, Geneva, Switzerland. [PubMed]
- 24.Zapata-Estrella, H., C. Hummel-Newell, G. Sanchez-Burgos, et al. 2006. Control of Trypanosoma cruzi infection and changes in T-cell populations induced by a therapeutic DNA vaccine in mice. Immunol. Lett. 103:186-191. [DOI] [PubMed] [Google Scholar]