Abstract
C57BL/6 mice develop an allergic bronchopulmonary mycosis following intratracheal inoculation of Cryptococcus neoformans 24067. We determined that only low levels of tumor necrosis factor alpha (TNF-α) are produced in the lungs following infection. Thus, the objective of the present studies was to determine whether treatment with a TNF-α-expressing adenoviral vector (adenoviral vector with the murine TNF-α transgene under the control of the human cytomegalovirus promoter [AdTNFα]) could switch the type 2 (T2) T-cell response/T1 T-cell response balance toward the T1 T-cell response. AdTNFα induced an increase in TNF-α expression at days 3 and 7. At days 7 to 14, the number of cryptococcal lung CFU continued to increase in both untreated and control adenoviral vector (empty adenovirus type 5 backbone)-treated mice, but the number was ultimately 100-fold lower following AdTNFα treatment. AdTNFα markedly increased neutrophil and macrophage numbers, and pulmonary eosinophilia did not develop. CXCL1, CXCL2, and gamma interferon were also up-regulated, while eotaxin, interleukin-4 (IL-4), and IL-5 were down-regulated. AdTNFα treatment also increased the number of CD80+ and CD40+ cells and decreased the number of CD86+ cells (CD11b+ and CD11c+) in the lungs. Major histocompatibility complex class II levels on CD11b+ cells were increased. Whole-lung expression of inducible nitric oxide synthase was increased, while YM2 expression and acidic mammalian chitinase expression were decreased. None of these effects were observed with the control (empty) adenoviral vector. Overall, these results support the hypothesis that early TNF-α expression promotes a shift in T-cell and macrophage polarization from T2/alternatively activated macrophages toward T1/classically activated macrophages, resulting in control of the fungal infection and prevention of the allergic response.
C57BL/6 mice are susceptible to pulmonary Cryptococcus neoformans 24067 infection and develop a chronic fungal infection in their lungs (1). The immune response to C. neoformans in these mice is a type 2 (T2) T-cell allergic response characterized by a pulmonary eosinophil infiltrate and increased production of T2 cytokines (2). Following intratracheal infection, C57BL/6 mice produce more interleukin-5 (IL-5) and less gamma interferon (IFN-γ) than resistant mouse strains (i.e., CBA/J, BALB/c, and C.B-17) (11-13). C57BL/6 mice can clear C. neoformans and develop T1 T-cell responses in the lungs if production of IL-4 or IL-10 is absent (9), whereas an infection in IFN-γ−/− mice becomes progressive (1). During a chronic infection, wild-type C57BL/6 mice harbor a stable burden of 106 to 107 cryptococci in the lungs and can survive >12 weeks postinfection (12, 13). As the infection persists in the lungs, large numbers of cryptococci are visible within macrophages, and significant amounts of eosinophilic Ym1/Ym2 crystals accumulate in the lungs (1, 7, 13). These crystals of chitinase-like proteins are produced by alternatively activated macrophages (M2), which are produced when macrophages are exposed to IL-4 and IL-13, typical of a T2 response. While normal classically activated macrophages (M1) are produced when mice are exposed to IFN-γ and lipopolysaccharide or tumor necrosis factor alpha (TNF-α), M1 express opsonic receptors and high levels of inducible nitric oxide synthase (iNOS) and nitric oxide synthase 2 (19).
TNF-α production is required for the development of T1 cell-mediated immunity to C. neoformans infection and precedes the inflammatory response in infected mice (3, 11, 14, 15). Development of a T1 response is required to clear a C. neoformans infection, and neutralization of early TNF-α in CBA/J mice produces a T2 shift preventing pulmonary clearance of C. neoformans (11). Anti-TNF-α-treated mice also fail to generate a delayed-type hypersensitivity response to C. neoformans antigen and exhibit elevated IL-5 production and pulmonary eosinophilia (11). These findings demonstrate that the absence of early TNF-α during C. neoformans infection shifts the T1/T2 balance of immunity toward a T2 response, resulting in chronic fungal infection. Interestingly, the pathology of the cryptococcal infection in anti-TNF-α-treated mice resembles the response described for susceptible C57BL/6 mice. The objective of the current study was to determine whether overexpression of TNF-α in the lungs could alter the T2/T1 balance of the T-cell response and diminish the chronic allergic response to C. neoformans infection, including M2 in the C57BL/6 mice.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were 6 to 8 weeks of age at the time of infection. Mice were housed in sterilized cages covered with a filter top. Food and water were given ad libitum. The mice were maintained by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor, MI) in accordance with regulations approved by the University of Michigan Committee on the Use and Care of Animals.
C. neoformans.
C. neoformans strain 24067 (= 52D) was obtained from the American Type Culture Collection (Manassas, VA). For injection, yeast cells were grown to stationary phase (72 h) at 37°C in Sabouraud dextrose broth (1% neopeptone and 2% dextrose; Difco, Detroit, MI) on a shaker. The cultures were washed in nonpyrogenic saline, counted on a hemocytometer, and diluted to obtain a concentration of 6.6 × 105 CFU/ml in sterile nonpyrogenic saline.
Adenoviral TNF-α vector.
The adenoviral vector containing the murine TNF-α transgene (AdTNFα) was prepared as previously described (22). Briefly, a human type 5 adenoviral vector, pADBg1 II, containing replication-defective deletions in the E1 region and partial deletions in the E3 region was used. An expression cassette containing the human cytomegalovirus promoter and a murine TNF-α cDNA spliced with a transcription termination signal at the end (22) was inserted into the E1 position of the vector. The control adenoviral vector (empty adenovirus type 5 backbone) (Ad-Ctrl) was the same as the vector described above except that it had an expression cassette containing only the human cytomegalovirus promoter.
In the dose-response study, adenovirus containing murine TNF-α was administered to mice intratracheally at a dose of 1 × 107, 1 × 108, or 5 × 108 PFU. For C. neoformans infection studies, mice received either control adenovirus or AdTNFα intratracheally at the time of infection (5 × 108 PFU).
Surgical intratracheal inoculation.
Mice were anesthetized by intraperitoneal injection of pentobarbital (0.074 mg/g) and restrained on a small surgical board. A small incision was made through the skin over the trachea, and the underlying tissue was separated. A 30-gauge needle, attached to a tuberculin syringe filled with diluted C. neoformans, was inserted into the trachea. A 30-μl (total volume) inoculum containing both 6.6 × 105 CFU of C. neoformans and 5 × 108 PFU of either control adenovirus or AdTNFα (or phosphate-buffered saline for the mice receiving only C. neoformans) was dispensed into the lungs. The skin was closed with cyanoacrylate adhesive.
CFU assay.
Small aliquots were collected from lung digests, and 10-fold dilutions were plated on Sabouraud dextrose agar. Plates were incubated at room temperature, and C. neoformans colonies were counted 48 h later to determine the number of CFU/organ.
Lung leukocyte isolation.
Individual lungs were excised, minced, and enzymatically digested for 45 min in 15 ml of digestion buffer (RPMI medium, 5% fetal calf serum, antibiotics, 1 mg/ml collagenase, 30 μg/ml DNase). Each cell suspension and undigested fragments were further dispersed by drawing the preparation up and down through the bore of a 10-ml syringe. The total cell suspension was pelleted, and erythrocytes were lysed in cold NH4Cl buffer (0.83% NH4Cl, 0.1% KHCO3, 0.037% Na2EDTA; pH 7.4). Leukocytes were washed with RPMI medium, resuspended in complete medium, and enumerated in the presence of trypan blue using a hemocytometer.
Lung leukocyte subsets.
Lung cell suspensions were cytospun onto glass slides and stained using Wright-Giemsa stain. A total of 200 neutrophils, mononuclear cells, and eosinophils from randomly chosen high-power fields were visually counted. The percentage of a specific leukocyte subset was multiplied by the total number of leukocytes to calculate the absolute number of the subset in the sample.
Detection of cytokine mRNA by RT-PCR.
Total RNA was prepared from whole-lung samples removed from C57BL/6 mice for reverse transcription (RT)-PCR for cytokine measurement. Macrophages were isolated via cell adherence to plates for RT-PCR for cytokine measurement as well. RNA was isolated using the TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's directions. The purified RNA was subsequently reverse transcribed, and DNA was amplified using a SuperScript One-Step RT-PCR kit (Invitrogen). The murine oligonucleotide primer sequences used for RT-PCR analysis are described in the supplemental material. Each RT-PCR amplification mixture was incubated at 45°C for 45 min. Then the reverse transcriptase was denatured, and the RNA-cDNA hybrid was denatured at 94°C for 2 min. The PCR conditions were 25 or 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and elongation at 68°C for 1 min. A final amplification step consisted of 72°C for 7 min. After amplification, the samples were separated on a 2% agarose gel containing ethidium bromide, and bands were visualized and photographed using UV transillumination.
Lung leukocyte cultures and cytokine production.
Isolated lung leukocytes (5 × 106 cells/ml) were cultured for 24 h in six-well plates containing 3 ml of complete medium with 5 μg/ml concanavalin A at 37°C in the presence of 5% CO2. Culture supernatants were assayed for IFN-γ, IL-4, and IL-5 production by an enzyme-linked immunosorbent assay (ELISA) performed according to the manufacturer's protocol (BD-PharMingen, San Diego, CA).
Flow cytometric analysis.
Cell suspensions were prepared from digests of whole lungs harvested from mice. Lung cells were incubated and stained with the following monoclonal antibodies purchased from BD Pharmingen: 2.4G2, anti-murine CD16/CD32 Fc block, rat immunoglobulin G2b (IgG2b); PerCp-Cy5.5-labeled anti-murine CD19, rat IgG2a; PerCp-Cy5.5-labeled anti-murine CD3ɛ chain, Armenian hamster IgG1, κ; fluorescein isothiocyanate-labeled anti-murine CD11c, Armenian hamster IgG1, λ2; fluorescein isothiocyanate-labeled anti-murine CD11b, rat IgG2b, κ; phycoerythrin (PE)-labeled anti-murine major histocompatibility complex class II (MHC-II) 1-Ab(Aβb), IgG2a, κ; PE-labeled anti-murine Gr-1, rat IgG2b, κ; PE-labeled anti-murine CD86, rat IgG2a, κ; PE-labeled anti-murine CD80, Armenian hamster IgG2, κ; and PE-labeled anti-murine CD40, rat IgG2a, κ. Cells were incubated for 30 min on ice, washed in staining buffer, fixed in 2.5% paraformaldehyde in buffered saline, and analyzed by flow cytometry. After gating for live cells and exclusion of B and T cells, CD11b+ and CD11c+ cells were analyzed for coexpression with CD40, CD86, CD80, and MHC-II. Further, after gating for live cells, CD11c expression was gated against Gr-1 expression. Isotype control antibodies were used to subtract the background. The percentages of expression for CD40, CD86, CD80, and MHC-II were determined for both CD11b+ and CD11c+ cells.
Statistics.
Statistical significance was determined using the unpaired two-tailed Student t test or one-way analysis of variance corrected for multiple comparisons where appropriate. P values less than 0.05 were considered statistically significant. Calculations were performed using either the Prism 3.0 software program for Windows (GraphPad Software) or Microsoft Excel spreadsheet.
RESULTS
Increasing expression of TNF-α in the lungs during C. neoformans infection by delivery of a TNF-α-expressing adenoviral vector.
The first objective was to determine the level of TNF-α expression during C. neoformans 24067 infection in C57BL/6 mice. Whole-lung RNA was prepared from mice at days 3 and 7 postinfection (intratracheal) and from samples from uninfected mice. TNF-α expression was analyzed by RT-PCR. We did not detect TNF-α expression in uninfected mice and observed only slight induction of TNF-α in the lungs following infection with C. neoformans (Fig. 1). There also did not appear to be increased expression of TNF-α between days 3 and 7 postinfection (Fig. 1).
FIG. 1.
TNF-α expression in the lungs of C. neoformans-infected mice in the presence or absence of TNF-α-expressing adenoviral vector. C57BL/6 mice were inoculated on day 0 with either C. neoformans alone (Cneo only), C. neoformans plus empty adenoviral vector (Cneo + Ctrl Adv), or C. neoformans plus TNF-α-expressing adenoviral vector (Cneo + TNFα Adv). Whole-lung RNA was analyzed by RT-PCR on day 0 (uninfected), day 3, and day 7 postinfection. Each lane represents one of at least three animals per group. Uninf, uninfected.
The second objective was to determine the dose of intratracheally delivered TNF-α-expressing adenoviral vector (AdTNFα) that produced significant TNF-α levels in the lungs. Various doses of AdTNFα or the adenoviral vector control (Ad-Ctrl) were delivered intratracheally into uninfected mice, and bronchoalveolar lavage fluid (BALF) was collected on day 2 posttreatment and assayed by a TNF-α ELISA. TNF-α production from the adenoviral vector was dose dependent. Low levels of TNF-α could be detected in the lungs of mice treated with 1 × 108 PFU. However, we observed a significant increase in the TNF-α levels in mice treated with 5 × 108 PFU of AdTNFα but not in mice treated with Ad-Ctrl (data not shown). Thus, a dose of 5 × 108 PFU was chosen for additional studies.
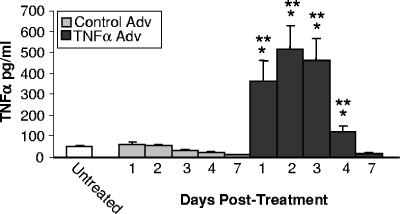
We next examined the kinetics of TNF-α production in the lungs of uninfected mice following intratracheal delivery of AdTNFα. TNF-α levels were increased in the BALF as early as day 1, and the maximal levels of TNF-α were observed at day 2 posttreatment (Fig. 2). The increased levels of TNF-α persisted for at least 4 days following AdTNFα administration, but the levels returned to untreated levels by day 7. In contrast, treatment of uninfected mice with Ad-Ctrl did not increase TNF-α levels in the BALF at any time point tested (Fig. 2).
FIG. 2.
Kinetics of TNF-α production in the lungs following intratracheal administration of TNF-α-expressing adenoviral vector. C57BL/6 mice were inoculated with either empty adenoviral vector (Control Adv) or TNF-α-expressing adenoviral vector (TNFα Adv) on day 0, and TNF-α levels in the BALF were analyzed by ELISA at the times indicated. The results are expressed as the means ± standard errors of the means. One asterisk, P < 0.05 for a comparison with the untreated mice at the same time point; two asterisks, P < 0.05 for a comparison with the Control Adv-treated mice at the same time point. Each group contained five mice.
Our next set of experiments examined whether intratracheal delivery of AdTNFα along with C. neoformans 24067 increased the expression of TNF-α in the lungs compared to the expression with C. neoformans infection alone or infection with C. neoformans plus Ad-Ctrl. At day 3 postinfection, there was a striking increase in the level of TNF-α expression in the lungs of mice also treated with AdTNFα compared to the level in untreated or Ad-Ctrl-treated mice (Fig. 1). It should be noted that while Ad-Ctrl did not increase TNF-α expression in uninfected mice (Fig. 2), it did slightly increase TNF-α expression in C. neoformans-infected mice (Fig. 1), but not to the level seen in C. neoformans-infected mice treated with AdTNFα. At day 7, TNF-α expression remained high in the mice treated with AdTNFα but was low in the untreated and Ad-Ctrl-treated C. neoformans-infected mice. This time was longer than that observed for AdTNFα-treated uninfected mice (Fig. 2), opening the possibility that host TNF-α expression may also be induced by early expression of AdTNFα. Thus, AdTNFα treatment was able to markedly increase the otherwise low TNF-α expression in the lungs of C. neoformans-infected C57BL/6 mice.
Effect of intratracheal delivery of a TNF-α-expressing adenoviral vector on pulmonary growth of C. neoformans.
Since AdTNFα could increase TNF-α expression, the next question was whether this had a positive effect on host defenses and control of pulmonary C. neoformans infection. The number of lung CFU increased over 300-fold from day 0 to day 3 in all three groups of mice, and the levels were not significantly different for untreated, Ad-Ctrl-treated, and AdTNFα-treated mice (Fig. 3). However, by day 7 the number of lung CFU was significantly lower in mice treated with AdTNFα than in the other two groups of mice (Fig. 3). Between days 7 and 14, the number of cryptococcal lung CFU continued to increase in both untreated and Ad-Ctrl-treated mice. However, mice that had received AdTNFα continued to control the levels of C. neoformans in the lungs, resulting in a nearly 100-fold-lower number of lung CFU in AdTNFα-treated mice than in untreated and Ad-Ctrl-treated mice (Fig. 3). Thus, while AdTNFα did not augment clearance of the infection by resident alveolar macrophages (day 3), it did augment control of the pulmonary C. neoformans infection during the adaptive phase of the response (days 7 to 14).
FIG. 3.
Effect of TNF-α-expressing adenoviral vector on pulmonary growth of C. neoformans. C57BL/6 mice were inoculated on day 0 with either C. neoformans alone (C.neo), C. neoformans plus empty adenoviral vector (C.neo + Control Adv), or C. neoformans plus TNF-α-expressing adenoviral vector (C.neo + TNFα Adv) and analyzed on days 3, 7, and day 14. One asterisk, P < 0.05 for a comparison with the C.neo group at the same time point; two asterisks, P < 0.05 for a comparison with the C.neo + Control Adv group at the same time point. The results are expressed as the means ± standard errors of the means. Each group contained eight mice from two separate experiments as described in Materials and Methods.
Effect of intratracheal delivery of a TNF-α-expressing adenoviral vector on the pulmonary inflammatory response to C. neoformans.
The next objective was to evaluate the effect of AdTNFα on the T2 cell-mediated pulmonary inflammatory response to C. neoformans in C57BL/6 mice. Inflammatory infiltrates into the lungs of the three groups of mice were analyzed on days 7 and 14 postinfection by differential cell staining and flow cytometry (5). In untreated C. neoformans-infected mice, there was a small number of neutrophils in the lungs at day 7 but very few neutrophils at day 14 (Fig. 4 and 5). At both time points, significant numbers of eosinophils had been recruited into the lungs and there was a large influx of mononuclear leukocytes (largely monocytes). Treatment of mice with Ad-Ctrl did not affect the composition or kinetics of the inflammatory response at the time points examined (Fig. 4 and 5). However, treatment with AdTNFα markedly changed the composition of the granulocyte response. Significantly greater numbers of neutrophils were recruited into the lungs at both time points, and the pulmonary eosinophilia did not develop (Fig. 4). This shift in the granulocyte response in AdTNFα-treated mice was additionally confirmed by flow cytometry (Fig. 5). In addition, AdTNFα treatment also augmented macrophage recruitment into the lungs of C. neoformans-infected C57BL/6 mice (Fig. 4 and 5). In summary, AdTNFα treatment at the onset of infection significantly changed the composition of the inflammatory response, consistent with a shift from T2 to T1 polarization of the immune response.
FIG. 4.
Effect of TNF-α-expressing adenoviral vector on pulmonary inflammation following C. neoformans infection. The groups are the same as those described in the legend to Fig. 3. Whole-lung leukocytes were prepared and enumerated as described in Materials and Methods, using Wright-Giemsa staining to distinguish neutrophils (Neut), eosinophils (Eos), and mononuclear cells (Mono). Eosinophil numbers were additionally confirmed by flow cytometry (see Fig. 5). One asterisk, P < 0.05 for a comparison with the C.neo group. The leukocyte cell numbers on days 7 and 14 for all groups were significantly different from the numbers for uninfected mice (P < 0.05); the only exception was eosinophils in the C.neo + TNFα Adv group.
FIG. 5.
Flow cytometric analysis of the effect of TNF-α-expressing adenoviral vector on pulmonary inflammation following C. neoformans infection (day 7). The groups are the same as those described in the legend to Fig. 3. Whole-lung leukocytes were prepared as described in Materials and Methods. (A) Analysis of eosinophil influx by forward scatter (FSC)/side scatter (SSC) and Gr-1/CD11c staining. Eosinophils are indicated by arrow E. Arrows 1, 2, and 3 indicate neutrophils, eosinophils, and macrophages, respectively. (B) Analysis of Gr-1 and CD11c expression on SSChi FSClo cells. (C) SSC/FSC analysis of Gr-1hi CD11clo (panels N) and Gr-1lo CD11chi (panel M) cells.
Effect of intratracheal delivery of a TNF-α-expressing adenoviral vector on the development of T2 versus T1 cell-mediated pulmonary immunity.
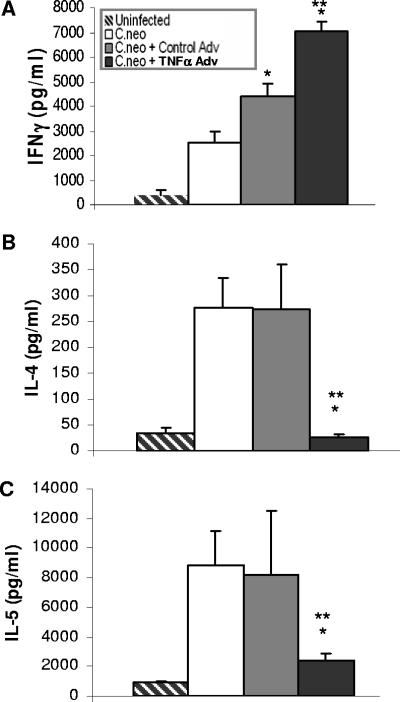
Our analysis of the pulmonary inflammatory response suggested that treatment with AdTNFα at the onset of C. neoformans infection in C57BL/6 mice might promote the development of T1 versus T2 cell-mediated pulmonary immunity, a result that would be consistent with our previously published finding that early production of TNF-α is required for the development of T1 cell-mediated immunity to C. neoformans (10, 11, 14). To address this possibility, we analyzed the expression of a large number of cytokines involved in the regulation of cell-mediated immunity and inflammatory cell recruitment. There was up-regulation of CXCL1 and CXCL2 and down-regulation of eotaxin (Fig. 6), consistent with the increased number of neutrophils and decreased number of eosinophils in AdTNFα-treated mice (Fig. 4 and 5). More importantly, treatment with AdTNFα increased IFN-γ expression and decreased IL-4 and IL-5 expression compared to the expression in untreated or Ad-Ctrl treated mice (Fig. 6). These changes in T1/T2 gene expression were also seen at the level of cytokine protein production by isolated lung leukocytes in culture (Fig. 7). AdTNFα treatment also resulted in increased numbers of CD80+ and CD40+ cells and decreased numbers of CD86+ cells in both the CD11b+ and CD11c+ populations in the lungs (Fig. 8). Overall, these data support the model that AdTNFα treatment at the onset of infection promotes a shift in the balance of cell-mediated immunity away from T2 and toward T1.
FIG. 6.
Effect of TNF-α-expressing adenoviral vector on pulmonary cytokine expression following C. neoformans infection. Whole-lung mRNA expression was analyzed by RT-PCR, and the groups are the same as described in the legend to Fig. 1. CD11b expression is also shown along with chemokine expression as an indication of macrophage recruitment. Each lane represents one of at least three animals per group. TGFβ, transforming growth factor β; GMCSF, granulocyte-macrophage colony-stimulating factor.
FIG. 7.
Effect of TNF-α-expressing adenoviral vector on cytokine production by lung leukocytes from C. neoformans-infected mice (day 7). Lung leukocytes were isolated from a whole-lung enzymatic digest as described in Materials and Methods and then cultured with the T-cell mitogen concanavalin A for 24 h. Culture supernatants were assayed by ELISA. The groups are the same as those described in the legend to Fig. 3. Each group contained eight mice from two separate experiments as described in Materials and Methods. One asterisk, P < 0.05 for a comparison with the C.neo group at the same time point; two asterisks, P < 0.05 for a comparison with the C.neo + Control Adv group at the same time point.
FIG. 8.
Effect of TNF-α-expressing adenoviral vector on MHC-II and costimulatory molecule expression on pulmonary (A) CD11c+ and (B) CD11b+ leukocytes following C. neoformans infection (day 7). Lung leukocytes were isolated from a whole-lung enzymatic digest as described in Materials and Methods, and the groups are the same as those described in the legend to Fig. 3.
Effect of intratracheal delivery of a TNF-α-expressing adenoviral vector on macrophage polarization.
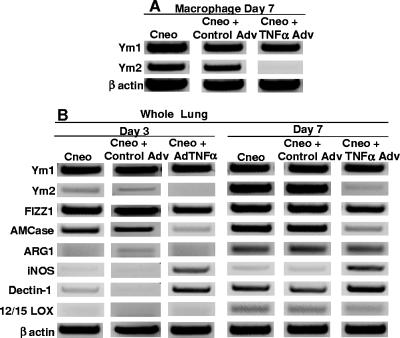
The downstream effects of changes in T-cell polarization include changes in macrophage polarization. Thus, we also examined the effects of the three treatments on the differentiation of macrophages in the lungs of C. neoformans-infected mice. There was no difference in macrophage differentiation marker expression between untreated and Ad-Ctrl-treated C. neoformans-infected mice (Fig. 8 and 9). However, there was an increase in surface MHC-II levels and a decrease in YM2 expression on CD11b+ cells in the lungs of AdTNFα-treated mice compared to untreated or Ad-Ctrl-treated mice (Fig. 8 and 9). In addition, there was up-regulation of iNOS and down-regulation of YM2 and acidic mammalian chitinase in the lungs of AdTNFα-treated mice (Fig. 8). Overall, these results suggest a shift from M2 toward M1 differentiation and are consistent with the increased expression of IFN-γ and decreased expression of IL-4 in AdTNFα-treated mice.
FIG. 9.
Effect of TNF-α-expressing adenoviral vector on (A) macrophage expression of YM1 and YM2 and (B) whole-lung expression of macrophage differentiation markers following C. neoformans infection (days 3 and 7). mRNA expression was analyzed by RT-PCR, and the groups are the same as those described in the legend to Fig. 1. (A) Leukocytes were prepared from whole lungs as described in Materials and Methods and enriched by plate adherence. (B) Whole-lung mRNA was isolated as described in Materials and Methods. Each lane represents one of at least three animals per group. AMCase, acidic mammalian chitinase.
DISCUSSION
These studies demonstrated that delivery of a TNF-α-expressing adenoviral vector can increase the expression of TNF-α in the lungs during the afferent phase of the immune response. We have shown that pulmonary TNF-α levels are low in C57BL/6 mice following C. neoformans 24067 infection. However, treatment with a TNF-α-expressing adenoviral vector promotes control of a C. neoformans 24067 infection in C57BL/6 mice. The mechanism underlying this enhanced immunity involves a shift in the T1/T2 balance away from a T2 response toward a T1 response. These results demonstrate the critical role of TNF-α as an early signal for the development of protective T1 cell-mediated pulmonary immunity to C. neoformans. Furthermore, these studies also identified that induction of this signal molecule appears to be defective following infection with C. neoformans 24067.
Intratracheal delivery of AdTNFα was effective in stimulating a shift in CD11b+ and CD11c+ cell differentiation, as shown by the increased expression of CD80, CD40, and MHC-II and the concomitant decrease in the expression of CD86. TNF-α plays a key role in dendritic cell maturation, and these cells are central for driving protective T1 responses to C. neoformans (3, 4, 10, 16, 20, 23, 24, 26). Stimulation of an antifungal response by immature dendritic cells can result in an immune deviation similar to that produced by transient TNF-α deficiency (10). It has been previously reported that encapsulated C. neoformans is a poor inducer of CD80 on macrophages (25). The current studies suggest that signaling from TNF-α can promote up-regulation of CD80 expression on macrophages during their encounters with C. neoformans in vivo. In some studies, overexpression of CD80 (B7-1) can drive the polarization of T-cell responses toward T1, while anti-CD80 treatment can have the opposite effect and induce a T2 response (17, 21). We also noted a reduction in CD86-expressing CD11c+ and CD11b+ following treatment with AdTNFα. It has been reported that inhibiting CD86 signaling by anti-CD86 (B7-2) can shift the balance of cell-mediated immunity towards T1 (17, 21). Finally, activation of CD40 on dendritic cells can increase the production of IL-12 (6), an observation that is consistent with the observed increase in CD40 expression following AdTNFα treatment. Overall, these studies highlight the importance of TNF-α signaling during C. neoformans infection for antigen-presenting cell differentiation and T1 cell-mediated immunity development.
There is significant interest in the role of macrophage differentiation during chronic infection (8, 19), and these studies implicate a role for TNF-α in driving the differentiation of M1 over M2. We have previously reported that in this model of murine allergic bronchopulmonary mycosis large numbers of M2 developed in the lungs during infection (1, 9). These M2 express high levels of arginase and the chitinase-like molecule YM2 and low levels of iNOS. The pathology of chronic pulmonary C. neoformans infection in C57BL/6 mice is consistent with M2-mediated damage, as characterized by YM2 expression leading to crystal deposition, large numbers of intracellular cryptococci, and low NO production (7, 13, 18). IFN-γ-deficient C57BL/6 mice have even higher numbers of M2 in their lungs during a cryptococcal infection, which correlates with progressive infection (1). While it remains to be determined what the long-term effect of AdTNFα treatment is on the pathology and chronicity of cryptococcal infection in C57BL/6 mice, we predict that the allergic response never develops and the infection is controlled with minimal damage to the pulmonary architecture.
Supplementary Material
Acknowledgments
We thank Theodore J. Standiford (University of Michigan) for providing the TNF-α-expressing adenoviral vector used in this study. In addition, we are indebted to the University of Michigan Vector Core for providing technical expertise, advice, and stocks of the control adenoviral backbone vector for these studies.
This work was supported in part by National Institutes of Health grants R01-AI059201 (to G.B.H.), R01-AI064479 (to G.B.H.), R01-HL51082 (to G.B.T.), and T32-HL07749 (to J.E.M., A.C.H.-P., and R.P.) and by a Department of Veterans Affairs merit grant (to G.B.T.).
Editor: A. Casadevall
Footnotes
Published ahead of print on 23 July 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. [DOI] [PubMed] [Google Scholar]
- 2.Arora, S., and G. B. Huffnagle. 2005. Immune regulation during allergic bronchopulmonary mycosis: lessons taught by two fungi. Immunol. Res. 33:53-68. [DOI] [PubMed] [Google Scholar]
- 3.Bauman, S., G. B. Huffnagle, and J. Murphy. 2003. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 71:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. [DOI] [PubMed] [Google Scholar]
- 5.de Heer, H. J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M. A. Willart, H. C. Hoogsteden, and B. N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp. Med. 200:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallarino, F., U. Grohmann, C. Vacca, R. Bianchi, M. C. Fioretti, and P. Puccetti. 2002. CD40 ligand and CTLA-4 are reciprocally regulated in the Th1 cell proliferative response sustained by CD8(+) dendritic cells. J. Immunol. 169:1182-1188. [DOI] [PubMed] [Google Scholar]
- 7.Feldmesser, M., Y. Kress, and A. Casadevall. 2001. Intracellular crystal formation as a mechanism of cytotoxicity in murine pulmonary Cryptococcus neoformans infection. Infect. Immun. 69:2723-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez, Y., S. Arora, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 174:1027-1036. [DOI] [PubMed] [Google Scholar]
- 10.Herring, A. C., N. R. Falkowski, G. H. Chen, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect. Immun. 73:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herring, A. C., J. Lee, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoag, K., N. Street, G. Huffnagle, and M. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13:487-495. [DOI] [PubMed] [Google Scholar]
- 13.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 14.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNFα is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 15.Kawakami, K., X. Qifeng, M. Tohyama, M. H. Qureshi, and A. Saito. 1996. Contribution of tumor necrosis factor-alpha (TNF-alpha) in host defence mechanism against Cryptococcus neoformans. Clin. Exp. Immunol. 106:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, R. M., J. Chen, L. E. Yauch, and S. M. Levitz. 2005. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect. Immun. 73:592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 18.Lovchik, J., M. F. Lipscomb, and C. R. Lyons. 1997. Expression of lung inducible nitric oxide synthase protein does not correlate with nitric oxide production in vivo in a pulmonary immune response against Cryptococcus neoformans. J. Immunol. 158:1772-1778. [PubMed] [Google Scholar]
- 19.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677-686. [DOI] [PubMed] [Google Scholar]
- 20.Pietrella, D., C. Corbucci, S. Perito, G. Bistoni, and A. Vecchiarelli. 2005. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect. Immun. 73:820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Retini, C., T. R. Kozel, D. Pietrella, C. Monari, F. Bistoni, and A. Vecchiarelli. 2001. Interdependency of interleukin-10 and interleukin-12 in regulation of T-cell differentiation and effector function of monocytes in response to stimulation with Cryptococcus neoformans. Infect. Immun. 69:6064-6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standiford, T., J. Wilkowski, T. Sisson, N. Hattori, B. Mehrad, K. Bucknell, and T. Moore. 1999. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum. Gene Ther. 10:899-909. [DOI] [PubMed] [Google Scholar]
- 23.Syme, R. M., J. C. Spurrell, E. K. Amankwah, F. H. Green, and C. H. Mody. 2002. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcγ receptor II for presentation to T lymphocytes. Infect. Immun. 70:5972-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traynor, T. R., A. C. Herring, M. E. Dorf, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659-4666. [DOI] [PubMed] [Google Scholar]
- 25.Vecchiarelli, A., C. Monari, C. Retini, D. Pietrella, B. Palazzetti, L. Pitzurra, and A. Casadevall. 1998. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86) expression on human monocytes. Eur. J. Immunol. 28:114-121. [DOI] [PubMed] [Google Scholar]
- 26.Vecchiarelli, A., D. Pietrella, P. Lupo, F. Bistoni, D. C. McFadden, and A. Casadevall. 2003. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J. Leukoc. Biol. 74:370-378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.