Abstract
Brucellosis is a zoonotic disease with a worldwide distribution that can be transmitted via intentional or accidental aerosol exposure. In order to engineer superior vaccine strains against Brucella species for use in animals as well as in humans, the possibility of challenge infection via aerosol needs to be considered to properly evaluate vaccine efficacy. In this study, we assessed the use of an aerosol chamber to infect deep lung tissue of mice to elicit systemic infections with either Brucella abortus or B. melitensis at various doses. The results reveal that B. abortus causes a chronic infection of lung tissue in BALB/c mice and peripheral organs at low doses. In contrast, B. melitensis infection diminishes more rapidly, and higher infectious doses are required to obtain infection rates in animals similar to those of B. abortus. Whether this difference translates to severity of human infection remains to be elucidated. Despite these differences, unmarked deletion mutants BAΔasp24 and BMΔasp24 consistently confer superior protection to mice against homologous and heterologous aerosol challenge infection and should be considered viable candidates as vaccine strains against brucellosis.
Brucellosis is a zoonosis affecting numerous species of domestic animals, wildlife, and humans (13-15). Humans are commonly infected as a result of contact with infected animals, ingestion of contaminated animal products such as milk, milk products, or meat, or laboratory exposure. Abattoir workers may also acquire the disease via aerosol exposure (2, 3, 13-15, 18, 31). The Brucella species most pathogenic to humans include Brucella melitensis, B. suis, B. abortus, and B. canis, all of which are distributed worldwide but are most common in Mediterranean countries, the Middle East, India, Mexico, Central Asia, and Central and South America (3, 10, 13, 17, 22, 23). Brucellosis has been reported to be the most common zoonotic infection worldwide, with over 500,000 new infections reported annually (22, 23). Human disease manifests as prolonged febrile illness (undulant fever), flu-like symptoms, night sweats, headache, depression, and arthritis, and infection can lead to chronic illness, such as meningitis and endocarditis (2, 3, 8, 14, 22, 31).
Documented evidence of aerosol transmission of these organisms has emphasized the recent focus on the use of Brucella as a potential bioterrorism agent (5, 20). In the 1950s, B. suis was the first agent weaponized in the United States, and Brucella has been evaluated for this purpose by several other countries as well (3, 22). It has been estimated that as few as 10 to 100 organisms comprise an infectious aerosol dose in humans, and Brucella is therefore considered highly infectious when it is delivered in this manner (3). As a result, B. melitensis, B. abortus, and B. suis have been classified as category B agents by the Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases (1, 22). The absence of a safe and efficacious vaccine for use in humans underscores the concern.
In order to engineer superior vaccine strains against Brucella species for use in animals as well as humans, the potential for challenge infection via aerosol exposure needs to be considered to properly evaluate vaccine efficacy. Previous studies have considered intranasal infection of mice and guinea pigs as models for aerosol exposure (1, 2, 10, 12, 13, 18, 29). In this study, we evaluated the kinetics of systemic infection in the mouse model after aerosol exposure of deep lung tissue to both B. abortus and B. melitensis in order to establish a novel exposure route in the mouse model. This route of infection was utilized to evaluate the protective efficacy of selected Brucella mutants previously shown by our lab to elicit significant protection in mouse and goat models (14, 15).
MATERIALS AND METHODS
Bacteria and bacterial culture.
The B. abortus 2308 and B. melitensis 16M wild-type strains used for challenge, as well as unmarked deletion strains used for vaccination studies, were routinely grown on tryptic soy agar (TSA) (Difco Laboratories) at 37°C in an atmosphere containing 5% (vol/vol) CO2. Virulent B. abortus strain S2308 was obtained from Billy Deyoe at the National Animal Disease Center in Ames, IA. B. melitensis biovar 1 (16M) was originally obtained from ATCC and was reisolated from an aborted goat fetus (14). Unmarked deletion strains used in this study (BAΔasp24, BMΔasp24, BAΔvirB2, BMΔvirB2, BAΔmanBA, and BMΔmanBA) were engineered previously and tested for survival and protective efficacy in BALB/c mice (15). All bacterial strains were stored frozen at −80°C in medium supplemented with 50% (vol/vol) glycerol and grown on TSA for immediate use in each experiment. Bacteria were harvested into phosphate-buffered saline (PBS) (pH 7.4; Gibco) to obtain the final concentration needed for each experiment, as estimated turbidometrically using a Klett meter. Serial dilution was performed retrospectively to accurately determine the number of organisms in the inoculum. Lung, liver, and spleen samples from mice were plated onto Farrell's medium to select for Brucella. Farrell's medium is TSA supplemented with 5 mg/liter nalidixic acid, 25,000 IU/liter bacitracin, 100 mg/liter cycloheximide, 5000 IU/liter polymyxin B sulfate, 20 mg/liter vancomycin, 100,000 IU/liter nystatin (Brucella selective supplement; Oxoid), 10% (vol/vol) horse serum, and 2% (wt/vol) dextrose).
Kinetics of aerosol infection of Brucella in mice.
The survival or persistence of wild-type strain B. abortus 2308 or B. melitensis 16M was evaluated using groups of 6- to 8-week-old female BALB/c mice (Jackson Laboratories) following aerosol exposure via a Madison aerosol chamber (College of Engineering Shops, University of Wisconsin, Madison) (30) to three different doses of Brucella added to the chamber nebulizer: 5 × 107, 5 × 108, and 5 × 109 CFU/ml. The actual numbers of infectious organisms inhaled by the mice at each dose were determined directly by euthanizing a group of four or five mice immediately after removal from the chamber via carbon dioxide asphyxiation, homogenizing the lungs in 1 ml PBS, and plating the preparations onto Farrell's medium to determine the number of CFU in the combined lung tissue for each mouse. For kinetic studies, mice were euthanized at 1, 2, 4, 6, or 8 weeks postinfection. At each time point, the lungs, liver, and spleen were collected and weighed, homogenized in 1 ml PBS, and serially diluted, and 200 μl of each dilution was plated onto Farrell's medium. Recovered bacteria were enumerated to evaluate the persistence of each strain.
Efficacy studies.
The mouse model was used to evaluate the efficacies of unmarked deletion mutants (previously shown to protect mice against virulent Brucella by intraperitoneal [i.p.] challenge) against subsequent aerosol challenge. Groups of four or five female 6- to 8-week-old BALB/c mice were vaccinated via i.p. injection of 1 × 106 CFU/ml of unmarked deletion mutant or PBS for naïve controls. Mice were subsequently challenged with an aerosol chamber dose of 5 × 109 CFU/ml of the homologous wild-type strain at 20 weeks postvaccination. Four weeks after the virulent challenge (corresponding to 24 weeks postvaccination), the mice were euthanized, and the lungs, liver, and spleen were extracted, weighed, homogenized in 1 ml PBS, serially diluted, and plated onto Farrell's medium to measure recovery of the challenge organism.
To evaluate cross-species protection, groups of four or five female 6- to 8-week-old BALB/c mice were vaccinated with either the BAΔasp24 or BMΔasp24 unmarked mutant strain and challenged as described above with the heterologous wild-type strain 20 weeks postvaccination. Four weeks after the virulent challenge, the mice were euthanized, and the challenge organisms recovered were enumerated as described above.
For both experiments, vaccine efficacy is described in the text as a measure of protective immunity or units of protection (U), representing differences in bacterial burden in the spleen of challenge organism for naïve and vaccinated mice (log10 wild type recovered from unvaccinated mice − log10 wild type recovered from vaccinated mice).
Statistical analysis.
Data from aerosol infection kinetics and efficacy studies were expressed as mean CFU ± standard error and are presented below in graphs as the log10 Brucella CFU recovered per organ. Culture-negative organs were assigned a value of 4 CFU/organ, which is below the limit of detection of 5 CFU/organ. Spleen weight data from aerosol kinetics studies were plotted as the mean spleen weight (in mg) ± standard error. The statistical significance of differences between vaccinated animals was evaluated by analysis of variance (ANOVA) by comparing the spleen weights for a group of five naïve, unvaccinated BALB/c control mice (data not shown) to the spleen weights for all mice receiving the same chamber dose inoculum, followed by Dunnett's multiple-comparison test.
In efficacy studies vaccinated and subsequently challenged mice were compared to mice receiving PBS as a vaccine control that were challenged with the wild-type organism. The statistical significance of differences between vaccinated animals was analyzed by ANOVA followed by Tukey's honestly significant difference posttest comparing all groups to one another. For all ANOVAs, P values less than 0.05 were considered statistically significant.
RESULTS
Kinetics of aerosol infection with B. abortus.
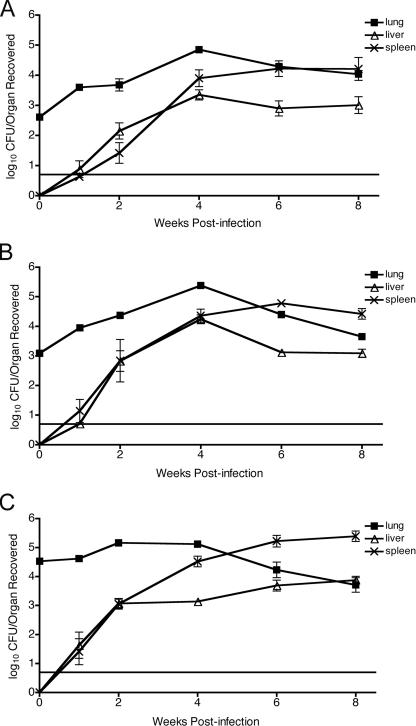
Mice were infected with three different doses of B. abortus 2308 via aerosol challenge to evaluate the kinetics of infection in the lungs, livers, and spleens. Mice receiving a dose of 5 × 107 CFU/ml added to the chamber nebulizer actually inhaled much less challenge organism into the total lung tissue; the average was determined to be 415 organisms per mouse (2.62 logs) (Fig. 1A). At this dose, lung colonization with 2308 gradually increased over the first 4 weeks postchallenge and then gradually decreased over the following 4 weeks to 90% of the maximum value. Despite this slight decrease, colonization by the organism in the other tissues was consistent with a chronic infection. Colonization of the liver, although barely detectable at 1 week postchallenge, steadily increased over the first 4 weeks postchallenge and then declined negligibly between weeks 4 and 8. The spleens of infected mice displayed a colonization pattern similar to that of the livers, although the total number of CFU recovered was consistently higher. Spleen colonization increased between weeks 4 and 8, consistent with a persistent infection.
FIG. 1.
Kinetics of clearance of B. abortus 2308 from BALB/c mice. Four or five female BALB/c mice were infected with aerosolized 2308 in a Madison aerosol chamber using three different chamber doses, 5 × 107 CFU/ml (A), 5 × 108 CFU/ml (B), or 5 × 109 CFU/ml (C). The initial lung colonization was evaluated immediately after challenge to determine the quantity of Brucella inhaled for each chamber dose. Mice were euthanized at 1, 2, 4, 6, or 8 weeks postchallenge to determine the numbers of Brucella persisting in the lungs, livers, and spleens. The recovery of organisms is plotted as the total CFU/organ (means ± standard errors). The solid line at 0.7 log represents the lower limit of detection, which is ≥5 CFU.
Mice receiving doses of 5 × 108 and 5 × 109 CFU/ml added to the chamber nebulizer on average inhaled 1,266 and 34,560 organisms per mouse (3.1 and 4.54 logs), respectively (Fig. 1B and 1C). Other than the difference in the numbers of organisms inhaled and a 0.5- to 1.0-log increase in lung colonization, the difference in the persistence of the organism in each of the tissues evaluated was negligible.
Kinetics of aerosol infection with B. melitensis.
As described above for B. abortus, mice were infected with three different doses of B. melitensis 16M via the aerosol route to evaluate the kinetics of infection in the lungs, livers, and spleens. Mice receiving a dose of 5 × 107 CFU/ml added to the chamber nebulizer inhaled an average of 805 organisms per mouse (2.91 logs) (Fig. 2A). Mice receiving a dose of 5 × 108 CFU/ml added to the chamber nebulizer inhaled an average of 7,988 organisms per mouse (3.9 logs) (Fig. 2B). Mice receiving a dose of 5 × 109 CFU/ml added to the chamber nebulizer inhaled an average of 12,520 organisms per mouse (4.10 logs) (Fig. 2C). It is not clear whether the failure to record a 10-fold increase in inhaled organisms at the highest dose was the result of the viscosity of the inoculum (i.e., the more concentrated culture did not pass through the nebulizer as well as lower doses) or the breathing patterns of the mice in the last chamber run.
FIG. 2.
Kinetics of clearance of B. melitensis 16M from BALB/c mice. Four or five female BALB/c mice were infected with aerosolized 16M in a Madison aerosol chamber using three different chamber doses, 5 × 107 CFU/ml (A), 5 × 108 CFU/ml (B), or 5 × 109 CFU/ml (C). The initial lung colonization was determined immediately after challenge to determine the quantity of Brucella inhaled for each chamber dose. Mice were euthanized at 1, 2, 4, 6, or 8 weeks postchallenge to determine the numbers of Brucella persisting in the lungs, livers, and spleens. The recovery of organisms is plotted as the total CFU/organ (means ± standard errors). The solid line at 0.7 log represents the lower limit of detection, which is ≥5 CFU.
At the lowest dose, lung, liver, and spleen colonization with 16M displayed an erratic colonization pattern with large variations between mice, despite the fact that these mice received on average a higher dose than animals infected with B. abortus. Overall, the increased levels of colonization in all tissues (aside from the initial lung colonization immediately after exposure) peaked by 4 weeks after exposure, although the levels were lower than the challenge dose. Tissue colonization followed the same profile over the first 4 weeks, with liver and spleen colonization subsequent to lung colonization, and reached a peak by 4 weeks postexposure. However, in contrast to colonization by B. abortus, systemic colonization of the liver and spleen waned over the next 4 weeks, and there was a 90 to 99% reduction in the number of CFU.
Splenomegaly induced by aerosol exposure.
The colonization profiles described above parallel those observed for i.p. inoculations and, except for lung involvement, confirm that introduction via aerosol exposure results in a similar course of systemic infection. As an alternative measure of disease in the mice, spleen weights were also recorded to observe splenomegaly patterns. Mice infected with B. abortus did not have significant splenomegaly until 6 weeks postinfection with the 5 × 108 and 5 × 109 CFU/ml chamber doses or until 8 weeks postinfection with the lower dose (5 × 107 CFU/ml) (Fig. 3A). This inflammation correlated with the levels of bacteria in the spleen, which began to peak or plateau at 4 to 6 weeks postinfection depending upon the dose. This pattern is analogous to that observed following i.p. inoculation, where splenomegaly mirrored spleen colonization but was delayed.
FIG. 3.
Evaluation of splenomegaly induced by Brucella aerosol challenge. Spleen weights of mice infected in B. abortus (A) and B. melitensis (B) aerosol kinetics studies were recorded as a measure of splenomegaly induced by the infection. The data are the averages and standard errors for all mice in a treatment group. Statistical significance was determined by ANOVA, followed by Dunnett's multiple-comparison test comparing the spleen weights of a group of five unchallenged BALB/c mice to the spleen weights of all mice receiving the same chamber inoculum dose. The dotted line represents the average spleen weight of uninfected mice (96 mg). One asterisk, P < 0.05; two asterisks, P < 0.01.
Mice infected with the lowest chamber dose of 16M did not display significant splenomegaly, which correlated with the erratic infection observed (Fig. 3B). Mice infected at the 5 × 108-CFU/ml chamber dose presented with significant splenomegaly 6 weeks postinfection, subsequent to the peak bacterial load observed in the spleen. Mice infected at the highest chamber dose, 5 × 109 CFU/ml, exhibited splenomegaly earlier, at 4 weeks postinfection, paralleling the increased spleen colonization evident at 1 week postchallenge.
Protective efficacy against homologous B. abortus aerosol challenge.
To evaluate the vaccine potential of selected B. abortus unmarked mutants BAΔasp24, BAΔvirB2, and BAΔmanBA against virulent aerosol infection, the level of protection provided against wild-type colonization was assessed. Protective efficacy was expressed as a value relative to the nonvaccinated controls challenged with the wild type at each time point, which was obtained by subtracting the mean number of CFU/organ recovered from vaccinated mice from the mean number of CFU/organ recovered from age-matched nonvaccinated aerosol-challenged controls. The highest chamber dose, 5 × 109 CFU/ml, was chosen for the challenge inoculum, and mice were challenged with the wild type (S2308) 20 weeks after i.p. vaccination with each mutant. Colonization by the wild type was determined 4 weeks after challenge (corresponding to 24 weeks postvaccination) in the spleen, liver, and lungs.
As described previously for i.p. challenge and as described above, the spleens of mice typically have the highest bacterial burden and spleen tissue is the most useful tissue for monitoring Brucella infection. In these experiments the bacterial burden in the spleen following aerosol challenge was reduced 2.02 U by vaccination with BAΔasp24 and by 0.86 U by vaccination with BAΔvirB2 relative to the burden in naïve mice (P < 0.001 and P < 0.05, respectively) (Fig. 4C). In contrast, the rough BAΔmanBA mutant was unable to elicit significant protective immunity relative to the immunity of naïve mice (−0.06 U; P > 0.05).
FIG. 4.
Evaluation of the efficacy of vaccination against B. abortus aerosol challenge. Groups of four or five female 6- to 8-week-old BALB/c mice were vaccinated via i.p. injection of 1 × 106 CFU/ml of unmarked deletion mutant BAΔasp24, BAvirB2, BAΔmanBA, or BMΔasp24 or PBS for naïve controls. Mice were subsequently challenged with an aerosol chamber dose of 5 × 109 CFU/ml B. abortus 2308 at 20 weeks postvaccination (heterologous challenge for BMΔasp24). Four weeks after the virulent challenge, the mice were euthanized, and lungs (A), livers (B), and spleens (C) were extracted to measure the recovery of the challenge organisms. Data are expressed as the log10 recovery of 2308 from the entire organ, and the results for individual mice in each treatment group are shown. The solid line at 0.7 log represents the lower limit of detection, which is ≥5 CFU. For comparisons with naïve control animals using ANOVA with Tukey's honestly significant difference posttest, one asterisk indicates that the P value is <0.05 and two asterisks indicates that the P value is <0.01.
At 24 weeks postvaccination, less protection was observed in the lungs of mice vaccinated with BAΔasp24 (0.83 U) and BAΔvirB2 (0.43 U), although the protection was significantly greater than that observed for naïve controls (P < 0.001 and P < 0.05, respectively) (Fig. 4A). The rough mutant, BAΔmanBA, protected mouse lungs to a lesser degree (0.35 U, which was not significant).
Despite the large size of the liver, the colonization was 2 logs less than that observed in either the spleen or lung, and although the total recovery of wild-type organisms from livers was reduced by an average of 1.04 U, there were too few animals to provide statistical significance (Fig. 4B). Furthermore, the variation between mice in the BAΔasp24-vaccinated group was substantial, possibly masking the degree of protection afforded in the liver, since for two of five mice no challenge organisms were recovered from the liver. No protection was evident in BAΔvirB2- or BAΔmanBA-vaccinated animals (−0.03 and −0.44 U of protection, respectively).
Protective efficacy against homologous B. melitensis aerosol challenge.
Mice were vaccinated i.p. with B. melitensis unmarked deletion strains BMΔasp24, BMΔvirB2, and BMΔmanBA and allowed to rest for 20 weeks prior to aerosol challenge with 16M. The highest chamber dose, 5 × 109 CFU/ml 16M, was chosen for the challenge inoculum. The mice were euthanized 4 weeks postchallenge (corresponding to 24 weeks postvaccination).
Spleens of mice were highly protected against aerosol 16M challenge by BMΔasp24 (3.99 U), and for three of five mice no recoverable 16M was detected in the spleen (P < 0.001) (Fig. 5C). BMΔmanBA-vaccinated mice were also significantly protected compared to naïve controls (1.59 U) (P < 0.05). BMΔvirB2 was unable to elicit significant protective immunity in the spleens (0.92 U; P > 0.05).
FIG. 5.
Evaluation of the efficacy of vaccination against B. melitensis aerosol challenge. Groups of four or five female 6- to 8-week-old BALB/c mice were vaccinated via i.p. injection of 1 × 106 CFU/ml of unmarked deletion mutant BMΔasp24, BMvirB2, BMΔmanBA, or BAΔasp24 or PBS in naïve controls. Mice were subsequently challenged with an aerosol chamber dose of 5 × 109 CFU/ml B. melitensis 16M at 20 weeks postvaccination (heterologous challenge for BAΔasp24). Four weeks after the virulent challenge, the mice were euthanized, and lungs (A), livers (B), and spleens (C) were extracted to measure recovery of the challenge organism. Data are expressed as the log10 recovery of 16M from the entire organ, and the results for individual mice in each treatment group are shown. The solid line at 0.7 log represents the lower limit of detection, which is ≥5 CFU. For comparisons with naïve control animals using ANOVA with Tukey's honestly significant difference posttest, one asterisk indicates that the P value is <0.05 and two asterisks indicates that the P value is <0.01.
Lung colonization in mice indicated that there were much lower levels of protection against virulent 16M challenge by vaccination with BMΔasp24 (1.72 U) and BMΔvirB2 (1.22 U), but the levels were significantly greater than those for naïve controls (P < 0.001 for both) (Fig. 5A). The rough mutant, BMΔmanBA, protected mouse lungs to a lesser degree (0.25 U, which was not significant).
In contrast to B. abortus colonization, B. melitensis reached higher levels in the tissues, providing statistically significant results. As a result, significant levels of protection against 16M aerosol infection were observed after vaccination with BMΔasp24 (2.78 U), and three of five mice had no recoverable 16M in their livers (P < 0.001) (Fig. 5B). The livers of mice vaccinated with BMΔmanBA also exhibited significant protection compared to naïve controls (1.83U) (P < 0.001). BMΔvirB2 also protected the livers (0.51 U, which was not significant).
Protective efficacy against heterologous aerosol challenge with B. abortus and B. melitensis.
Heterologous challenges of vaccinated mice were performed to evaluate if the highly protective mutants, BAΔasp24 and BMΔasp24, elicit cross-species protection. When mice were challenged with 2308, the BMΔasp24 mutant significantly protected mouse lungs (1.42 U) (P < 0.001) and spleens (3.13 U) (P < 0.01) (Fig. 4A and 4C). In this case, organisms were cultured from only two of four mouse spleens. The livers were not significantly protected, although there was an evident reduction (0.96 U) (Fig. 4B). When mice were challenged with 16M, the BAΔasp24 mutant significantly protected the lungs (1.51 U) (Fig. 5A) (P < 0.001). In the liver, mice were protected (2.65 U), and the challenge organism was not recovered from three of five mice (Fig. 5B) (P < 0.001). The spleens of mice were significantly protected (4.25 U), and the challenge organism was not recovered from three of five mice (Fig. 5C) (P < 0.001).
DISCUSSION
Human brucellosis is often associated with aerosol transmission and is therefore considered a biowarfare threat. Protection afforded by novel Brucella vaccine strains should therefore consider challenge via inhalation of infectious organism to evaluate vaccine efficacy. Aerosol chambers have been successfully used to study infections with several different organisms, including Mycobacterium tuberculosis, Bacillus anthracis, and Coxiella burnetti in mice and guinea pigs (11, 16, 25-27). Aerosol exposure has also been utilized previously to infect rhesus macaques with B. melitensis, but this is the first evaluation of aerosol infection in mice, which have been widely used to model human infections (17).
In this study, a Madison aerosol chamber was utilized to infect groups of mice with B. abortus or B. melitensis. Use of the Madison chamber leads to infection of deep lung tissue, specifically the delivery of droplet nuclei to alveolar spaces, as might be experienced as a result of inhalation during aerosol exposure (26, 30). Ranges of inoculum doses were evaluated to determine differences in the levels of infectivity between the two bacterial species, as well as to establish the kinetics of systemic infection. Under these controlled conditions in an enclosed environment, it was found that as little as a 5-min exposure to 5 ×107 CFU/ml is sufficient to cause infection in the lungs and systemically in mice. However, consistent results for B. melitensis required exposure to doses that were 10 to 100 times greater. Although organisms were detected on the fur of animals using cotton swabs, the recovery data suggested that the levels were in the range from 100 to 1,000 organisms per mouse, which is well below the oral dose necessary for infection (12).
It has been previously reported that variation in the Brucella exposure dose in primates alters the kinetics of trafficking to peripheral organs (18). This effect was also evident here for the murine model after aerosol inoculation. B. abortus infection of mice via the aerosol route results in immediate colonization of lung tissue that is sustained or increases over time, indicating a capacity to replicate within the lung. Bacterial colonization of livers and spleens was delayed as long as 2 weeks before noticeable colonization in these organs became evident, and this colonization occurred without diminution within the lungs, consistent with replication of the organism. B. melitensis infections of mice (including lung tissue) appeared to be variable early in infection at lower doses compared to B. abortus infections. Significant systemic colonization of spleens and livers was evident as early as 1 week postinfection only in the group receiving the highest dose and occurred 2 weeks postinfection with lower challenge doses.
Comparison of the durations of Brucella infections in mice exposed by the i.p. and intravenous routes also revealed a more persistent and chronic infection with B. abortus than with B. melitensis (6, 7, 9, 15, 21). Although the present studies considered time points up to only 8 weeks postinfection, a similar trend was observed with the aerosol exposure described here. It may be concluded that B. abortus infection via the aerosol route elicits a persistent and relatively constant bacteremia in BALB/c mice, requiring a much lower inoculum dose than that required for B. melitensis. Persistence in the lung and the chronic nature of the infection appear to extend the period of optimum systemic colonization for both organisms.
Splenomegaly is a well-known clinical manifestation associated with Brucella infection that correlates with increased numbers of mononuclear cells and appears to be dependent on the bacterial burden (9, 17-19, 28). Splenomegaly was evident after aerosol exposure with both B. abortus, with which splenomegaly was delayed until approximately 6 weeks postexposure, and B. melitensis, with which splenomegaly was evident earlier at 4 weeks postexposure. Overall, the gross size of the spleens was not as great as the gross sizes observed when other routes of infection were used, although splenomegaly remained a significant marker of disease in these mice (4, 9, 28).
The highest chamber dose, 5 × 109 CFU/ml, was chosen as the challenge inoculum in efficacy trials. This dose closely mimics the doses used in previous efficacy studies for the same vaccine strains when mice were challenged with 1 × 104 CFU/mouse via the i.p. route (15). The mice in the present experiments received 3.3 × 104 CFU 2308/mouse or 1.3 × 104 CFU 16M/mouse via the aerosol route, and therefore the levels of protection afforded, particularly in the spleens, could be compared for the two studies. It is important to note, however, that in previous studies mice were euthanized 1 week postchallenge, whereas here they were euthanized 4 weeks postchallenge. The 4-week time point was chosen to allow adequate infection of the peripheral organs in order to properly evaluate efficacy.
It is interesting to observe that, particularly for B. abortus challenges, the protection afforded to the lungs against infection is not as marked (although for three vaccine strains it is significant) as the protection afforded to the spleen. There are several possible explanations for this observation. The first and most obvious possible explanation is the use of the i.p. route of vaccination. Although not known with certainty, organisms are not expected to traffic to lung tissue, and therefore the lungs may not elicit the same immune response upon challenge infection that would be expected if they were primed by vaccination. Alternatively, it is possible that a lack or diminishment of an inflammatory response in the lungs masks the efficacy of vaccination. It has been previously demonstrated that lungs of mice inoculated intranasally with 16M, although colonized, do not show substantial histologic changes associated with the infection or an indication of inflammatory responses (18). In addition, rhesus macaques infected with 16M via the aerosol route did not develop significant pathological lesions in lung tissues (17). Epithelial cells of the lungs have been shown to control inflammation and immune responses in the airways and alveoli (24). Protection against aerosol challenge may therefore be enhanced if the vaccine strain itself is delivered via mucosal routes, including oral or nasal delivery.
Another possible explanation for the persistence of B. abortus in the lung tissue, even in vaccinated mice, is that B. abortus challenge was performed with a dose that overwhelmed the immune response. Particularly for B. abortus, we demonstrated that persistent infection also resulted from aerosol exposure at doses that were 10- to 100-fold reduced, and therefore lower doses could be used for challenge.
In this study, we established a novel infection model of Brucella infection in BALB/c mice that parallels natural exposure. We showed that consistent B. abortus aerosol infection of mice is possible using as few as 4 × 102 organisms per animal, whereas infection with B. melitensis requires between 8 × 103 and 1 × 104 organisms per mouse to be consistent. It was also demonstrated that the BAΔasp24 and BMΔasp24 unmarked deletion mutants, which have been previously shown to elicit superior protection against i.p. challenge, also evoke significant protection against B. abortus and B. melitensis homologous and heterologous aerosol challenges in lungs, livers, and spleens. The BMΔasp24 mutant was also previously tested for safety in pregnant goats and did not cause abortion (14). As such, the Δasp24 deletion mutants remain excellent candidates for further evaluation due to their protective ability, while they remain safe for pregnant animals. Future studies should include vaccination of mice intranasally to enhance mucosal immunity for improvement of memory responses against aerosol infection with Brucella.
Acknowledgments
This work was supported by The National Center for Foreign Animal and Zoonotic Diseases (FAZD) grant N00014-04-1-0 from DHS/ONR and from NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (NIH grant U54 AI057 156-0110).
We thank David McMurray for his assistance and guidance using the Madison aerosol chamber. We also thank Allison Rice-Ficht, Jianwu Pei, and Xicheng Ding for assistance with the mouse experiments.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Bhattacharjee, A. K., M. J. Izadjoo, W. D. Zollinger, M. P. Nikolich, and D. L. Hoover. 2006. Comparison of protective efficacy of subcutaneous versus intranasal immunization of mice with a Brucella melitensis lipopolysaccharide subunit vaccine. Infect. Immun. 74:5820-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., L. Van de Verg, M. J. Izadjoo, L. Yuan, T. L. Hadfield, W. D. Zollinger, and D. L. Hoover. 2002. Protection of mice against brucellosis by intranasal immunization with Brucella melitensis lipopolysaccharide as a noncovalent complex with Neisseria meningitidis group B outer membrane protein. Infect. Immun. 70:3324-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossi, P., A. Tegnell, A. Baka, F. Van Loock, J. Hendriks, A. Werner, H. Maidhof, and G. Gouvras. 2004. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Eur. Surveill. 9:E15-E16. [PubMed] [Google Scholar]
- 4.Campos, E., S. L. Cravero, L. Delgui, I. Mora, N. Kahn, A. I. Arese, and O. L. Rossetti. 2002. Brucella abortus INTA2, a novel strain 19 (Delta)bp26::luc (Delta)bmp18 double mutant lacking drug resistance markers. Vet. Microbiol. 87:1-13. [DOI] [PubMed] [Google Scholar]
- 5.Davos, D. E., C. F. Cargill, M. R. Kyrkou, J. A. Jamieson, and G. E. Rich. 1981. Outbreak of brucellosis at a South-Australian abattoir. 2. Epidemiological investigations. Med. J. Aust. 2:657-660. [PubMed] [Google Scholar]
- 6.Edmonds, M. D., A. Cloeckaert, and P. H. Elzer. 2002. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet. Microbiol. 88:205-221. [DOI] [PubMed] [Google Scholar]
- 7.Enright, F. M., L. N. Araya, P. H. Elzer, G. E. Rowe, and A. J. Winter. 1990. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet. Immunol. Immunopathol. 26:171-182. [DOI] [PubMed] [Google Scholar]
- 8.Godfroid, J., A. Cloeckaert, J. P. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313-326. [DOI] [PubMed] [Google Scholar]
- 9.Guilloteau, L. A., J. Dornand, A. Gross, M. Olivier, F. Cortade, Y. L. Vern, and D. Kerboeuf. 2003. Nramp1 is not a major determinant in the control of Brucella melitensis infection in mice. Infect. Immun. 71:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoover, D. L., R. M. Crawford, L. L. Van De Verg, M. J. Izadjoo, A. K. Bhattacharjee, C. M. Paranavitana, R. L. Warren, M. P. Nikolich, and T. L. Hadfield. 1999. Protection of mice against brucellosis by vaccination with Brucella melitensis WR201(16MΔpurEK). Infect. Immun. 67:5877-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, M. A., C. S. Green, L. Lowchyj, G. M. Lee, V. K. Grippe, M. F. Smith, Jr., L. Y. Huang, E. T. Harvill, and T. J. Merkel. 2005. MyD88-dependent signaling contributes to protection following Bacillus anthracis spore challenge of mice: implications for Toll-like receptor signaling. Infect. Immun. 73:7535-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izadjoo, M. J., A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2004. Oral vaccination with Brucella melitensis WR201 protects mice against intranasal challenge with virulent Brucella melitensis 16M. Infect. Immun. 72:4031-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahl-McDonagh, M. M., P. H. Elzer, S. D. Hagius, J. V. Walker, Q. L. Perry, C. M. Seabury, A. B. den Hartigh, R. M. Tsolis, L. G. Adams, D. S. Davis, and T. A. Ficht. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169-5177. [DOI] [PubMed] [Google Scholar]
- 15.Kahl-McDonagh, M. M., and T. A. Ficht. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 74:4048-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurray, D. N., S. S. Allen, A. Jeevan, T. Lasco, H. Cho, T. Skwor, T. Yamamoto, C. McFarland, and T. Yoshimura. 2005. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinburgh) 85:295-301. [DOI] [PubMed] [Google Scholar]
- 17.Mense, M. G., R. H. Borschel, C. L. Wilhelmsen, M. L. Pitt, and D. L. Hoover. 2004. Pathologic changes associated with brucellosis experimentally induced by aerosol exposure in rhesus macaques (Macaca mulatta). Am. J. Vet. Res. 65:644-652. [DOI] [PubMed] [Google Scholar]
- 18.Mense, M. G., L. L. Van De Verg, A. K. Bhattacharjee, J. L. Garrett, J. A. Hart, L. E. Lindler, T. L. Hadfield, and D. L. Hoover. 2001. Bacteriologic and histologic features in mice after intranasal inoculation of Brucella melitensis. Am. J. Vet. Res. 62:398-405. [DOI] [PubMed] [Google Scholar]
- 19.Mielke, M. E. 1991. T cell subsets in granulomatous inflammation and immunity to L. monocytogenes and B. abortus. Behring Inst. Mitt 88:99-111. [PubMed] [Google Scholar]
- 20.Miller, C. D., J. R. Songer, and J. F. Sullivan. 1987. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am. Ind. Hyg. Assoc. J. 48:271-275. [DOI] [PubMed] [Google Scholar]
- 21.Montaraz, J. A., and A. J. Winter. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pappas, G., P. Panagopoulou, L. Christou, and N. Akritidis. 2006. Biological weapons: Brucella as a biological weapon. Cell. Mol. Life Sci. 63:2229-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 24.Pilette, C., Y. Ouadrhiri, V. Godding, J. P. Vaerman, and Y. Sibille. 2001. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18:571-588. [DOI] [PubMed] [Google Scholar]
- 25.Russell-Lodrigue, K. E., M. W. Poels, G. Q. Zhang, D. N. McMurray, and J. E. Samuel. 2005. Hepatitis associated with C. burnetii isolates. Ann. N. Y. Acad. Sci. 1063:176-180. [DOI] [PubMed] [Google Scholar]
- 26.Russell-Lodrigue, K. E., G. Q. Zhang, D. N. McMurray, and J. E. Samuel. 2006. Clinical and pathologic changes in a guinea pig aerosol challenge model of acute Q fever. Infect. Immun. 74:6085-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, D., E. Wiegeshaus, and V. Balasubramanian. 2000. Animal models for experimental tuberculosis. Clin. Infect. Dis. 31(Suppl. 3):S68-S70. [DOI] [PubMed] [Google Scholar]
- 28.Tobias, L., D. O. Cordes, and G. G. Schurig. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet. Pathol. 30:119-129. [DOI] [PubMed] [Google Scholar]
- 29.Van De Verg, L. L., A. B. Hartman, A. K. Bhattacharjee, B. D. Tall, L. Yuan, K. Sasala, T. L. Hadfield, W. D. Zollinger, D. L. Hoover, and R. L. Warren. 1996. Outer membrane protein of Neisseria meningitidis as a mucosal adjuvant for lipopolysaccharide of Brucella melitensis in mouse and guinea pig intranasal immunization models. Infect. Immun. 64:5263-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith. 1970. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422-429. [DOI] [PubMed] [Google Scholar]
- 31.Yagupsky, P., and E. J. Baron. 2005. Laboratory exposures to brucellae and implications for bioterrorism. Emerg. Infect. Dis. 11:1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]