Abstract
The licensed smallpox vaccine Dryvax is used as the standard in comparative immunogenicity and protection studies of new smallpox vaccine candidates. Although the correlates of protection against smallpox are unknown, recent studies have shown that a humoral response against the intracellular mature virion and extracellular enveloped virion (EV) forms of vaccinia virus is crucial for protection. Using a recombinant Semliki Forest virus (rSFV) vector system, we expressed a set of full-length EV proteins for the development of EV antigen-specific enzyme-linked immunosorbent assays (ELISAs) and the production of monospecific antisera. The EV-specific ELISAs were used to evaluate the EV humoral response elicited by Dryvax and the nonreplicating modified vaccinia virus Ankara (MVA) in mouse vaccination experiments comparing doses and routes of vaccination. Quantitatively similar titers of antibodies against EV antigens A33R, A56R, and B5R were measured in mice vaccinated with Dryvax and MVA when MVA was administered at a dose of 108 plaque-forming units. Further, a substantial increase in the EV-specific antibody response was induced in mice inoculated with MVA by using a prime-boost schedule. Finally, we investigated the abilities of the EV-expressing rSFV vectors to elicit the production of polyclonal monospecific antisera against the corresponding EV proteins in mice. The monospecific serum antibody levels against A33R, A56R, and B5R were measurably higher than the antibody levels induced by Dryvax. The resulting polyclonal antisera were used in Western blot analysis and immunofluorescence assays, indicating that rSFV particles are useful vectors for generating monospecific antisera.
The licensed smallpox vaccine Dryvax is a replication-competent vaccinia virus that rapidly induces a potent and long-lasting protective immune response against variola virus, the etiological agent that causes smallpox, and other closely related orthopoxviruses. Despite the efficacy of such live smallpox vaccines, there is a reluctance to vaccinate the general population in response to a possible threat of smallpox release due to substantial risk of adverse reactions, many with severe outcomes (14, 28). Serious adverse events are more likely in individuals with weakened immune systems (the very young and elderly), active or a history of eczema, immunodeficiencies due to human immunodeficiency virus infection or immunosuppressive therapies, and heart disease (19, 43). Therefore, the development of new effective alternatives to traditional smallpox vaccines with a safer profile is a high priority for public health agencies.
One candidate vaccine developed in Germany to overcome vaccine-associated complications is the modified vaccinia virus Ankara (MVA) vaccine, which was attenuated by >500 passages of the virus in embryonated chicken eggs (39, 41). Recent MVA-based vaccination studies that evaluated safety as well as the abilities of the virus vaccines to induce an immune response have heightened interest in MVA as a new-generation smallpox vaccine (12, 39, 55). Although MVA has a superior safety profile compared to standard vaccinia virus-based vaccines, the ability of MVA to protect against smallpox is essentially unknown since MVA vaccinations were administered in a region of the world where smallpox was not endemic (37, 54). In the case of traditional vaccines, the primary end point that predicts protective immunity is the formation of a vesicle, or a take, at the site of vaccination, whereas MVA-based vaccines do not produce a take due to attenuation and the route of vaccination (18, 38, 53). Demonstrating the protective efficacy of MVA, as well as other new vaccine candidates, will be difficult since clinical studies with smallpox challenge are no longer possible (49). Therefore, efficacy evaluation of candidate vaccines will necessitate comparative studies with Dryvax in surrogate challenge studies using relevant animal models. For such evaluations, improved assays will be needed to better assess and quantify the elicited immune response and to identify biological markers that can help predict protective efficacy (49).
Immunogenicity studies have shown that the protective response to vaccinia virus targets two infectious forms of the virus that are antigenically and structurally different, termed intracellular mature virions (MV) and extracellular enveloped virions (EV) (3, 9, 30, 58). MV represent the majority of virus progeny that accumulates in the infected cell that can be released by cell lysis. A small fraction of MV acquire a double additional membrane with several integral membrane viral glycoproteins that include A33R, A34R, A56R, and B5R, giving rise to intracellular EV. Intracellular EV are transported to the plasma membrane, where EV progeny are released on the periphery of the cell by exocytosis. The abundant MV form is environmentally stable and plays a principal role in host-to-host spread of the virus, whereas the EV form is critical in the dissemination of virus within the host (52). Recent investigations into the humoral arm of the protective immune response revealed that antibodies target numerous MV antigens, including the membrane-associated proteins A27L, L1R, H3L, and D8L that play a role in virus attachment and entry, as well as the EV-specific antigens A33R, A56R, and B5R found on the outer membrane (10, 25, 42). The significance of these findings has been reinforced by recent immunization studies using subunit vaccines based on recombinant DNA and proteins that have demonstrated the importance of eliciting an immune response against MV and EV in providing protection against a virus infection (17, 22, 36, 63).
We have recently compared the abilities of MVA and Dryvax to protect against a lethal poxvirus challenge using a murine model (40). The results of these studies have indicated that an effective humoral response elicited by MVA or Dryvax immunization plays an important role in protection against a virus infection using the intranasal challenge model, although the correlates of the protective immunity against smallpox infection have not been defined. Further, our recent investigations into the humoral response induced by Dryvax smallpox vaccine in humans, as represented by the human vaccinia virus immune globulin (VIG), have identified numerous MV antigen targets, as well as EV-specific antigen targets A33R, A56R, and B5R (25). Such studies suggest that quantifying the antibody response against a subset of EV antigens will be an essential step in evaluating new vaccine candidates (7, 25, 30). Here, we describe the development of a set of EV antigen-specific enzyme-linked immunosorbent assays (EV-ELISAs) using affinity-purified full-length EV proteins expressed in mammalian cells by using a Semliki Forest virus (SFV) replicon expression system (31). We demonstrate the utility and specificity of the recombinant EV (rEV) proteins A33R, A56R and B5R as immobilized substrates in a series of Western blot and ELISA experiments. Using these assays, we show that quantitatively similar antibody levels against these EV proteins are produced in mice vaccinated with Dryvax and with a 1-log-higher dose of MVA and that a higher EV antibody response results when mice are vaccinated with MVA using a prime-boost regimen. Finally, we investigate the abilities of the EV-expressing rSFV vectors to elicit the production of monospecific polyclonal antisera for use in immunological assays such as Western blotting and immunofluorescence.
MATERIALS AND METHODS
Cell lines and viruses.
Baby hamster kidney cells (BHK-21; ATCC, CCL-10) used in producing rSFV were grown in Glasgow minimum essential medium (G-MEM-BHK-21 medium; Gibco) supplemented with 5% fetal bovine serum (FBS), 20 mM HEPES-buffered solution (pH ∼7.2; Gibco), 10% (29.5 g/liter) tryptose phosphate (Difco), 2 mM l-glutamine, and 25 μg/ml gentamicin. Primary chicken embryo fibroblast (CEF) and HeLa cells (ATCC, CCL2) were maintained in Eagle's minimal essential medium (E-MEM) supplemented with 10% FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin. BHK-21, BS-C-1 (ATCC, CCL-26), and Vero (ATCC, CCL-81) cells used in propagating vaccinia virus were grown in Dulbecco's minimal essential medium (D-MEM) supplemented with 10% FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin. Dryvax virus stocks used for immunizations and ELISAs were prepared by infecting Vero cells or BS-C-1 and HeLa cells, respectively, with virus derived from a reconstituted vial of Dryvax (manufactured by Wyeth) as described previously (25). Vaccinia virus strain WR was propagated in BS-C-1 cells. A recombinant WR strain vaccinia virus called vA5Lint that lacks the hemagglutinin (HA) A56R gene was propagated in BS-C-1 cells. Modified MVA was propagated in CEF cells and titered by plaque assay with CEF cells or BHK-21 cells as described previously (11). The vaccinia virus stocks were purified by sedimentation through a 36% (wt/vol) sucrose cushion by centrifugation as described previously (11a). The vaccinia virus stocks were titered by plaque assay done with BS-C-1 cells.
Plasmid construction and PCR.
SFV expression vectors (a kind gift from Wolfgang Resch) were constructed by using a two-step cloning procedure employing the Gateway cloning system (Invitrogen). The four EV-specific A33R, A34R, A56R, and B5R open reading frames (ORFs) were amplified by PCR using vaccinia virus (Dryvax) genomic DNA as template DNA and appropriate primer pairs, indicated below. The forward primers (f) contained a 5′-CACCATG sequence that serves as a directional cloning element and as a Kozak consensus sequence. The reverse primers (r) encode the sequence corresponding to the protein C epitope tag (prC) (Roche Applied Science) or V5 epitope tag (V5) (Invitrogen) and translational stop codon. The indicated oligonucleotides were used to amplify the following EV-specific ORFs appended with either a C-terminal prC or a C-terminal V5 epitope tag (vaccinia virus coding sequences are in bold, termination codons are underlined, prC or V5 epitope tag-coding sequences are in plain font, and the TOPO directional cloning sequence elements are in italics): A33R ORF, (f) 5′-CACCATGATGACACCAGAAAACGACGAAGAGC and (r-prC) 5′-TTATTTGCCGTCAATAAGACGTGGATCAACCTGATCTTCGTTCATTGTTTTAACACAAAAATACTTTCTAAC or (r-V5) 5′-TTATGTTGAGTCCAAACCTAGAAGTGGATTGGGAATTGGTTTGCCGTTCATTGTTTTAACACAAAAATACTTTCTAAC; A34R ORF, (f) 5′-CACCATGAAATCGCTTAATAGACAAACTGTAAG and (r-prC) 5′-TTATTTGCCGTCAATAAGACGTGGATCAACCTGATCTTCCTTGTAGAATTTTTTAACACATAGTACAG or (r-V5) 5′-TTATGTTGAGTCCAAACCTAGAAGTGGATTGGGAATTGGTTTGCCGTTCATTGTTTTAACACAAAAATACTTTCTAAC; A56R ORF, (f) 5′-CACCATGACACGATTACCAATACTTTTG and (r-prC) 5′-TTATTTGCCGTCAATAAGACGTGGATCAACCTGATCTTCGACTTTGTTCTCTGTTTTGTATTTACG or (r-V5) 5′-TTATGTTGAGTCCAAACCTAGAAGTGGATTGGGAATTGGTTTGCCGACTTTGTTCTCTGTTTTGTATTTACG; B5R ORF, (f) 5′-CACCATGAAAACGATTTCCGTTGTTACGTTG and (r-prC) 5′-TTATTTGCCGTCAATAAGACGTGGATCAACCTGATCTTCCGGTAGCAATTTATGGAACTTATATTGGTC or (r-V5) 5′-TTATGTTGAGTCCAAACCTAGAAGTGGATTGGGAATTGGTTTGCCCGGTAGCAATTTATGGAACTTATATTG GTC. The PCR products were purified by using a QIAquick PCR purification kit (QIAGEN) and cloned into the pENTR/D-TOPO (Invitrogen) Gateway entry vector, resulting in the set of plasmids pENTR-A33R-prC, -A34R-prC, -A56R-prC, and -B5R-prC (the set of plasmids referred to as pENTR-EV-prC). Another set of pENTR vectors was made that contains A33R, A34R, A56R, and B5R genes with a C-terminal V5 epitope tag (the set of plasmids referred to as pENTR-EV-V5). The four EV-prC genes were cloned into the vector pSFV-destination (a generous gift of Wolfgang Resch) by combining pENTR-EV-prC with pSFV-destination in an LR recombination reaction, resulting in plasmids pSFV-A33R-prC, -A34R-prC, -A56R-prC, and -B5R-prC. The pENTR-EV-V5 plasmids were used to generate pSFV-A33R-V5, -A34R-V5, -A56R-V5, and -B5R-V5. The gene encoding enhanced green fluorescent protein (GFP) was inserted into pSFV-destination by following the two-step cloning procedure. All plasmids were grown in Escherichia coli TOP10 (Invitrogen), and the relevant DNA portions synthesized by PCR were confirmed by restriction enzyme digestion and confirmation of the DNA sequence.
In vitro transcription and production of rSFV particles.
In vitro transcription reactions with bacteriophage SP6 RNA polymerase were typically done in a 100-μl final volume (using a RiboMAX SP6 RNA production system; Promega) containing 6 μg of linearized recombinant pSFV plasmids. In vivo packaging of the self-replicating SFV RNA vectors (replicons) expressing the individual EV genes into replication-deficient SFV particles was performed by combining the rSFV RNA vector and two helper RNAs (i.e., Helper C and Helper S) and cotransfecting the RNA mixture into BHK-21 cells by electroporation as described previously (32, 51). Cells transfected with the RNA mixture were incubated at 37°C for 24 h, rSFV particles were harvested by collecting the cell culture medium, fresh medium was added to the cell monolayers, the cells were allowed to incubate for another 24 h, and the rSFV particles were harvested. The medium containing the rSFV virus particles was clarified by centrifugation at ∼500 × g for 15 min, and the virus stocks were stored at −80°C.
For the immunization studies, rSFV particles encoding GFP and A33R, A34R, A56R, and the B5R ORF containing the C-terminal V5 epitope tag were prepared as described above. After the cell culture medium containing the rSFV particles was clarified, the virus particles were purified by two successive sedimentations through a 20% sucrose cushion as described previously (16), except that the ultracentrifugation was done by using a Beckman SW40 (30,000 rpm. for 90 min at 4°C). The final rSFV pellets were resuspended in TNE buffer (50 mM Tris-HCl [pH 7.4]-100 mM NaCl-0.5 mM EDTA), and aliquots of the viral particle suspensions were stored at −80°C. The amount of infectious units (I.U.) per ml of each rSFV suspension was determined by infecting BHK-21 cells with 10-fold serial dilutions of rSFV particles as described previously (32), and cells positive for GFP expression or rEV protein expression, which were stained with V5 monoclonal antibody (MAb) as a primary antibody followed by goat anti-mouse immunoglobulin (Ig) conjugated with r-phycoerythrin (Southern Biotechnology Associates, Inc.), were visualized by fluorescence microscopy and counted.
Expression of EV proteins and affinity purification.
Mammalian cell expression of recombinant A33R, A34R, A56R, and B5R proteins was carried out with 90% confluent BHK-21 cells that were seeded in 10 to 15 162-cm2 cell culture flasks. Cell monolayers were infected with rSFV-EV particles in culture medium diluted fivefold in MEM supplemented with 0.2% (wt/vol) bovine serum albumin, 20 mM HEPES-buffered solution (pH ∼7.2; Gibco/Invitrogen), and 2 mM l-glutamine. The infected cells were incubated for ∼2 h at 37°C with rocking every 15 min; after absorption, the inocula were removed, and fresh G-MEM culture medium was overlaid onto the cell monolayers and incubation continued for ∼24 h. To harvest the infected cells from each 162-cm2 flask, the monolayers were washed with phosphate-buffered saline (PBS); the cells were then dislodged in 5 ml PBS containing 5 mM EDTA at room temperature, resuspended in PBS, and pooled. The pooled cells were pelleted by centrifugation, and the cell pellet was resuspended in PBS and then repelleted in a 15-ml conical tube. Cytoplasmic extracts were typically prepared by resuspending the cell pellet in 5 ml hypotonic buffer (10 mM HEPES [pH 7.5]-10 mM NaCl-0.1 mM EDTA)-0.4% (vol/vol) NP-40, 0.05% (wt/vol) deoxycholate, and a 2× working concentration of Complete protease inhibitor cocktail (EDTA-free tablets; Roche Applied Science). The cell suspension was incubated on ice for 15 min, the cell debris was sedimented by centrifugation (∼3,000 × g) for 15 min, a 5% (vol/vol) glycerol final concentration was added to the cell lysate supernatant, and it was frozen at −80°C. For immunoaffinity purification, the cell lysate was mixed with equal volumes of 2× extract buffer (100 mM Tris-HCl [pH 7.5]-300 mM NaCl-2% [vol/vol] NP-40-0.1% [wt/vol] deoxycholate, 2 mM CaCl2)-2× working concentration of Complete protease inhibitor cocktail. The diluted cell lysate was clarified by filtration, using a 0.45-μm syringe filter (Acrodisc; Gelman Sciences). Five to 6 ml of clarified lysate was mixed with 1 ml of anti-prC MAb affinity matrix (Roche Applied Science), previously equilibrated in 1× extract buffer, in a 10-ml column, and the recombinant proteins were bound to the affinity matrix using a batch preparation procedure for 3 h at 4°C. Column washes and protein elution were done as described in the manufacturer's protocol (Roche Applied Science). The peak protein fractions were combined with 5% (vol/vol) glycerol, final concentration, and frozen at −80°C.
Mouse antisera used in Western blot analysis and ELISA.
Mouse polyclonal antisera were produced in male BALB/cByJ mice. Anti-vaccinia virus hyperimmune serum was prepared from mice inoculated with a single intradermal (i.d.) inoculation of 108 plaque-forming units (PFU) of sucrose cushion-purified MVA followed by a single i.d. inoculation of 107 PFU of sucrose-cushioned Dryvax and then inoculated with an intranasal dose of 5 × 106 PFU of WR strain vaccinia virus, given 3 weeks apart. Anti-Dryvax hyperimmune serum was prepared by inoculating mice with three intraperitoneal (i.p.) doses of 107 PFU of Dryvax, given 3 weeks apart. Anti-MV hyperimmune serum was prepared by inoculating mice subcutaneously (s.c.) with three doses of 4.0 × 107 PFU equivalents of noninfectious intracellular WR strain vaccinia virus (MV), administered at 3-week intervals. The MV particles used in the immunizations were purified by sedimentation through a 36% sucrose cushion, followed by CsCl density gradient centrifugation, as described previously (45). The purified MV particles were titrated by plaque assay on BS-C-1 cells prior to their inactivation by trioxsalen treatment of the virus suspension and UV irradiation done in a UV Stratalinker 1800 for 15 min. To verify that the MV particles were rendered noninfectious, the treated MV particles were checked by plaque assay on BS-C-1 cells. Anti-vA5Lint immune serum was prepared by inoculating mice i.p. with three doses of 107 PFU of live vA5Lint, a recombinant WR strain vaccinia virus that lacks the A56R (HA) gene, given at 3-week intervals.
Western blot analysis.
Protein samples were mixed in 4× NuPAGE LDS sample buffer (NP0007; Invitrogen)-200 mM β-mercaptoethanol to the final working concentration and analyzed by electrophoresis in a 4 to 12% gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel (NuPAGE Bis-Tris gel in 4-morpholineethanesulfonic acid [MES]-SDS running buffer; Invitrogen), and the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). After being blocked with either Tris-buffered saline-1 mM CaCl2 (TBS-CaCl2; used for CaCl2-dependent antibody binding) or PBS containing 5% (wt/vol) powdered milk (Bio-Rad) and 0.1% Tween 20 for 1 h, the membranes were incubated with various primary antibodies followed by washes with either TBS-CaCl2 or PBS containing 0.1% Tween 20. If required, the membrane was incubated with appropriate secondary antibodies conjugated with horseradish peroxidase (HRP) for 45 min. The proteins were detected by chemiluminescence (SuperSignal West Dura extended-duration substrate; Pierce). The primary antibodies used were as follows: prC MAb (Roche Applied Science), anti-prC MAb conjugated to HRP, anti-Dryvax mouse antiserum, anti-MV mouse antiserum, and anti-vA5Lint antiserum. Sheep anti-mouse antibody conjugated to HRP (Amersham Pharmacia Biotech) was used as a secondary antibody.
For protein samples from vaccinia virus-infected cells, BS-C-1 monolayers in six-well plates were infected with vaccinia virus (i.e., vA5Lint, Dryvax, or WR strain vaccinia virus) at a multiplicity of infection of 5 for 1 h at 37°C. After absorption, the inocula were removed and replaced with E-MEM containing 10% FBS and incubation was continued for ∼18 h. The cells were harvested and lysed in hypotonic buffer containing 2 mM MgCl2 and 0.02 U of DNase I per ml. The cell lysates were mixed with 4× NuPAGE LDS sample buffer-200 mM β-mercaptoethanol to the final working concentration and analyzed by electrophoresis in 4 to 12% gradient SDS-polyacrylamide gel (NuPAGE Bis-Tris gel in MES-SDS running buffer; Invitrogen) and by Western blotting using monospecific antiserum anti-A33R, anti-A56R, or anti-B5R and anti-mouse Ig conjugated to HRP.
ELISA.
Serum antibodies specifically against EV proteins in mice were detected by using an ELISA. Affinity-enriched A33R, A34R, A56R, and B5R proteins were diluted in PBS, and 100 μl of protein per well of 96-well Immulon 2HB plates (ThermoLab Systems) was dispensed. The plates were incubated overnight at 4°C, and afterwards, the plates were blocked by dispensing 200 μl of PBS containing 10% FBS and incubated for 2 h at 37°C. The quantification of the amounts of individual EV protein preparations was hampered due to the sensitivity thresholds of standard protein detection assays. Therefore, the optimal dilutions of A33R, A34R, A56R, and B5R proteins used to coat the 96-well Immulon 2HB plates were initially determined empirically by testing various dilutions of proteins in pilot ELISA experiments using anti-prC MAb and an anti-vaccinia virus mouse hyperimmune serum. The total titers of the antibodies against EV proteins in the serum samples were measured by performing twofold dilutions and incubating for 2 h at 37°C, followed by incubating the plates with 1:4,000 dilutions of goat anti-mouse IgG antibody conjugated to HRP (Southern Biotechnology Associates, Inc.) for 1 h at 37°C. The immune antisera and goat anti-mouse IgG antibody-HRP were diluted in PBS containing FBS and 0.05% (vol/vol) Tween 20. In between incubations with antisera and antibody conjugates, the plates were washed with PBS containing 0.05% Tween 20. After washing of excess HRP-conjugated antibody, the plates were developed with ABTS-H2O2-peroxidase substrate (Kirkegaard & Perry Laboratories, Inc.) for 45 min at room temperature, the reactions were stopped by adding a 1% SDS solution, and the absorbance (optical density at 405 nm [OD405]) was measured in a Versamax microplate reader (Molecular Devices Corporation, Sunnyvale, CA). The antibody end point titers were defined as the highest serum dilution that resulted in an absorbance value exceeding 0.05 above those of the control sera (i.e., mock immune serum and preimmune serum), as described previously (40).
Comet reduction assay.
Confluent Vero cells were prepared in 24-well plates and infected with 80 PFU per well of vaccinia virus, IHD-J strain, for ∼2 h at 37°C with rocking done every 15 min. After absorption, the inocula were removed and replaced with serum-free medium containing a 1:50 dilution of mouse immune serum, incubation was continued for ∼40 h postinfection, and the monolayers were stained with crystal violet to visualize the plaques.
Mice, immunizations, and challenge.
Male BALB/cByJ mice were obtained from the Jackson Laboratory, Bar Harbor, ME, and maintained in the animal facility as previously described (40). Inoculations of the vaccines into the mice were performed using three different protocols as previously described (40): (i) intramuscularly (i.m.), the virus suspension was injected into the quadriceps muscles of the hind limbs; (ii) subcutaneously (s.c.), the virus suspension was injected under the skin at the base of the tail; and (iii) i.d., the virus suspension was delivered with a Biojector 2000 jet injector device (Bioject Inc., Portland, OR).
For the inoculation of mice with sucrose cushion-purified SFV particles, the mice were inoculated s.c. with two doses of 106 IU of rSFV particles administered 3 weeks apart. Antisera were collected 3 weeks after each inoculation, and the specific antibody levels were determined by ELISA. Three weeks after the last immunization, the mice were challenged intranasally with a dose of 5 × 106 PFU (25 times the 50% lethal dose) of WR strain vaccinia virus, as previously described (40).
Indirect immunofluorescence staining.
BS-C-1 cells were seeded and grown to confluence on glass coverslips and infected with Dryvax at a multiplicity of infection of 0.5. To fix and permeabilize infected cells, the cells were washed once with TBS (Tris-buffered saline, pH 7.4) containing 1 mM CaCl2, fixed with 4% paraformaldehyde in TBS and 1 mM CaCl2 for 15 min, permeabilized with 0.1% Triton X-100, and quenched with 100 mM glycine for 10 min. The coverslips were incubated for 1 h with the following monospecific antisera diluted in diluent (20% FBS-0.1% [vol/vol] Triton X-100-TBS-1 mM CaCl2, pH 7.4): anti-A33R at a dilution of 1:200, anti-A56R at a dilution of 1:200, and anti-B5R at a dilution of 1:50. The washed coverslips were then incubated with a goat anti-mouse Ig conjugated with R-phycoerythrin (Southern Biotechnology Associates, Inc.). After being washed, the coverslips were mounted in Fluoromount-G (Southern Biotechnology Associates, Inc.) and images of the stained plaques were acquired by fluorescence microscopy using the Zeiss AxioVision system (Carl Zeiss Vision GmbH).
RESULTS
Expression, purification, and characterization of rEV proteins.
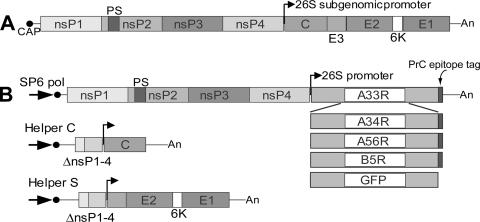
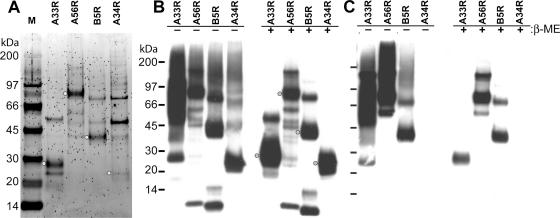
While five EV-associated proteins have been identified, only A33R, A56R, A34R, and B5R are displayed on the surface of the outer membrane of EV (52). Therefore, in order to study the antigen-specific antibody response to the EV components of vaccinia virus, we constructed eukaryotic expression vectors for the production of the individual EV antigens A33R, A56R, A34R, and B5R. Vaccinia virus naturally replicates and expresses its genes in the cytoplasm of mammalian cells. In order to maximize our chances of obtaining accurate expression of each vaccinia virus gene and authentic mammalian posttranslational modification of the EV protein products produced, we used an SFV expression system (31) to express full-length EV antigens in BHK-21 cells. The expression system is based on the in vivo production of rSFV particles by transfecting cells with the three individual RNAs transcribed from the self-replicating expression vector, the Helper C vector, and the Helper S vector (Fig. 1) (51). The resulting rSFV particles are infectious but replication defective, containing a self-replicating RNA which encodes the genes for the viral replicase (nonstructural genes [nsPs]) and the subgenomic promoter followed by a heterologous gene corresponding to one of the four EV proteins with a prC epitope tag at the C terminus (Fig. 1B). The prC epitope tag was used to facilitate the immunological identification and one-step purification by immunoaffinity chromatography of the rEV proteins expressed in the rSFV-infected cells. After affinity chromatography was performed, the purity and recovery of the rEV proteins were determined by SDS-polyacrylamide gel electrophoresis (PAGE), silver or SYPRO Ruby protein gel staining, and Western blotting (Fig. 2A and B). As determined by SYPRO Ruby-stained SDS-polyacrylamide gels, the A33R, A34R, A56R, and B5R proteins eluted from the affinity columns were substantially enriched; protein bands at the appropriate molecular masses corresponding to A33R, A34R, A56R, and B5R could be clearly discerned (Fig. 2A). Western blot analysis using an antibody to the prC epitope tag demonstrated prominent protein bands at the appropriate molecular mass for each ORF after purification (Fig. 2B, right panel). In the absence of a reducing agent, higher-molecular-mass forms corresponding to multimers were observed (Fig. 2B, left panel), as has been observed previously for these proteins (24, 44, 47). Western blot analysis using hyperimmune mouse antiserum against the NYCBH strain of vaccinia virus (Dryvax) detected three of the four EV proteins (Fig. 2C). A band corresponding to A34R was not detected in either reducing or nonreducing conditions. These observations show that the expression and purification scheme allowed for the recovery of full-length EV proteins A33R, A34R, A56R, and B5R of the expected molecular masses. Of the four affinity-enriched EV proteins, A33R, A56R, and B5R were recognized by the anti-Dryvax hyperimmune serum, suggesting that these recombinant proteins might be suitable antigens in developing quantitative ELISAs.
FIG. 1.
Diagram of SFV genome and replicon constructs. (A) A diagram of the plus-stranded native RNA genome of SFV. (B) Representations of rSFV replicon vector constructs that encode prC epitope-tagged EV A33R, A34R, A56R, B5R, and GFP genes. The nonstructural genes encoding the replicase complex are indicated by nsP1, nsP2, nsP3, and nsP4. Helper vectors designated Helper C and Helper S encode the genes for the capsid (C) and the spike proteins (E1, E2, E3, and 6K), respectively. Large black arrow (SP6 pol) denotes the SP6 RNA polymerase promoter region; small black arrow denotes the 26S subgenomic promoter; black circle at 5′-end denotes CAP structure. An, polyA; PS, packaging signal; ΔnsP1-4, deleted nonstructural genes.
FIG. 2.
Anti-Dryvax immune mouse serum recognizes rEV proteins. (A) Affinity-purified recombinant A33R, A34R, A56R, and B5R were analyzed under reducing conditions by using SDS-PAGE and SYPRO Ruby protein gel staining. Stained gel with lanes corresponding to (M) marker proteins and the indicated EV proteins is shown. Recombinant A33R, A34R, A56R, and B5R were treated with (+) and without (−) β-mercaptoethanol (β-ME) and were analyzed by SDS-PAGE and Western blotting using an anti-prC MAb (1/1,000 dilution) to the C-terminal prC epitope tag (B) or using a mouse anti-Dryvax antiserum (anti-Dryvax; 1/500 dilution) (C). Protein bands were detected by chemiluminescence after membranes were incubated with an anti-mouse IgG antibody conjugated to HRP (1/2,500 dilution). The positions and masses of the marker proteins are indicated on the left. Dots show the positions of EV protein bands that are consistent with the reported molecular mass for each of the indicated proteins (52).
Development of EV-specific antibody ELISAs.
Preliminary experiments to determine the feasibility of using rSFV-expressed EV proteins as ELISA antigens were performed using (i) the anti-PrC epitope tag MAb and (ii) a vaccinia virus hyperimmune serum. When the vaccinia virus hyperimmune serum was used as an ELISA primary antibody at low dilutions ranging from 1:320 to 1:2,500, plates coated with EV A33R, A56R, or B5R antigen elicited high OD values of >1, whereas plates coated with A34R antigen resulted in no detectible cross-reactivity at a similar dilution range of the vaccinia antiserum. When ELISA plates coated with each of the four EV antigens were probed with anti-prC MAb at a 1:400 dilution, comparable cross-reactivities were obtained with the A33R and A34R antigens (OD, >1.0) and with the A56R and B5R antigens (OD, 0.3 to 0.6). The specific end point antibody titers in the vaccinia virus antiserum against the A33R, A56R, and B5R antigens were reproducibly calculated at dilutions of >1:40,000. In contrast, uninoculated-mouse serum dilutions used at 1:40 or greater elicited low nonspecific background results (OD, <0.020) against all four EV antigens. These ELISA results were consistent with the results of the Western blotting analysis using the anti-Dryvax hyperimmune serum (Fig. 2C). Thus, the EV-specific ELISAs based on the recombinant A33R, B5R, and A56R antigens were further evaluated for their specificities and utilities in studying the antibody response against EV.
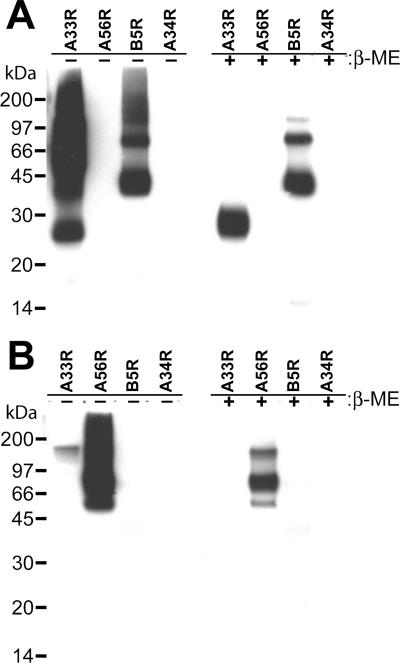
The specificities and utilities of the EV-specific ELISAs using the affinity-purified recombinant proteins were investigated by using a panel of murine hyperimmune antisera raised against (i) vaccinia virus, (ii) a recombinant WR strain vaccinia virus lacking the A56R gene (vA5Lint), and (iii) inactivated MV particles. The sensitivity of each EV antigen-specific ELISA was evaluated with antisera obtained from mice injected with a single dose of Dryvax (106 PFU). Antiserum anti-vA5Lint was produced by inoculating BALB/c mice with live vA5Lint (61) to obtain a hyperimmune antiserum directed against all the vaccinia virus EV proteins except A56R. The anti-MV antiserum was generated by immunizing mice with a UV-inactivated, purified MV particle preparation. In Western blot analyses performed with anti-vA5Lint and anti-MV, anti-vA5Lint was found to detect both purified A33R and B5R blotted proteins but not A56R. The anti-MV antiserum strongly detected A56R, whereas B5R was weakly detected and A33R was not detected (Fig. 3). Again, as observed in the results shown in Fig. 2, the intensities of the bands were greater in the lanes corresponding to the nonreduced samples of A33R and A56R.
FIG. 3.
Analysis of immune serum from mice immunized with an HA deletion mutant vaccinia virus strain and noninfectious MV particles. (A) Hyperimmune antiserum anti-vA5Lint was obtained from BALB/c mice inoculated i.p. with a recombinant WR-strain vaccinia virus that lacks the A56R (HA) gene, vA5Lint, using a prime-boost schedule. (B) Hyperimmune antiserum anti-MV was obtained from mice inoculated s.c. with noninfectious WR strain vaccinia virus MV particles (4.0 × 107 PFU equivalents) using a prime-boost schedule. A33R, A34R, A56R, and B5R protein preparations were treated with (+) and without (−) β-mercaptoethanol (β-ME) prior to SDS-PAGE and protein transfer onto PVDF membranes. The PVDF membranes were incubated with mouse immune sera, followed by a second incubation with an anti-mouse IgG antibody conjugated to HRP. Protein bands were detected by chemiluminescence. Masses of protein markers are indicated on the left.
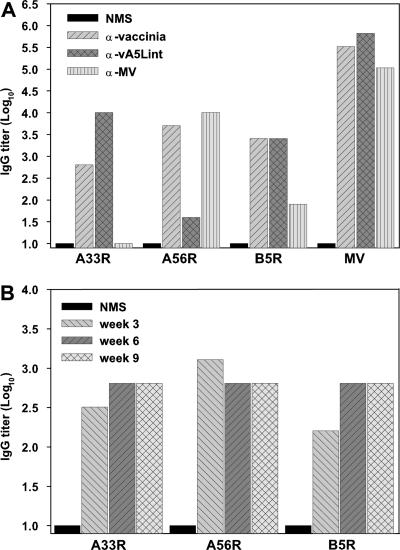
When UV-inactivated Dryvax MV was used as an ELISA antigen, similar high vaccinia virus-specific antibody titers were measured in all three vaccinia virus hyperimmune antisera compared to the titer in uninoculated-mouse serum (Fig. 4A). In contrast, when the affinity-purified A33R, A56R, or B5R EV proteins were used as ELISA antigens, the amount of EV-specific antibody measured depended upon the source of the hyperimmune sera. The A33R-specific ELISA detected high A33R antibody responses in the vaccinia virus and the anti-vA5Lint antisera but no appreciable A33R response in the anti-MV antiserum. The A56R-specific ELISA measured high A56R antibody titers in the vaccinia virus and anti-MV antisera but a low A56R titer in the anti-vA5Lint antiserum, as might be expected, since the vA5Lint virus has an insertion into the A56R gene. The B5R-specific ELISA detected high B5R antibody responses in the vaccinia virus and the anti-vA5Lint antisera, but a low B5R antibody titer in the anti-MV antiserum. Thus, each EV-specific ELISA measured a high end point titer for the respective antigen in a vaccinia virus hyperimmune serum with a negligible background in uninoculated-mouse serum (2.8 log10, 3.7 log10, and 3.4 log10 for A33R, A56R, and B5R, respectively, relative to the titer in uninoculated-mouse serum). In addition, using antiserum designed to be deficient in A56R antibody (anti-vA5Lint antiserum) resulted in an expected decrease in measured A56R-specific antibody. Using antiserum designed to be deficient in total EV antibody (anti-MV antiserum) resulted in the predicted decreases in the measured A33R- and B5R-specific antibodies. These observations were consistent with the results of Western blot analysis shown in Fig. 3. Taken together, these ELISA results indicated that affinity-purified A33R, A56R, and B5R proteins can specifically quantify antibody responses directed against the different EV proteins in vivo.
FIG. 4.
EV-specific antibody response induced by vaccinia virus. (A) A set of pooled anti-vaccinia virus antisera referred to as anti-vaccinia, anti-vA5Lint, and anti-MV was produced in BALB/c mice (in groups of three) that were inoculated with the MVA-Dryvax combination (107 PFU), live vA5Lint (107 PFU), and noninfectious WR strain MV particles (4.0 × 107 PFU equivalents), respectively, using a prime-boost schedule. Antisera were collected 3 weeks after the last inoculation, and pooled antisera were analyzed by ELISA using plates coated with EV proteins A33R, A56R, or B5R or UV-inactivated Dryvax MV particles. As a control, ELISA plates were probed with normal (uninoculated) mouse serum (NMS). (B) A group of mice were immunized i.d. with a single dose of 106 PFU of Dryvax, and antisera were collected at weeks 3, 6, and 9 postvaccination and pooled. Levels of antibodies to indicated EV proteins were measured by ELISA. The results shown in panels A and B were derived from a single experiment. α, anti.
The utility of the EV-ELISAs was further evaluated by measuring the time course of the EV-specific humoral response in mice following a single i.d. vaccination with 106 PFU of Dryvax; pooled serum samples were collected at 3-week intervals up to 9 weeks postvaccination, and antibody titers were quantified. As evaluated by the EV-specific ELISAs, the mice generated a potent and consistent antibody response against all three EV proteins over the 9-week period after vaccination (Fig. 4B). The EV-specific antibody response was qualitatively similar to the total vaccinia virus-specific antibody response observed over a 9-week period using an ELISA that contained UV-inactivated Dryvax MV (40). Again, these observations indicated the sensitivity and the utility of the rSFV-expressed EV proteins A33R, A56R, and B5R to serve as ELISA antigens in quantifying the EV-specific humoral response.
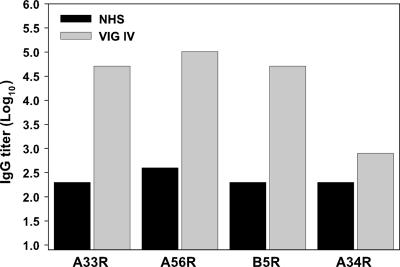
EV-specific antibody titers in VIG.
We next asked whether the EV-specific ELISAs were suitable for measuring the anti-EV antibody response in vaccinated humans. Toward this end, the antigen-specific ELISAs were used to quantify the levels of antibody titers developed against A33R, A56R, and B5R in VIG-IV, an enriched hyperimmune serum from donors vaccinated with the licensed smallpox vaccine, Dryvax (62). When conditions similar to those described above for the murine system were used, EV-specific ELISAs measured high antibody titers against the EV proteins A33R, A56R, and B5R in VIG, of 4.71 log10, 5.01 log10, and 4.71 log10, respectively (Fig. 5). In each case, the EV-specific antibody was approximately 2 to 2.5 logs higher than in the control human serum, which was determined to not react with any of the rEV proteins in Western blot analysis. In contrast, the A34R antibody titer measured in VIG-IV was virtually indistinguishable from the background level in the control human serum (Fig. 5). These results are consistent with the previously reported Western blot data showing that VIG-IV recognizes the EV proteins A33R, A56R, and B5R but not A34R (25) and suggest that the rSFV-expressed EV proteins A33R, A56R, and B5R can be used to develop ELISAs suitable for measuring the EV antibody response in human as well as mouse vaccine sera.
FIG. 5.
EV-specific antibody titers in VIG. The EV-specific antibody titer in human VIG-IV was determined by ELISA for each indicated EV protein. Control human serum (NHS) is indicated.
EV neutralization activity in hyperimmune sera.
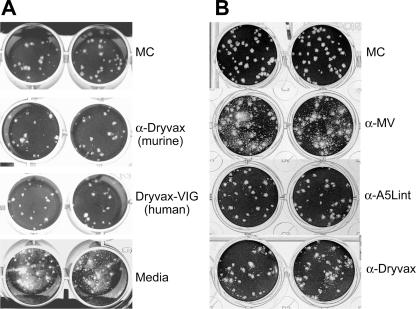
Neutralizing antibodies to vaccinia virus EV proteins are thought to inhibit the spread of virus by blocking released EV particles from reinfecting cells and/or by preventing the release of cell-associated EV particles into the media (29, 57). To determine whether the EV antibody responses measured by EV antigen-specific ELISA correlated with anti-EV neutralizing activity, the anti-Dryvax hyperimmune sera derived from mice (i.e., anti-Dryvax) and humans (i.e., VIG) were incubated with Vero cells previously infected with vaccinia virus strain IHD-J in a comet inhibition assay (Fig. 6A). In the presence of murine and human Dryvax hyperimmune sera, an appreciable inhibition of satellite plaque formation was observed. Similarly, the murine antisera produced to the A56R-defective vA5Lint, shown by ELISA to be deficient in A56R-specific antibodies, possessed considerable EV-neutralizing activity (Fig. 6B). In contrast, the presence of the anti-MV antiserum, which was shown to be greatly reduced in A33R- and B5R-specific ELISA titers, had little effect, if any, on the propagation of satellite plaques (Fig. 6B). Thus, the data indicated that the A33R- and B5R-specific antibody titers in hyperimmune antiserum (i.e., anti-vA5Lint) correlated with EV neutralization, while relatively high antibody titers against A56R in the hyperimmune serum anti-MV did not appear to play a role in EV neutralization.
FIG. 6.
EV neutralization activity detected in anti-vaccinia virus hyperimmune sera. (A) Comet inhibition assays were performed using Vero cell monolayers infected with vaccinia virus (IHD-J) that were overlaid with medium alone, medium containing methyl cellulose (MC), or medium containing a 1/50 dilution of hyperimmune sera generated against Dryvax in mice (anti-Dryvax) or humans (Dryvax-VIG); (B) infected Vero cell monolayers were overlaid with medium containing a 1/50 dilution of mouse hyperimmune antisera anti-MV, anti-A5Lint, and anti-Dryvax (described in Fig. 3 legend). α, anti.
EV-specific antibody responses elicited by Dryvax and MVA immunizations.
An immune response elicited against EV by smallpox vaccines is thought to be important in controlling viral spread within the host (3, 6, 56). The availability of sensitive, antigen-specific ELISA for the EV proteins A33R, A56R, and B5R should allow a quantitative comparison of the EV immune response between candidate smallpox vaccines. We therefore decided to use the quantitative EV-ELISAs to compare the magnitudes and durations of the humoral responses generated against the EV components of vaccinia virus elicited by Dryvax and the candidate smallpox vaccine MVA in a series of immunization experiments described previously (40). To evaluate the roles of dose and route of inoculation in the EV response, mice were immunized with a single dose of PBS given i.m., 106 PFU of Dryvax given i.d., or 107 PFU or 108 PFU of MVA given i.m., s.c., and i.d. As previously reported, the i.d. dose of 106 PFU Dryvax and the dose of 107 PFU or 108 PFU MVA, administered by various routes of vaccination, elicited antibody responses to total vaccinia virus that peaked around week 6 and remained steady over a 15-week period, and the observed antibody responses were not appreciably affected by the route of vaccination. In addition, the total vaccinia virus-specific antibody responses elicited by the MVA immunizations were similar to or greater than the responses elicited by the Dryvax immunization (40). Since peak antibody titers to vaccinia virus were detected at week 6 among the different vaccination groups, we evaluated the pooled immune sera from week 6, obtained from two separate experiments, for specific EV antibody responses to A33R, A56R, and B5R. As shown in Table 1, among the different routes of vaccination tested, mice immunized i.d. with 107 PFU of MVA developed antibody responses against EV antigens A33R, A56R, and B5R that were similar to the responses elicited by i.d. immunization with 106 PFU of Dryvax. At the dose of 108 PFU of MVA given by various routes of inoculation, the highest antibody levels against A33R and B5R were elicited using the i.d. route relative to the i.d. administration of 106 PFU of Dryvax, whereas the highest response against A56R was elicited using the i.m. route. The observed differences in antibody titer were consistent but not significant, possibly due to the number of animals used in each experimental group. These results demonstrated that a dose of 108 PFU of MVA is able to induce an EV-specific antibody response similar to or slightly greater than that to 106 PFU of Dryvax. Additionally, based on the observed trend, the i.d. route of vaccination appeared to be the most effective compared to i.m. and s.c. routes of vaccination.
TABLE 1.
Comparison of EV-specific antibody titers elicited by a single immunization with Dryvax or MVAa
| Group | Type of immunization | Routea | Dose | Antibody titer (log10 ± SD) against EV antigen:
|
||
|---|---|---|---|---|---|---|
| A33R | A56R | B5R | ||||
| 1 | PBS | i.m. | NA | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 |
| 2 | Dryvax | i.d. | 106 | 3.40 ± 0.42 | 3.25 ± 0.21 | 2.50 ± 1.27 |
| 3 | MVA | i.m. | 107 | 2.95 ± 0.21 | 3.11 ± 0.00 | 2.95 ± 0.21 |
| 4 | MVA | s.c. | 107 | 2.05 ± 0.21 | 2.95 ± 0.21 | 3.11 ± 0.42 |
| 5 | MVA | i.d. | 107 | 3.25 ± 0.64 | 3.11 ± 0.00 | 3.26 ± 0.21 |
| 6 | MVA | i.m. | 108 | 3.40 ± 0.00 | 3.41 ± 0.00 | 3.26 ± 0.21 |
| 7 | MVA | s.c. | 108 | 3.10 ± 0.00 | 3.11 ± 0.00 | 3.56 ± 0.21 |
| 8 | MVA | i.d. | 108 | 3.86 ± 0.21 | 2.95 ± 0.21 | 3.71 ± 0.00 |
BALB/c mice were immunized with a single dose of PBS, Dryvax, or MVA at week 0 by the indicated routes of vaccination. Antisera were collected at week 6 and pooled, and the antibody levels against the indicated EV antigens were measured by ELISA. The results are the average of two independent immunization experiments in which each group contained three mice.
Next, we investigated the effects of different dosing regimens of MVA vaccination on the EV-specific humoral response. Since the i.d. inoculation appeared to generate a good EV-specific antigen response after a single dose, we compared that response to the EV-specific antibody response elicited using this route of inoculation in a prime-boost regimen (Table 2). Two groups of mice were immunized with two i.d. doses of 108 PFU of MVA which were injected either 3 weeks or 6 weeks apart (Table 2). For comparison, three groups of mice (groups of 3) were immunized with a single i.m. dose of PBS or a single i.d. dose of 106 PFU or 107 PFU of Dryvax. Pooled immune sera were obtained on week 12 of the study and used to measure EV-specific antibody titers by A33R, A56R, and B5R single-antigen ELISAs. Mice immunized with a single i.d. dose of 107 PFU of Dryvax had slightly higher specific titers compared to mice that received a 106 PFU dose of Dryvax given i.d. Mice immunized with two doses of MVA developed a greater EV antigen-specific response than those given the Dryvax immunization (Table 2), with the highest specific antibody response observed in the MVA group that was boosted at week 6. There was a statistical difference between the B5R-specific antibody response induced by (106 PFU) Dryvax and by two (108 PFU) MVA inoculations administered 3 weeks apart (P, <0.0001). There was a statistical difference between the A33R- and B5R-specific antibody responses to two (108 PFU) MVA inoculations administered 6 weeks apart compared to the responses to a single (106 PFU) Dryvax inoculation (P, <0.019 and P, <0.026, respectively). Thus, the data indicated that adopting a prime-boost schedule in MVA immunization can substantially increase the EV-specific antibody response elicited compared to the response to a single Dryvax immunization.
TABLE 2.
Comparison of EV-specific antibody titers elicited by single immunizations and prime-boost immunizationsa
| Group | Type of immunization | Route | Dose | Wk(s) | Antibody titer (log10 ± SD) against EV antigen:
|
||
|---|---|---|---|---|---|---|---|
| A33R | A56R | B5R | |||||
| 1 | PBS | i.m. | NA | 0 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 |
| 2 | Dryvax | i.d. | 106 | 0 | 2.80 ± 0.00 | 2.80 ± 0.00 | 2.50 ± 0.00 |
| 3 | Dryvax | i.d. | 107 | 0 | 3.26 ± 0.21 | 3.11 ± 0.42 | 2.95 ± 0.64 |
| 4 | MVA | i.d. | 108 | 0, 3 | 3.11 ± 0.42 | 3.56 ± 0.21 | 4.01 ± 0.00 |
| 5 | MVA | i.d. | 108 | 0, 6 | 3.86 ± 0.21 | 3.86 ± 0.63 | 4.31 ± 0.42 |
BALB/c mice were immunized with a single dose of PBS, Dryvax, or MVA at week 0 using indicated routes of vaccination. Mice in groups 4 and 5 received two doses of MVA given 3 weeks apart and 6 weeks apart, respectively. Mice in group 6 were immunized once with Dryvax by scarification at week 0. In groups 1 through 5, the results represent the average of two independent immunization experiments in which each group contained three mice.
rSFV-EV expression vectors elicit high titers of antigen-specific antisera.
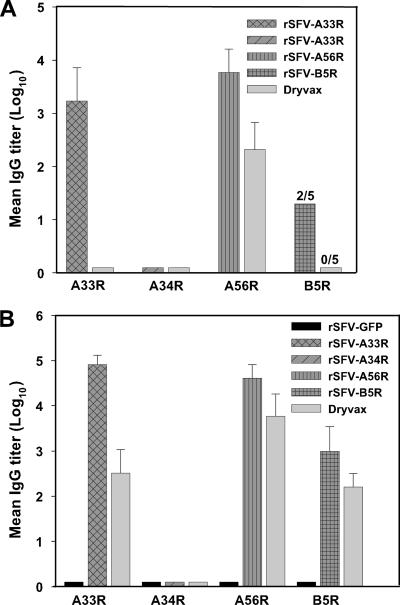
In addition to using these rSFV expression vectors to produce EV antigens as reagents to measure the EV-specific humoral response generated by vaccines, we suspected that these rSFV expression vectors could be used to generate monospecific antisera against each of the EV antigens. Such antigen-specific polyclonal antisera would be important for dissecting the immune response to individual EV antigens. rSFV particles have been used successfully in several immunization studies and shown to induce long-lasting immune responses to heterologous antigens in different animal models (34). The immune response in mice after two s.c. injections, given 3 weeks apart, of 106 infectious rSFV particles expressing A33R, A34R, A56R, B5R, or GFP was evaluated. For comparison, mice were immunized with two s.c. doses of 106 PFU of Dryvax, given 3 weeks apart. Immune sera were collected 3 weeks after each vaccination, and antibody titers against EV antigens were measured by ELISA. All mice inoculated with a single dose of rSFV-A33R or rSFV-A56R seroconverted, two of the five mice in the rSFV-B5R group seroconverted, and mice that received rSFV-A34R had no measurable antibodies (Fig. 7A). In contrast, only antibodies against A56R could be detected in mice immunized with one s.c. dose of Dryvax (Fig. 7A). Higher antibody titers against EV proteins A33R, A56R, and B5R were obtained in mice boosted with rSFV-based vaccines and Dryvax (Fig. 7B). Mice boosted with rSFV-A34R, however, did not develop measurable antibodies against A34R. Collectively, rSFV-A33R, -A56R, and -B5R inoculations induced a greater antibody response to the individual EV proteins than the Dryvax vaccinations, with rSFV-A33R eliciting the greatest antibody response. As for the control, immune serum collected from mice inoculated with rSFV-GFP particles generated no detectable antibody activity against immobilized EV proteins in the specific ELISAs. These results suggested that rSFV infectious vectors are suitable reagents for eliciting the production of EV-monospecific antisera.
FIG. 7.
Antibody response to rSFV infectious vectors expressing EV proteins. Mice in groups of five were inoculated s.c., using a prime-boost regimen, with 106 I.U. of indicated rSFV particles expressing GFP, A33R, A34R, A56R, or B5R or with 106 PFU of Dryvax. The inoculations were administered 3 weeks apart, and immune sera were collected 3 weeks after each injection and analyzed by single-EV-antigen ELISAs. (A) Antibody levels in antisera measured after the first inoculations. (B) Antibody levels measured after the second inoculations. Note: two out of five (2/5) mice inoculated once with rSFV-B5R seroconverted, whereas that none of the five (0/5) mice inoculated once with Dryvax had detectable antibody against B5R. After the second inoculation, all mice seroconverted.
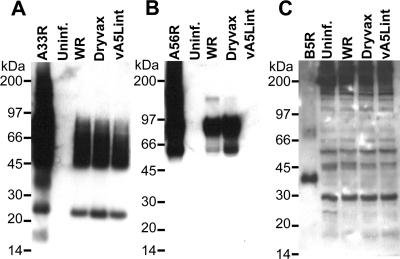
Next, we investigated the ability of each monospecific EV antiserum to recognize natural A33R, A56R, and B5R antigens produced in cells infected with different vaccinia viruses by using SDS-PAGE and Western blot analysis. The monospecific antiserum anti-A33R detected affinity-purified A33R, as well as protein bands of similar sizes from B-SC-1 cells that were infected with the WR strain of vaccinia virus, the recombinant WR strain vaccinia virus vA5Lint lacking the HA gene (A56R), and Dryvax, but not from uninfected cell lysates (Fig. 8A). The monospecific antiserum anti-A56R recognized protein bands of ∼60 kDa and ∼90 kDa, corresponding to affinity-purified A56R and A56R from WR strain vaccinia virus- and Dryvax-infected cell lysates but not from cells infected with vA5Lint or mock-infected cells (Fig. 8B). In contrast, the monospecific antiserum anti-B5R only detected highly enriched affinity-purified B5R corresponding to a ∼40-kDa band from rSFV-infected cells, but no other protein bands at ∼40 kDa were detected from vaccinia virus-infected cell lysates (Fig. 8C).
FIG. 8.
Western blot analysis of vaccinia virus-infected cells using rSFV vector-generated EV-specific antisera. Immune sera were collected from mice inoculated with two s.c. doses of 106 I.U. of rSFV particles encoding either A33R, A56R, or B5R and analyzed by Western blotting using uninfected and vaccinia virus-infected B-SC-1 cell lysates. B-SC-1 cells were infected at a multiplicity of infection of 5 with WR strain vaccinia virus (WR), Dryvax, and recombinant WR strain vaccinia virus vA5Lint (described in Fig. 3 legend). Indicated cell lysates were analyzed by Western blotting using antisera anti-A33R (A), anti-A56R (B), or anti-B5R (C) and an anti-mouse Ig-HRP conjugate. Indicated affinity-purified EV proteins A33R, A56R, and B5R were used as positive controls, and uninfected-cell lysate (uninf.) was used as a negative control. Molecular masses are indicated on the left. α, anti.
To further characterize the rSFV vector-generated A33R-, A56R-, and B5R-specific antisera, immunofluorescence staining was performed with B-SC-1 cell monolayers infected with Dryvax (data not shown). The monospecific antisera anti-A33R and anti-A56R strongly stained vaccinia virus plaques but not the surrounding uninfected cells. Similarly, antiserum anti-B5R stained vaccinia virus plaques, albeit at a lower intensity than the antisera anti-A33R and anti-A56R. While monospecific antisera anti-A33R and anti-A56R were capable of detecting A33R and A56R in Western blot and vaccinia virus plaques by immunofluorescence staining, the anti-B5R antiserum detected B5R in vaccinia virus plaques efficiently by immunofluorescence staining and not in Western blotting. The immunofluorescence data, taken together with the ability of monospecific antisera to detect A33R, A56R, and B5R antigens, indicated that the rSFV vectors express biologically active EV proteins, making these reagents suitable for generating a panel of EV-monospecific antisera.
DISCUSSION
Vaccination with live vaccinia virus-based vaccines (e.g., Dryvax) elicits long-lasting and robust protection against orthopoxvirus infection and targets both the EV and MV forms of the virus (8, 21, 46, 58). However, there is little information regarding the actual correlates of the protective immune response against smallpox infection (2). Previous studies of the protective humoral immune response against smallpox as represented by VIG, an antiserum collected from Dryvax vaccinees and used to treat vaccination adverse events (26, 62), have identified numerous virus antigen targets recognized by antibodies in VIG. In addition to numerous MV proteins recognized by VIG serum antibodies, the EV-specific proteins A33R, B5R, and A56R were detected in Western blot analysis by antibodies in VIG (4, 9, 25). Recent studies with DNA or protein candidate vaccines have reinforced the importance of an immune response against EV proteins, as well as MV envelope proteins, to provide the best protection against virus infection (17, 22, 23, 63).
In order to quantify the specific antibody response directed against the EV proteins, we developed a set of EV-specific ELISAs based on rEV antigens corresponding to the natural A33R, A34R, A56R, and B5R ORFs. While our studies were in progress, other laboratories reported the development of EV-specific ELISAs using truncated forms of A33R and B5R proteins produced in insect cells or truncated forms of A33R, B5R, and A56R produced in Chinese hamster ovary cells (17, 30, 63). Although these truncated EV antigens were found to be viable ELISA substrates in quantifying the EV-specific antibody responses, we elected to use an SFV expression system to produce full-length EV antigens in BHK-21 cells, where mammalian-specific posttranslation modification may be conferred, and subsequently immunoaffinity purified the proteins by virtue of the C-terminal epitope tag (31). Natural EV proteins are transmembrane proteins that acquire numerous mammalian-specific posttranslational modifications that may influence their correct folding. Protein expression was verified by Western blot analysis using primary antibodies directed against the epitope tag. Western blot analysis using anti-Dryvax serum as the primary antibody recognized prominent protein bands of the expected sizes for the corresponding EV A33R, A56R, and B5R. The predominant EV protein species recognized by Western blot analysis with the epitope tag antibody or anti-vaccinia virus sera appeared as numerous diffuse bands after SDS-PAGE under reducing and nonreducing conditions. These multiply reactive EV protein species probably represent unmodified, multimeric, posttranslationally modified EV proteins and cleavage products from EV protein precursors from rSFV-infected cell lysates, as previously described for these proteins (24, 44, 47). The response to the A33R and A56R protein species treated under nonreducing conditions in the Western blot analysis using the anti-Dryvax serum was much stronger than that observed under reducing conditions (Fig. 2). Serum antibodies reacted predominantly with the high-molecular-mass forms of A33R and A56R protein species. The effect was less pronounced for B5R. These results suggest that a strong antibody response is elicited against epitopes present only in the oligomeric forms of A33R and A56R. The purified A56R and B5R preparations also contained small-molecular-mass protein fragments of less than 14 kDa that were only detected using the epitope tag antibody, suggesting that these viral peptide fragments at the C-terminal end were probably not very antigenic in vivo. No protein bands corresponding to A34R were detected after Western blot analysis using serum from mice immunized with Dryvax or the SFV recombinant expressing A34R. Western blot analysis using the epitope tag antibody demonstrated that the A34R protein made by the SFV vector is abundant and migrates with the expected molecular mass (Fig. 2B). Although we cannot formally rule out the possibility that recombinant A34R is lacking posttranslational modifications that affect its antigenicity, the most likely explanation is that A34R is not very antigenic, since VIG does not detect A34R when used as the primary antibody in Western blot analysis (25). Moreover, these results are consistent with data showing that antibodies raised against A34R did not neutralize EV (20).
To optimize the utility of each of the four EV proteins as an immobilized antigen on an ELISA plate, we tested the ability of each EV protein to quantify antibody titers using the epitope tag antibody as a primary ELISA antibody and a panel of vaccinia virus antisera. The results from these ELISA experiments recapitulated the results seen for the Western blot experiments in that antibodies to all four proteins were detected using the epitope tag as the primary antibody in an ELISA, while none of the tested vaccinia virus hyperimmune sera detected A34R. The specificities of the A33R, A56R, and B5R ELISAs were demonstrated by using the murine antisera produced by using vA5Lint (a recombinant WR strain vaccinia virus that lacks the A56R gene) or UV-inactivated MV particles. While Western blot analysis demonstrated that anti-vA5Lint immune serum detected A33R and B5R but not A56R, the A56R ELISA also detected a trace amount of activity against A56R in the anti-vA5Lint immune serum. Since vA5Lint was constructed from the pVOTE vectors (60, 61) that retain a portion of the A56R gene, the low A56R activity seen in the ELISA is likely directed against a truncated product of the A56R ORF. Western blot analysis using the anti-MV antiserum did not detect A33R and weakly detected B5R. Unexpectedly, however, the anti-MV antiserum contained sufficient antibody activity to recognize the A56R protein on a Western blot and to be quantified in the A56R-specific ELISA. In addition, the A56R and B5R ELISAs measured considerable A56R antibody, as well as detectable B5R antibody, in the anti-MV serum. Since there was no detectable live virus in the UV-inactivated MV sample used to produce the MV hyperimmune serum (limit of detection, <100 PFU/ml), it is unlikely that replicating vaccinia virus was responsible for the generation of A56R and B5R antibodies in this antiserum. Although it is possible that residual A56R and B5R protein was present in the UV-inactivated MV preparation, it seems more likely that, since inactivated virus is still capable of infecting cells, these proteins were synthesized following limited early gene expression. All three EV gene products appear to be transcribed at both early and late times postinfection (13, 48). The relative immune responses generated by the UV-inactivated particles can be explained by the observation that the product of the A56R gene partially localizes on the cell surface (33), whereas the B5R protein sequesters within the Golgi apparatus of the infected cell (33, 59). The absence of an A33R antibody response in the anti-MV serum can be explained by the observation that the expression of A33R is predominantly late (44, 48). The sum of the experiments with the panel of hyperimmune antisera and the antisera from mice immunized with a single dose of 106 Dryvax demonstrated that the SFV-recombinant A33R, A56R, and B5R proteins were suitable antigens for use in sensitive and quantitative EV-ELISAs, particularly in the mouse model of vaccination.
Since previous reports have shown that numerous vaccinia virus proteins are recognized by VIG, including the EV proteins A33R, A56R, and B5R (9, 25), we also evaluated the EV-specific ELISAs in a limited number of experiments for their utility in quantifying EV antibody titers in human sera. While the ELISAs for A33R, A56R, and B5R demonstrated antibody titers approximately 250-fold-higher than those in the control human serum, there were considerable background amounts using the conditions established for the murine vaccination experiments. Interestingly, the A34R-specific ELISA detected virtually no antibody above the control human serum background, consistent with previous results using Western blot analysis. Nevertheless, the results suggested that similar sensitive quantitative EV-ELISAs could be developed for human vaccine trials using the rSFV EV reagents.
We also conducted a series of EV neutralization assays in order to determine if the humoral response measured by the EV-specific ELISAs correlated with biological activity against the EV form of the vaccinia virus. As demonstrated in the comet inhibition assay, both the mouse and human anti-Dryvax immune sera were able to inhibit new infections due to EV by blocking the spread of virus from the infected cells. Similar inhibitory activity was observed with the hyperimmune serum raised against vA5Lint, which is only missing the A56R protein, but no inhibitory activity was detectable using the hyperimmune serum raised against purified UV-inactivated MV particles, which is greatly reduced in anti-A33R and anti-B5R antibodies but contains relatively high anti-A56R titers. The results suggested that A56R antibodies are not important for EV-neutralizing activity. Although our data do not discriminate between A33R-specific and B5R-specific antibodies in EV neutralization, it has recently been reported that polyclonal antibodies and MAbs directed against A33R or B5R possess EV neutralization activity (1, 4, 17, 35) and that the response against B5R is thought to constitute the primary EV neutralization activity of VIG (4, 20). While the comet inhibition assay provides a reliable and simple test to detect anti-EV antibody activity, it is not quantitative or particularly sensitive. In our hands, for example, we were unable to reliably measure comet inhibition using antisera from mice immunized with a single dose of 106 Dryvax, thus limiting the assay's usefulness for comparing EV responses to candidate vaccines. Similarly, the A33R, A56R, and B5R monospecific antisera generated by rSFV vector immunization were unable to limit satellite plaque formation in a comet inhibition assay at any dilution (data not shown).
One of the major goals of the present study was to develop sensitive assays for quantifying the EV antibody response to candidate smallpox vaccines such as MVA (50). We have recently compared MVA to Dryvax in its ability to protect against a lethal poxvirus challenge in a small animal model (40). The results of these studies indicated that an effective humoral response elicited by MVA or Dryvax immunization plays an important role in protection, but the role of individual antigens in protective immunity is still largely unknown. The results from the experiments in the present study demonstrated that the EV-ELISAs are useful for quantifying the antibody response to individual EV antigens and can distinguish the responses to different vaccines and vaccination protocols. For example, the A33R and B5R responses following a two-dose vaccination with MVA (108 PFU) at 0 and 6 weeks were significantly higher than with a single dose of Dryvax (106 PFU). As specific EV vaccine markers are identified and correlated with protection, these quantitative assays will be increasingly valuable for assessing their efficacies in preclinical animal studies.
Based on previous immunization studies done with SFV-based vaccines (5, 15), we suspected that these rSFV expression vectors could be used to generate monospecific antisera against each of the EV antigens. Following rSFV vector immunization, a strong serum antibody response against each of the expressed EV proteins except for A34R was observed. This response was greater than the corresponding specific-antibody titers elicited by two doses of Dryvax (106 PFU). The absence of A34R-specific antibodies following rSFV-A34R immunization is consistent with other results that suggest that the A34R protein does not induce a substantial antibody response. The monospecific antisera elicited by the rSFV-A33R and -A56R vectors reacted strongly with protein bands in Western blot analysis derived from vaccinia virus infected-cell lysates that corresponded to A33R and A56R. In the immunofluorescence studies, all three monospecific antisera specifically stained Dryvax-infected cells, albeit the staining by the B5R-monospecific antiserum appeared less intense than the staining by the A33R- and A56R-monospecific antisera. Thus, the rSFV vectors expressing EV proteins are capable of generating polyclonal EV-monospecific antisera that can be used for dissecting the EV immune response.
Recent studies have reported that the induction of antibody responses against A33R and B5R antigens is important in the protective humoral response (7, 17, 20, 22). An antibody response against A33R correlates with protection (17, 20, 22); an anti-B5R antibody response is primarily responsible for the EV neutralization activity in VIG (4, 30), and B5R antibodies are able to partially protect mice in passive immunization and challenge studies (7, 17, 20). These results not only suggest the value of antigen-specific assays to quantify the response to EV antigens following vaccination, they also suggest that the rSFV-A33R and rSFV-B5R vectors, or the antibodies produced by them, might confer protection against a poxvirus infection in animal models. Although we have not explored this concept in depth, the animals used to produce the monospecific EV antisera were challenged intranasally with 25 times the 50% lethal dose of WR strain vaccinia virus (data not shown). Mice inoculated with rSFV-A33R exhibited partial protection (two of five survived) against the lethal intranasal challenge with pathogenic vaccinia virus WR, even though the prechallenge A33R-specific antiserum from these mice contained no comet inhibition activity. This partial protection afforded by the rSFV-A33R inoculations is consistent with previous reports. In contrast, all mice inoculated with rSFV-A56R and rSFV-B5R particles were susceptible to the vaccinia virus WR challenge dose. The induction of an A56R-specific response was not expected to be critical for protection, given that it has been reported that one-third of EV particles lack A56R antigens (27) and that the hyperimmune antiserum (anti-A5Lint) that was deficient in A56R antibodies exhibited potent comet inhibition activity. At the present time, we do not have enough data to speculate on the reason for the inability of the rSFV-B5R-immunized animals to resist challenge. Further immunization experiments are planned with the rSFV-EV vectors, both singly and in combination. Nevertheless, the preliminary results point out the usefulness of these vectors in dissecting the protective immune response to vaccinia virus EV proteins.
In summary, the evaluation of new-generation smallpox vaccines will require the use of animal models as surrogate markers for vaccine efficacy (49). Although the correlates for protection against smallpox are unknown, investigations of DNA-based and protein subunit vaccines have shown the requirement for both MV and EV components for maximum efficacy (17, 22, 63), and assays will be needed to accurately quantify the EV humoral response after vaccination. The assays described here using the A33R, A56R, or B5R proteins were sensitive, specific, and reproducible. The proteins appeared to be bona fide copies of the vaccinia virus EV proteins, as antisera derived from vaccinia virus-based immunizations recognized the rSFV-expressed EV proteins in Western blotting, and the proteins were able to adopt the oligomeric forms observed by natural vaccinia virus EV proteins under nonreducing conditions. The EV-specific assays allowed a quantitative comparison of the EV antibody responses following Dryvax and MVA vaccination in a mouse model; similar quantitative comparisons of other candidate smallpox vaccines can be used to correlate protection against lethal challenge with EV-directed antibody response. The rSFV-EV expression vectors will also be valuable as tools for dissecting the protective immune response to vaccination, as vectors for generating polyclonal monospecific antisera, and possibly as experimental vaccines.
Acknowledgments
We thank Amy Woerner for the cloning of the A56R gene used in the SFV expression system and Wolfgang Resch and Peter Berglund for instructions in propagating and purifying rSFV particles. We thank Hana Golding and Carol Weiss for critical reading of the manuscript.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320-337. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen. Virol. 13:9-17. [DOI] [PubMed] [Google Scholar]
- 4.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 5.Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljestrom. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. [DOI] [PubMed] [Google Scholar]
- 6.Boulter, E. A. 1969. Protection against poxviruses. Proc. R. Soc. Med. 62:295-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 103:1882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969-4973. [DOI] [PubMed] [Google Scholar]
- 9.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl, P., N. Cooper, L. Wyatt, B. Moss, and M. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.13. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology, vol. 3. John Wiley and Sons, Inc., New York, NY. [DOI] [PubMed]
- 11a.Earl, P. L., N. Cooper, L. Wyatt, B. Moss, and M. W. Carroll. 1998. Generation of recombinant vaccinia virus, p. 16.17.1-16.17.19. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology, vol. 3. John Wiley and Sons, Inc., New York, NY.
- 12.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. [DOI] [PubMed] [Google Scholar]
- 13.Engelstad, M., S. T. Howard, and G. L. Smith. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801-810. [DOI] [PubMed] [Google Scholar]
- 14.Engler, R. J., J. Kenner, and D. Y. Leung. 2002. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J. Allergy Clin. Immunol. 110:357-365. [DOI] [PubMed] [Google Scholar]
- 15.Fleeton, M. N., P. Liljestrom, B. J. Sheahan, and G. J. Atkins. 2000. Recombinant Semliki Forest virus particles expressing louping ill virus antigens induce a better protective response than plasmid-based DNA vaccines or an inactivated whole particle vaccine. J. Gen. Virol. 81:749-758. [DOI] [PubMed] [Google Scholar]
- 16.Fleeton, M. N., B. J. Sheahan, E. A. Gould, G. J. Atkins, and P. Liljestrom. 1999. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J. Gen. Virol. 80:1189-1198. [DOI] [PubMed] [Google Scholar]
- 17.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part I. Background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin. Infect. Dis. 37:241-250. [DOI] [PubMed] [Google Scholar]
- 19.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 20.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 21.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 22.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 66:7217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones-Trower, A., A. Garcia, C. A. Meseda, Y. He, C. Weiss, A. Kumar, J. P. Weir, and M. Merchlinsky. 2005. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343:128-140. [DOI] [PubMed] [Google Scholar]
- 26.Kempe, C. H., T. O. Berge, and B. England. 1956. Hyperimmune vaccinal gamma globulin; source, evaluation, and use in prophylaxis and therapy. Pediatrics 18:177-188. [PubMed] [Google Scholar]
- 27.Krauss, O., R. Hollinshead, M. Hollinshead, and G. L. Smith. 2002. An investigation of incorporation of cellular antigens into vaccinia virus particles. J. Gen. Virol. 83:2347-2359. [DOI] [PubMed] [Google Scholar]
- 28.Lane, J. M., and J. Goldstein. 2003. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann. Intern. Med. 138:488-493. [DOI] [PubMed] [Google Scholar]
- 29.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83:209-222. [DOI] [PubMed] [Google Scholar]
- 30.Law, M., M. M. Putz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991-1000. [DOI] [PubMed] [Google Scholar]
- 31.Liljestrom, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 32.Liljeström, P., and H. Garoff. 1995. Expression of proteins using Semliki Forest virus vectors, vol. 2. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 33.Lorenzo, M. M., I. Galindo, G. Griffiths, and R. Blasco. 2000. Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon. J. Virol. 74:10535-10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundstrom, K. 2002. Alphavirus-based vaccines. Curr. Opin. Mol. Ther. 4:28-34. [PubMed] [Google Scholar]
- 35.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 37.Mayr, A., and K. Danner. 1978. Vaccination against pox diseases under immunosuppressive conditions. Dev. Biol. Stand. 41:225-234. [PubMed] [Google Scholar]
- 38.Mayr, A., H. Stickl, H. K. Muller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defense mechanism. Zentbl. Bakteriol. B 167:375-390. (In German.) [PubMed] [Google Scholar]
- 39.McCurdy, L. H., B. D. Larkin, J. E. Martin, and B. S. Graham. 2004. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin. Infect. Dis. 38:1749-1753. [DOI] [PubMed] [Google Scholar]
- 40.Meseda, C. A., A. D. Garcia, A. Kumar, A. E. Mayer, J. Manischewitz, L. R. King, H. Golding, M. Merchlinsky, and J. P. Weir. 2005. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339:164-175. [DOI] [PubMed] [Google Scholar]
- 41.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 42.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 43.Neff, J. M., J. M. Lane, V. A. Fulginiti, and D. A. Henderson. 2002. Contact vaccinia—transmission of vaccinia from smallpox vaccination. JAMA 288:1901-1905. [DOI] [PubMed] [Google Scholar]
- 44.Payne, L. G. 1992. Characterization of vaccinia virus glycoproteins by monoclonal antibody precipitation. Virology 187:251-260. [DOI] [PubMed] [Google Scholar]
- 45.Payne, L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Putz, M. M., I. Alberini, C. M. Midgley, I. Manini, E. Montomoli, and G. L. Smith. 2005. Prevalence of antibodies to vaccinia virus after smallpox vaccination in Italy. J. Gen. Virol. 86:2955-2960. [DOI] [PubMed] [Google Scholar]
- 47.Roper, R. L., L. G. Payne, and B. Moss. 1996. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 70:3753-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roper, R. L., E. J. Wolffe, A. Weisberg, and B. Moss. 1998. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 72:4192-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal, S. R., M. Merchlinsky, C. Kleppinger, and K. L. Goldenthal. 2001. Developing new smallpox vaccines. Emerg. Infect. Dis. 7:920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slifka, M. K. 2005. The future of smallpox vaccination: is MVA the key? Med. Immunol. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smerdou, C., and P. Liljestrom. 1999. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 53.Stickl, H., V. Hochstein-Mintzel, A. Mayr, H. C. Huber, H. Schafer, and A. Holzner. 1974. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA). Dtsch. Med. Wochenschr. 99:2386-2392. (In German.) [DOI] [PubMed] [Google Scholar]
- 54.Stittelaar, K. J., T. Kuiken, R. L. de Swart, G. van Amerongen, H. W. Vos, H. G. Niesters, P. van Schalkwijk, T. van der Kwast, L. S. Wyatt, B. Moss, and A. D. Osterhaus. 2001. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine 19:3700-3709. [DOI] [PubMed] [Google Scholar]
- 55.Stittelaar, K. J., G. van Amerongen, I. Kondova, T. Kuiken, R. F. van Lavieren, F. H. Pistoor, H. G. Niesters, G. van Doornum, B. A. van der Zeijst, L. Mateo, P. J. Chaplin, and A. D. Osterhaus. 2005. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 79:7845-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner, G. S., and E. J. Squires. 1971. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J. Gen. Virol. 13:19-25. [DOI] [PubMed] [Google Scholar]
- 57.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J. Gen. Virol. 78:2041-2048. [DOI] [PubMed] [Google Scholar]
- 58.Viner, K. M., and S. N. Isaacs. 2005. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 7:579-583. [DOI] [PubMed] [Google Scholar]
- 59.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 74:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 92:6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, O., E. J. Wolffe, A. S. Weisberg, and M. Merchlinsky. 1999. Vaccinia virus WR gene A5L is required for morphogenesis of mature virions. J. Virol. 73:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wittek, R. 2006. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int. J. Infect. Dis. 10:193-201. [DOI] [PubMed] [Google Scholar]
- 63.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]