Abstract
Sao is a Streptococcus suis surface protein recently identified as a potential vaccine candidate. In this study, recombinant Sao in combination with Quil A provided cross-protection against S. suis serotype 2 disease in mouse and pig vaccination protocols. Subcutaneous immunization of mice elicited strong immunoglobulin G (IgG) antibody responses. All four IgG subclasses were induced, with the IgG2a titer being the highest, followed by those of IgG1, IgG2b, and IgG3. Challenge of the mice with S. suis strain 31533 resulted in a mortality rate of 80% for the control group, which received Quil A only. In contrast, all of the mice immunized with Sao survived. In a pig vaccination protocol, intramuscular immunization with Sao also elicited significant humoral antibody responses, and both the IgG1 and IgG2 subclasses were induced, with a predominance of IgG2 production. In vitro assay showed that Sao-induced antibodies significantly promoted the ability of porcine neutrophils in opsonophagocytic killing of S. suis. An aerosol challenge of the pigs with S. suis strain 166 resulted in clinical signs characteristic of S. suis infection in diseased pigs. The vaccine group showed significantly better survival, lower clinical scores, and less S. suis recovery from postmortem tissue samples than did the control group. Furthermore, this study also revealed that although challenge S. suis strains express Sao size variants, recombinant Sao conferred cross-protection. These data demonstrate that recombinant Sao formulated with Quil A triggers strong opsonizing antibody responses which confer efficient immunity against challenge infection with heterologous S. suis type 2.
Streptococcus suis is an important pathogen of swine, causing meningitis, septicemia, arthritis, endocarditis, pneumonia, and substantial economical losses in the swine industry worldwide (17, 22, 46). It is also an important zoonotic agent for humans in contact with diseased pigs or their products, causing life-threatening diseases, as reported for a recent outbreak in China (42). Thirty-five serotypes have been described so far (17). Serotype 2 is the most prevalent type in association with diseases in most countries. The pathogenesis and virulence attributes of S. suis are not well defined, and attempts to control the infection are hampered by the lack of an effective vaccine (21).
Different types of vaccines have been developed or are presently under investigation. At present, inactivated commercial autogenous vaccines are used in the field, but results have been inconsistent (19, 34). Furthermore, safety data for autogenous vaccines are lacking, which has liability implications for the use of this type of material (18). Attenuated or avirulent live S. suis strains have been tested, and the results were also equivocal (6, 25, 52). In addition to bacterins and live vaccines, a number of purified bacterial components have been developed as vaccine candidates. The capsule polysaccharide is a critical virulence factor of S. suis. However, a vaccine based on capsular material was unsatisfactory due to its poor immunogenicity (13). Vaccination strategies using purified suilysin (26) or muramidase-released protein and extracellular protein factor (53) from S. suis serotype 2 have been shown to protect pigs from homologous and heterologous serotype 2 strains. However, a substantial number of virulent strains in some geographic regions do not express these proteins (14, 16, 44).
We recently identified a surface protein (Sao) which is highly conserved among S. suis species (36). Convalescent-phase swine sera have high titers of antibody against this protein, suggesting that Sao is a potent immunogen that is expressed during S. suis infection. These findings made Sao a candidate for use in a subunit vaccine. However, in a convenient test, immunization of piglets with recombinant Sao mixed with the oil-in-water Emulsigen reagent triggered a predominant production of immunoglobulin G1 (IgG1), and these antibodies lacked opsonophagocytic function and did not confer protection (36). This suggested that the quality of the type 1/type 2 immune response bias was inappropriate to mediate protection against S. suis. It is known that host protection against infection caused by S. suis, a highly encapsulated microorganism, is mediated primarily by opsonophagocytosis, which is mainly associated with a Th1-type immune response characterized by IgG2a production (5, 17). The vaccine formulation and components, such as adjuvants, can dramatically influence the vaccine-induced antibody response, including bias to type 1 or type 2 responses, which may have a significant effect on the protective efficacy of a vaccine (1, 30, 43). Evidence from vaccination using surface antigens of other gram-positive bacteria indicated that the efficiency of antibody-mediated opsonophagocytosis and protection can be improved dramatically by using Th1-directing adjuvants to promote a Th1-type immune response (2, 35). We therefore hypothesized that Sao may be protective in a vaccination protocol involving an optimal adjuvant and higher antigen dosage. In this study, the efficacy of recombinant Sao in combination with Quil A was demonstrated by protection against S. suis infection and disease in mice as well as pigs, the target species of this vaccine candidate.
MATERIALS AND METHODS
Bacterial strains.
Three S. suis strains of serotype 2 were used in this study. Strain S735 was used to clone the sao gene and to produce the protein (36). This strain is the reference strain and was originally isolated from a diseased pig in The Netherlands (27). Strains 166 and 31533 originated from pigs with meningitis (4, 32). Strain 31533 was chosen for challenging mice, and strain 166 was used for challenging pigs. Bacteria were grown on plates made with Todd-Hewitt broth (Difco, Detroit, MI) containing 2% agar or in liquid cultures of Todd-Hewitt broth.
Immunization and challenge of mice.
Recombinant Sao was produced and purified as previously described (36). Six-week-old female CD-1 mice were randomly assigned (according to body weight) to two groups of 10 mice and immunized subcutaneously twice at a 2-week interval with either 20 μg of purified Sao mixed with 20 μg of Quil A (Brenntag Biosector, Frederikssund, Denmark) adjuvant or 20 μg of Quil A only as a control in 100 μl of phosphate-buffered saline (PBS) per mouse. Ten days after the second vaccination, the animals were challenged intraperitoneally with 1 × 108 CFU per mouse of log-phase S. suis strain 31533 in 1 ml of Todd-Hewitt broth. This challenge model was confirmed to reproduce septic shock and meningitis similar to those induced by S. suis in pigs (unpublished data). Mice were monitored daily for clinical signs, such as abnormal behavior, rough hair coat, ataxia, and mortality, until day 14 after the infection. Blood samples were collected prior to each vaccination and the challenge for determining antibody responses. Guidelines from the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care were followed during the experiment, which followed a protocol that had been approved by the University of Montreal Committee on Animal Care.
Immunization and challenge of pigs.
Pigs were used to perform the immunization and protection experiment at the Vaccine and Infectious Disease Organization (University of Saskatchewan, Saskatoon, Canada) in accordance with principles outlined in the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care, using a protocol that was approved by the University Committee on Animal Care. Four-week-old piglets with an average weight of 7.79 kg from a herd that is free of S. suis serotype 2 were randomly assigned to two groups of 12 piglets each. Animals were injected intramuscularly twice at a 2-week interval with 1 ml of either 200 μg purified Sao mixed with 400 μg Quil A or 400 μg Quil A only in physiological saline as a control. Two weeks after the second injection, the immunized and control animals were challenged by aerosol with 1 ml (6.8 × 106 CFU) of a log-phase culture of S. suis strain 166 as previously described (38). Blood samples were collected prior to each injection and challenge for determination of antibody responses. Pigs were monitored for body temperature, clinical signs, and mortality for 10 consecutive days after challenge. A daily clinical score (from 0 to 8) was derived as the sum of attitude and locomotion scores for each pig, based upon signs of nervous, musculoskeletal, or respiratory disease. Attitude scores were given as follows: 0 = normal attitude and response to stimuli; 1 = inactive and slow to respond, with oculonasal secretions; 2 = only responsive to repeated stimuli; 3 = recumbent, nonresponsive, and unaware of surroundings; and 4 = dead. Locomotion scores were given as follows: 0 = normal gait and posture; 1 = slight incoordination, lameness, and/or joint swelling but rises without assistance; 2 = clearly uncoordinated or lame but stands without assistance; 3 = severe lameness and/or severe ataxia; and 4 = dead. Pigs having a clinical score of >2 on either scale were euthanized by lethal injection. A postmortem examination procedure was conducted for all pigs. Brain, tracheobronchial lymph node, and grossly affected joint samples from all pigs and blood samples from euthanized pigs were cultured for bacterial recovery.
ELISA.
Titers of Sao-specific total IgG and IgG subclasses in mouse and swine sera were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (36). Briefly, Polysorb immunoplates (Nunc, Rochester, NY) were coated overnight at 4°C with 100 μl per well of purified recombinant Sao at a concentration of 0.3 μg/ml in carbonate buffer. The plates were incubated with serial dilutions of test sera in PBS containing 0.05% Tween 20 for 1.5 h at room temperature. For determination of antibodies in mice, bound antibodies were detected by incubation with peroxidase-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b, or IgG3 antisera (Serotec, Kidlington, Oxford, United Kingdom) for 1 h at room temperature. For determination of swine total IgG, bound antibodies were detected by incubation with peroxidase-conjugated goat anti-swine IgG (heavy plus light chains) antisera (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 h at room temperature. For swine IgG1 and IgG2 detection, mouse anti-porcine IgG1 or IgG2 (Serotec) was used as the primary antibody, and peroxidase-conjugated goat anti-mouse IgG (heavy plus light chains) (Serotec) was used as the secondary antibody. The plates were developed with TMB substrate (Zymed, South San Francisco, CA). Absorbance was measured at 450 nm in an ELISA reader (Power Wave 340; Bio-Tek Instruments, Inc., Winooski, VT). The serum dilution that resulted in an optical density at 450 nm of 0.1 after background subtraction was considered the titer of that serum.
Opsonophagocytosis assay.
To investigate the role of Sao-specific antibody in protection, total IgG was purified from serum pools of control or Sao-vaccinated pigs after the second immunization by using a protein A column (Pharmacia, Uppsala, Sweden). Porcine neutrophils were isolated from pigs that belonged to a high-health-status herd. Complete normal serum from the healthy pig was used as a source of complement. An opsonophagocytosis assay was then performed as previously described (7). Briefly, S. suis strain 166 was suspended in complete normal porcine serum containing 25 μg/ml of purified IgG from either Sao-vaccinated pigs or control pigs and preopsonized for 30 min at 37°C. Neutrophils at a concentration of 5 × 106 cells/ml were mixed with 1 × 104 CFU/ml of bacteria in microtubes and incubated for 90 min at 37°C with 5% CO2. The neutrophil cells were lysed with sterile water, and viable bacterial counts were performed on Todd-Hewitt agar plates. Tubes with bacteria alone were treated similarly and used as controls. The tests were performed eight times. Results are expressed as percentages of killed bacteria.
Western blotting.
Fifty microliters of S. suis culture supernatant was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis in an 8% acrylamide gel. Proteins transferred to a nitrocellulose membrane (Bio-Rad, Mississauga, Ontario, Canada) were detected by incubation overnight at 4°C with a 1/200 dilution of pooled sera from the mice which received two doses of Sao or from the control animals in Tris-buffered saline-0.05% Tween 20 containing 5% skim milk. Sao-specific antibody was detected with peroxidase-conjugated goat anti-mouse IgG (Serotec) and visualized using 4-chloro-1-naphthol (Sigma, St. Louis, MO) as the substrate.
PCR and sequences.
The S. suis cell pellet from a 5-ml overnight culture was suspended in 1 ml of lysis buffer (50 mM Tris-HCl, 5 mM EDTA, 3% SDS, 1 mg/ml RNase, pH 8.5) and transferred to a 2-ml screw-cap tube containing 0.5 g of 0.1-mm glass beads (BioSpec, OK). The suspension was homogenized for 3 min at maximum speed using a mini-bead beater (BioSpec). The sample was centrifuged for 3 min at 16,000 × g in a microcentrifuge, and then the supernatant was used to extract the genomic DNA following standard procedure. The complete sao gene was amplified from the genomic DNA by using the pS1F (5′-ATGAATACTAAGAAATGG-3′) and pS1R (5′-AATTTACGTTTACGTGTA-3′) primer pair, and the DNA fragment flanking the repeating region in sao was amplified using the pS2F (5′-GAAATATCGAACCCCCTAAAG-3′) and pS2R (5′-CTTCGACTGTACCATTTTGGT-3′) primer pair. The PCR was performed for 5 min at 94°C, followed by 30 cycles of 1 min at 94°C, 30 s at 46°C, and 1 min at 72°C in a thermal cycler (Eppendorf Scientific Inc., Hamburg, Germany). The amplicons were analyzed in a 0.8% agarose gel and sequenced using the same primers.
Statistics.
Comparison between antibody titers and percentages of killed bacteria was done using the t test. The clinical scores were transferred by ranking, and the significance of the difference between groups was determined by the t test. Survival distributions were evaluated with chi-square analysis using the Kaplan-Meier method, and the significance of the difference was tested using the log rank test. The Fisher exact test was applied to compare the proportions of postmortem tissues from which S. suis was recovered. A P value of <0.05 was taken as significant.
RESULTS
Sao-specific IgG and IgG subclasses.
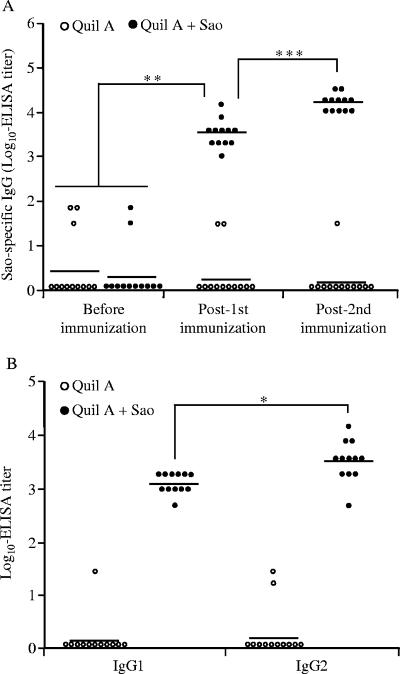
Subcutaneous immunization of mice with Sao in combination with Quil A elicited a significant humoral IgG response after primary immunization. The second immunization boosted the specific antibody response, which was significantly higher than the level after the primary immunization (P < 0.001) (Fig. 1A). Furthermore, all four tested IgG subclasses were induced in Sao-immunized mice, with the IgG2a titer being the highest, followed by those of IgG1, IgG2b, and IgG3, as measured in the sera 9 days after the second vaccination. The IgG2a titer was significantly higher than the titers of other IgG subclasses, including IgG1 (P < 0.05) (Fig. 1B). In contrast, Sao-specific IgG and its subclasses were below the limit of detection in sera of mice before vaccination and the mice in the control group.
FIG. 1.
Serum antibody responses in mice immunized with Quil A (open circles) or Quil A plus recombinant Sao (solid circles). (A) Total Sao-specific serum IgG. (B) IgG subclasses in sera 9 days after the second immunization. Antibody titers for individual mice are shown, with the average titer (n = 10) represented as a bar. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Similar patterns of immune responses were revealed for pigs that received 200 μg of Sao intramuscularly in combination with 400 μg of Quil A. Primary immunization of pigs triggered a strong antigen-specific IgG response. The serum IgG titer was significantly higher than that in control pigs receiving only Quil A or in pigs before immunization (P < 0.01). After a second dose of the vaccine, a significant increase in IgG level (P < 0.001) was seen in Sao-immunized pigs (Fig. 2A). Assessment of IgG subclasses demonstrated that while both IgG1 and IgG2 subclasses were induced in sera of Sao-immunized animals, the IgG2 response significantly dominated the IgG1 response (P < 0.05) (Fig. 2B).
FIG. 2.
Serum antibody responses in pigs immunized with Quil A (open circles) or Quil A plus recombinant Sao (solid circles). (A) Total Sao-specific serum IgG. (B) IgG subclasses in sera 13 days after the second immunization. Antibody titers for individual pigs are shown, with the average titer (n = 12) represented as a bar. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Protection of mice against S. suis strain 31533.
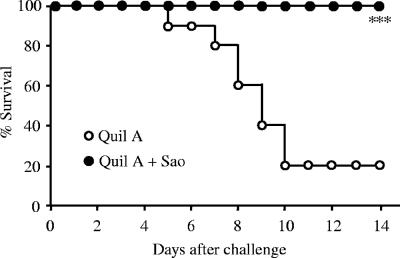
Sixteen hours after administration of the challenge infection with S. suis 31533, all mice in the nonimmunized control group exhibited clinical signs, such as a ruffled hair coat, suggesting fever, and a slow response to stimuli. Beginning about 4 days after the challenge, 8 of 10 mice in this group successively developed severe central nervous system signs, such as running in circles and opisthotonos. All eight of the ill mice died or met criteria for humane euthanasia due to the severity of their condition. In contrast, most of the mice in the Sao-vaccinated group showed only mild and transient rough hair after the challenge, and all mice survived the S. suis infection, resulting in complete protection from cause-specific mortality (P < 0.001) (Fig. 3).
FIG. 3.
Survival of mice immunized with Quil A (open circles) or Quil A plus recombinant Sao (solid circles) following challenge with S. suis 31533. Each group consists of 10 mice. ***, P < 0.001.
Protection of pigs against S. suis strain 166.
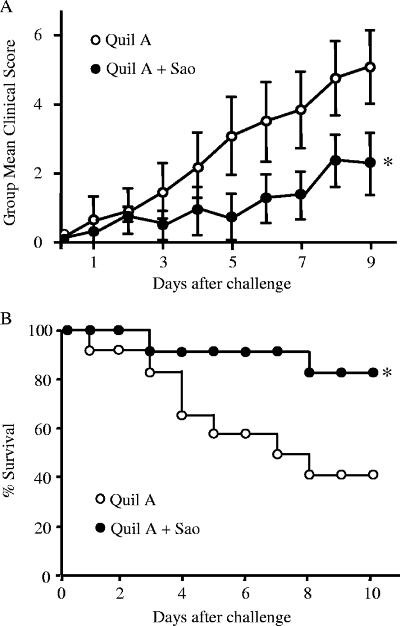
One pig in the Sao-immunized group was humanely killed because of an unrelated disease, and this pig was excluded from analysis of the effects of vaccination on disease. Aerosol challenge of the pigs with S. suis strain 166 resulted in diseases characteristic of S. suis infection. The mean accumulated clinical scores of two groups are presented in Fig. 4A, and clinical signs were significantly less severe in the Sao-vaccinated group than in the control group (P < 0.05). The body temperature data showed no significant difference between the two groups, although the Sao-vaccinated group tended to have lower temperatures (data not shown). In the control group, three pigs died and four more were euthanized due to high clinical scores prior to the end of the experiment, resulting in a survival rate of 42%. In contrast, only two pigs in the Sao-vaccinated group were euthanized, giving a survival rate of 82%. Comparison of survival curves showed that survival time for the Sao-vaccinated group was significantly longer than that for the control group (P < 0.05) (Fig. 4B). Bacterial culture of samples from blood, brain, tracheobronchial lymph nodes, and joints was done to monitor the infection level, and the recovery of bacteria with colonial morphology typical of the challenge strain is summarized in Table 1. Although the number of organs with detectable bacteria for the Sao-vaccinated group was less than that for the control group, only the proportion of positive brain tissue samples from immunized pigs was significantly lower than that from control pigs (P < 0.01) (Table 1).
FIG. 4.
Protection of pigs immunized with Quil A (open circles) or Quil A plus recombinant Sao (solid circles) following challenge with S. suis 166. (A) Clinical scores (daily means and standard deviations) of pigs after challenge. (B) Survival of pigs after challenge. The data are reported for 11 pigs in the Sao-vaccinated group and 12 pigs in the control group. *, P < 0.05.
TABLE 1.
Bacteriological analysis of postmortem samples from pigs immunized with Quil A or Quil A plus recombinant Sao
| Sample type | No. of samples with S. suis recovery/total no. of samples examined
|
P valueb | |
|---|---|---|---|
| Quil A | Quil A + Sao | ||
| Blood | 4/9a | 1/11 | 0.13 |
| Brain | 10/12 | 2/11 | 0.003** |
| Lymph node | 8/12 | 6/11 | 0.68 |
| Joint | 4/12 | 2/11 | 0.64 |
Postmortem blood samples could not be obtained from three pigs that died before they were observed.
**, P < 0.01.
Functional activity of Sao-induced antibodies.
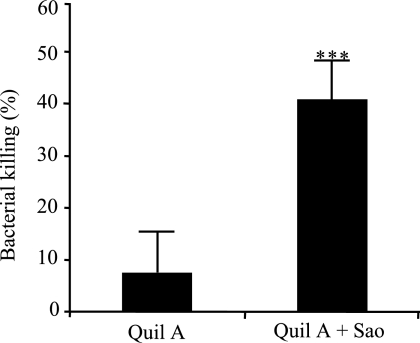
To determine the nature of protection, serum antibodies obtained from Quil A-plus-Sao-vaccinated pigs were compared with antibodies from pigs that received Quil A only for the ability to promote opsonization phagocytosis and killing of S. suis 166 by porcine neutrophils in vitro. As shown in Fig. 5, antibodies from Sao-vaccinated pigs mediated significantly more efficient opsonophagocytic killing of S. suis than antibodies from pigs that received adjuvant alone (P < 0.001).
FIG. 5.
Effect of antibodies on opsonophagocytic killing of S. suis by porcine neutrophils. The antibodies were purified from pooled sera of pigs immunized with Quil A or Quil A plus Sao. Data are expressed as mean percentages ± standard deviations of killed bacteria and are representative of eight independent experiments. ***, P < 0.001.
Immune recognition of Sao protein and its variants.
The specificity of Sao-induced antibodies was demonstrated by Western blotting with sera pooled from Sao-immunized mice, which recognized the Sao protein of wild-type S. suis strains as well as recombinant Sao (Fig. 6). In contrast, sera from nonimmunized control mice did not react with the proteins (data not shown). However, size variation of Sao was noted among the S. suis strains. While S. suis strain S735, from which the Sao protein antigen was produced, expressed an apparent 110-kDa Sao protein similar in size to the antigen, strains 166 and 31533 expressed Sao variants of approximately 100 kDa and 93 kDa, respectively (Fig. 6).
FIG. 6.
Western blot showing variation in Sao protein of S. suis. Culture supernatants of S. suis strain S735 (lane a), strain 166 (lane b), and strain 31533 (lane c) and the purified recombinant Sao protein that was used for immunization (lane d) were separated by SDS-polyacrylamide gel electrophoresis and then transferred to a membrane. The blot was incubated with sera pooled from mice after the second immunization with recombinant Sao. The molecular masses are indicated on the left.
Genetic analysis of Sao variants.
The genes encoding Sao variants were analyzed by PCR. As shown in Fig. 7, the size of sao varied among S. suis strains S735, 166, and 31533 and corresponded to the size of the Sao variants observed in the Western blot. One of the features of the Sao protein is the presence of 10 repeats of a 27-amino-acid sequence separated by 3-amino-acid spacers near the C-terminal end (36). Analysis of the PCR fragments spanning the repeating region of sao suggested that a variable number of repeats accounted for the size differences of sao (Fig. 7). Indeed, DNA sequence analysis revealed a deletion of a 270-bp nucleotide sequence, corresponding to three repeats plus the spacers, in strain 166 and a deletion of a 450-bp sequence, corresponding to five repeats plus the spacers, in strain 31533 compared with the sequence of sao in strain S735.
FIG. 7.
PCR amplification products of the full-length sao gene of S. suis and the DNA fragments flanking the repeats of sao. The variations of complete sao were correspondingly revealed in the differences in the DNA fragments spanning the repeats. Lanes: a, 1-kb DNA ladder (Life Technologies); b, strain S735; c, strain 166; d, strain 31533; and e, 100-bp DNA ladder (Invitrogen).
DISCUSSION
In our continued effort to search for an S. suis protein(s) useful in the development of a vaccine, a highly immunogenic surface protein, Sao, was identified from a virulent strain (S735) of S. suis serotype 2. In a convenient pig immunization protocol, Sao in combination with Emulsigen triggered a predominant production of IgG1 that did not confer protection against S. suis challenge infection (36). In this study, we first used a mouse model to test the protective efficacy of recombinant Sao in combination with Quil A and found that the experimental vaccine induced a predominant generation of IgG2a which confers protection against S. suis infection. This prompted us to further evaluate the protective capacity of Sao in combination with Quil A in pigs, the target host of this potential vaccine. Indeed, it significantly protected the pigs against S. suis infection and disease.
Induction of the appropriate type of antigen-specific immune responses is crucial for the success of vaccines. The IgG subclass produced as a consequence of immunization reflects the type of immune responses. In mice, serum IgG1 is associated with a Th2-type response, whereas serum IgG2a is associated with a Th1-type response, which is particularly effective at mediating bacterial opsonophagocytosis (48). Of the mouse IgG subclasses, IgG2a is the most effective at binding to FcγRI on phagocytic cells (41, 48). Thus, it is likely that predominant IgG2a production in the current mouse vaccination protocol contributed most to the observed protection. However, it is also possible that IgG2a is not the only effector of protection induced by vaccination with Sao. Some studies have shown that IgG2b and IgG3 are also associated with Th1-type immune responses and are critically involved in bacterial opsonophagocytosis and protection against infection with gram-positive pathogens (9, 31, 40, 47). In this pig immunization and challenge protocol, the Sao-induced immune response was characterized by predominant IgG2 production. Although the concept of “Th1/Th2” balance is not yet well documented for pigs, recent evidence showed that porcine IgG2 had greater complement-activating ability than did IgG1 (11).
Adjuvants play an important role in the efficacy of vaccines. The type of adjuvant used can direct the type of immune response generated to an administered antigen (43). It has been shown previously that an appropriate adjuvant is essential in determining the outcome of vaccination and that protection following vaccination is obtained only after switching immune responses to a predominantly Th1 type, such as the case with vaccines against Streptococcus pneumoniae (2, 39), Mycobacterium tuberculosis (37), Chlamydia pneumoniae (3), and Brucella abortus (20). The adjuvant Quil A has been shown to enhance antibody levels and, more importantly, to shift the response towards type 1, thus resulting in the induction of both bactericidal and opsonophagocytic antibodies (12, 29, 30, 50). In our modified pig protocol, it appears that Sao combined with Quil A triggers an adequate immune response bias which consequently leads to protection. To determine the nature of protection, Sao-induced antibodies were analyzed for the ability to promote opsonophagocytic killing of S. suis in the presence of white blood cells, an important immunological correlate of protective immunity against S. suis (7). We found that antibodies purified from the sera of pigs that received two doses of Sao vaccine in combination with Quil A exhibited strong opsonic capacity. Given our previous study showing that Sao combined with Emulsigen triggered a predominant production of IgG1 and that these antibodies lacked opsonophagocytic function (36), this result indicated that Sao in the present formulation may more adequately induce protective antibodies that are capable of triggering leukocyte effector. The enhanced level of opsonizing antibodies is likely related to the predominant generation of IgG2, directed by using Quil A adjuvant. However, it should be emphasized that the type of immune response induced could also be affected by the antigen dose (10, 15, 51). In contrast with our previous study, in which 100 μg of Sao/per pig was used, a dosage of 200 μg was applied in this trial. Although the exact factor(s) crucial in directing the immune response toward the adequate bias was not defined, this study did provide the basis of a suitable formulation for further clinical evaluation of the Sao protein as a vaccine candidate for control of S. suis disease in pigs.
S. suis strain S735, from which the sao gene was originally cloned, was not used for challenging the animals due to a controversial report about its virulence in experimental infection models (8, 49). We previously confirmed that a Sao-specific antibody raised in rabbits cross-reacted with cell lysates of S. suis strain 31533 (unpublished data) and strain 166 (36). Thus, these strains were chosen for challenging animals to investigate the cross-protection of recombinant Sao against heterologous S. suis field strains. The sera from the animals immunized with recombinant Sao recognized size variants of Sao expressed by S. suis field strains, suggesting that differences in Sao among the S. suis strains used in this study do not alter the immune recognition of the recombinant Sao-elicited antibody. One of the features of the Sao protein is the presence of a region of 10 repeats near the C-terminal end (36). Variation of repeat numbers has commonly been observed in bacterial proteins, such as EF of S. suis (45), the M protein of Streptococcus pyogenes (24, 28), and the alpha-like protein of group B streptococcus (33). Therefore, we assumed that the size difference of Sao occurred due to variation in the number of repeats. This was confirmed by DNA sequencing. It has been proposed that the size variation of gram-positive bacterial proteins, such as the M protein, is a mechanism by which organisms can escape from the host immune system (23, 24, 28). However, our study showed that Sao-specific antibody cross-reacted with Sao variants, and moreover, the Sao vaccine offers cross-protection against S. suis strains expressing Sao variants. This discrepancy may result from the structural difference between Sao and the M protein. In the M protein, the highly variable repeat region is present in the N-terminal half and the highly conserved region is present in the C-terminal half (27). Since the M protein is a C-terminally anchored protein, the N terminus extends outwards from the cell and epitopes close to the C terminus may be masked by other cell wall components. As a result, variation in the N terminus alters the ability of certain antibodies, originally produced in response to the parent protein, to bind to the mutant molecules or opsonize the mutant organisms (27). In contrast to the case for the M protein, the variable repeat region in Sao is located in the C-terminal half and the conserved region is located in the N-terminal half (36). Therefore, deletion of some repeats does not render them inaccessible to antibody binding.
In summary, we have shown that recombinant Sao in a vaccine formulation with Quil A triggers strong opsonizing antibody responses which confer protection against experimental S. suis infection. In addition, Sao protects against challenging strains expressing Sao size variants. These findings suggest that Sao is a potential candidate for development of a subunit vaccine against S. suis infection. However, an optimum vaccine formulation remains to be studied.
Acknowledgments
We thank Mariela Segura (McGill University) for scientific exchanges and Marylene Kobisch (AFSSA, Ploufragan, France) for providing strains 31533 and 166.
This work was supported by Valorisation Recherche Quebec (VRQ 2201-141) and the NSERC Canadian Research Network on Bacterial Pathogens of Swine (225155-00).
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Allison, A. C., and N. E. Byars. 1986. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J. Immunol. Methods 95:157-168. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandholtz, L., M. R. Kreuger, C. Svanholm, H. Wigzell, and M. E. Rottenberg. 2002. Adjuvant modulation of the immune responses and the outcome of infection with Chlamydia pneumoniae. Clin. Exp. Immunol. 130:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthelot-Herault, F., R. Cariolet, A. Labbe, M. Gottschalk, J. Y. Cardinal, and M. Kobisch. 2001. Experimental infection of specific pathogen free piglets with French strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 65:196-200. [PMC free article] [PubMed] [Google Scholar]
- 5.Brazeau, C., M. Gottschalk, S. Vincelette, and B. Martineau-Doize. 1996. In vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology 142:1231-1237. [DOI] [PubMed] [Google Scholar]
- 6.Busque, P., R. Higgins, F. Caya, and S. Quessy. 1997. Immunization of pigs against Streptococcus suis serotype 2 infection using a live avirulent strain. Can. J. Vet. Res. 61:275-279. [PMC free article] [PubMed] [Google Scholar]
- 7.Chabot-Roy, G., P. Willson, M. Segura, S. Lacouture, and M. Gottschalk. 2006. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 41:21-32. [DOI] [PubMed] [Google Scholar]
- 8.Charland, N., J. Harel, M. Kobisch, S. Lacasse, and M. Gottschalk. 1998. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144:325-332. [DOI] [PubMed] [Google Scholar]
- 9.Chu, R. S., T. McCool, N. S. Greenspan, J. R. Schreiber, and C. V. Harding. 2000. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect. Immun. 68:1450-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 11.Crawley, A., and B. N. Wilkie. 2003. Porcine Ig isotypes: function and molecular characteristics. Vaccine 21:2911-2922. [DOI] [PubMed] [Google Scholar]
- 12.DeVelasco, E. A., H. A. Dekker, P. Antal, K. P. Jalink, J. A. van Strijp, A. F. Verheul, J. Verhoef, and H. Snippe. 1994. Adjuvant Quil A improves protection in mice and enhances opsonic capacity of antisera induced by pneumococcal polysaccharide conjugate vaccines. Vaccine 12:1419-1422. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, S. D., F. Clifton-Hadley, and J. Tai. 1980. Streptococcal infection in young pigs. V. An immunogenic polysaccharide from Streptococcus suis type 2 with particular reference to vaccination against streptococcal meningitis in pigs. J. Hyg. 85:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galina, L., U. Vecht, H. J. Wisselink, and C. Pijoan. 1996. Prevalence of various phenotypes of Streptococcus suis isolated from swine in the USA based on the presence of muraminidase-released protein and extracellular factor. Can. J. Vet. Res. 60:72-74. [PMC free article] [PubMed] [Google Scholar]
- 15.Golding, B., and D. E. Scott. 1995. Vaccine strategies: targeting helper T cell responses. Ann. N. Y. Acad. Sci. 754:126-137. [DOI] [PubMed] [Google Scholar]
- 16.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 18.Haesebrouck, F., F. Pasmans, K. Chiers, D. Maes, R. Ducatelle, and A. Decostere. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100:255-268. [DOI] [PubMed] [Google Scholar]
- 19.Halbur, P., R. Thanawongnuwech, G. Brown, J. Kinyon, J. Roth, E. Thacker, and B. Thacker. 2000. Efficacy of antimicrobial treatments and vaccination regimens for control of porcine reproductive and respiratory syndrome virus and Streptococcus suis coinfection of nursery pigs. J. Clin. Microbiol. 38:1156-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, Y., R. Vemulapalli, and G. G. Schurig. 2002. Recombinant Ochrobactrum anthropi expressing Brucella abortus Cu, Zn superoxide dismutase protects mice against B. abortus infection only after switching of immune responses to Th1 type. Infect. Immun. 70:2535-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins, R., and M. Gottschalk. 2005. Streptococcal diseases, p. 769-783. In S. D. B. E. Straw, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 9th ed. Iowa State University Press, Ames, IA.
- 22.Higgins, R., M. Gottschalk, K. R. Mittal, and M. Beaudoin. 1990. Streptococcus suis infection in swine. A sixteen month study. Can. J. Vet. Res. 54:170-173. [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingshead, S. K., V. A. Fischetti, and J. R. Scott. 1987. A highly conserved region present in transcripts encoding heterologous M proteins of group A streptococci. Infect. Immun. 55:3237-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollingshead, S. K., V. A. Fischetti, and J. R. Scott. 1987. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol. Gen. Genet. 207:196-203. [DOI] [PubMed] [Google Scholar]
- 25.Holt, M. E., M. R. Enright, and T. J. Alexander. 1988. Immunisation of pigs with live cultures of Streptococcus suis type 2. Res. Vet. Sci. 45:349-352. [PubMed] [Google Scholar]
- 26.Jacobs, A. A., A. J. van den Berg, and P. L. Loeffen. 1996. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet. Rec. 139:225-228. [DOI] [PubMed] [Google Scholar]
- 27.Jacques, M., M. Gottschalk, B. Foiry, and R. Higgins. 1990. Ultrastructural study of surface components of Streptococcus suis. J. Bacteriol. 172:2833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, K. F., S. K. Hollingshead, J. R. Scott, and V. A. Fischetti. 1988. Spontaneous M6 protein size mutants of group A streptococci display variation in antigenic and opsonogenic epitopes. Proc. Natl. Acad. Sci. USA 85:8271-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karagouni, E. E., and L. Hadjipetrou-Kourounakis. 1990. Regulation of isotype immunoglobulin production by adjuvants in vivo. Scand. J. Immunol. 31:745-754. [DOI] [PubMed] [Google Scholar]
- 30.Kenney, J. S., B. W. Hughes, M. P. Masada, and A. C. Allison. 1989. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J. Immunol. Methods 121:157-166. [DOI] [PubMed] [Google Scholar]
- 31.Khan, A. Q., Q. Chen, Z. Q. Wu, J. C. Paton, and C. M. Snapper. 2005. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on Toll-like receptor 2. Infect. Immun. 73:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobisch, M., P. Morvan, R. Cariolet, G. Benevent, and J. P. Joly. 1995. Experimental infection of SPF piglets with Streptococcus suis serotype 2. J. Rech. Porc. France 27:97-102. [Google Scholar]
- 33.Lachenauer, C. S., R. Creti, J. L. Michel, and L. C. Madoff. 2000. Mosaicism in the alpha-like protein genes of group B streptococci. Proc. Natl. Acad. Sci. USA 97:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapointe, L., S. D'Allaire, A. Lebrun, S. Lacouture, and M. Gottschalk. 2002. Antibody response to an autogenous vaccine and serologic profile for Streptococcus suis capsular type 1/2. Can. J. Vet. Res. 66:8-14. [PMC free article] [PubMed] [Google Scholar]
- 35.Lefeber, D. J., B. Benaissa-Trouw, J. F. Vliegenthart, J. P. Kamerling, W. T. Jansen, K. Kraaijeveld, and H. Snippe. 2003. Th1-directing adjuvants increase the immunogenicity of oligosaccharide-protein conjugate vaccines related to Streptococcus pneumoniae type 3. Infect. Immun. 71:6915-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Y., G. Martinez, M. Gottschalk, S. Lacouture, P. Willson, J. D. Dubreuil, M. Jacques, and J. Harel. 2006. Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect. Immun. 74:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lun, S., J. Perez-Casal, W. Connor, and P. J. Willson. 2003. Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb. Pathog. 34:27-37. [DOI] [PubMed] [Google Scholar]
- 39.Lynch, J. M., D. E. Briles, and D. W. Metzger. 2003. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 71:4780-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaelsen, T. E., J. Kolberg, A. Aase, T. K. Herstad, and E. A. Hoiby. 2004. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 59:34-39. [DOI] [PubMed] [Google Scholar]
- 41.Neuberger, M. S., and K. Rajewsky. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012-1016. [DOI] [PubMed] [Google Scholar]
- 42.Normile, D. 2005. Infectious diseases. WHO probes deadliness of China's pig-borne disease. Science 309:1308-1309. [DOI] [PubMed] [Google Scholar]
- 43.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 44.Okwumabua, O., O. Abdelmagid, and M. M. Chengappa. 1999. Hybridization analysis of the gene encoding a hemolysin (suilysin) of Streptococcus suis type 2: evidence for the absence of the gene in some isolates. FEMS Microbiol. Lett. 181:113-121. [DOI] [PubMed] [Google Scholar]
- 45.Smith, H. E., F. H. Reek, U. Vecht, A. L. Gielkens, and M. A. Smits. 1993. Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type 2 are absent in pathogenic strains. Infect. Immun. 61:3318-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staats, J. J., I. Feder, O. Okwumabua, and M. M. Chengappa. 1997. Streptococcus suis: past and present. Vet. Res. Commun. 21:381-407. [DOI] [PubMed] [Google Scholar]
- 47.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 48.Unkeless, J. C., E. Scigliano, and V. H. Freedman. 1988. Structure and function of human and murine receptors for IgG. Annu. Rev. Immunol. 6:251-281. [DOI] [PubMed] [Google Scholar]
- 49.Vecht, U., H. J. Wisselink, N. Stockhofe-Zurwieden, and H. E. Smith. 1996. Characterization of virulence of the Streptococcus suis serotype 2 reference strain Henrichsen S 735 in newborn gnotobiotic pigs. Vet. Microbiol. 51:125-136. [DOI] [PubMed] [Google Scholar]
- 50.Verheul, A. F., J. A. Van Gaans, E. J. Wiertz, H. Snippe, J. Verhoef, and J. T. Poolman. 1993. Meningococcal lipopolysaccharide (LPS)-derived oligosaccharide-protein conjugates evoke outer membrane protein- but not LPS-specific bactericidal antibodies in mice: influence of adjuvants. Infect. Immun. 61:187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, P., K. Nakamura, Y. Mimura, K. Takeo, T. Tanaka, and M. Fujimoto. 1996. Immune response to different doses of a hapten of fluorescein isothiocyanate analyzed by two-dimensional affinity electrophoresis. Electrophoresis 17:1273-1279. [DOI] [PubMed] [Google Scholar]
- 52.Wisselink, H. J., N. Stockhofe-Zurwieden, L. A. Hilgers, and H. E. Smith. 2002. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet. Microbiol. 84:155-168. [DOI] [PubMed] [Google Scholar]
- 53.Wisselink, H. J., U. Vecht, N. Stockhofe-Zurwieden, and H. E. Smith. 2001. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet. Rec. 148:473-477. [DOI] [PubMed] [Google Scholar]