Abstract
The seventh cholera pandemic that started in 1961 was caused by Vibrio cholerae O1 strains of the El Tor biotype. These strains produce the pore-forming toxin hemolysin, a characteristic used clinically to distinguish classical and El Tor biotypes. Even though extensive in vitro data on the cytolytic activities of hemolysin exist, the connection of hemolysin to virulence in vivo is not well characterized. To study the contribution of hemolysin and other accessory toxins to pathogenesis, we utilized the model of intestinal infection in adult mice sensitive to the actions of accessory toxins. In this study, we showed that 4- to 6-week-old streptomycin-fed C57BL/6 mice were susceptible to intestinal infection with El Tor strains, which caused rapid death at high doses. Hemolysin had the predominant role in lethality, with a secondary contribution by the multifunctional autoprocessing RTX (MARTX) toxin. Cholera toxin and hemagglutinin/protease did not contribute to lethality in this model. Rapid death was not caused by increased dissemination due to a damaged epithelium since the numbers of CFU recovered from spleens and livers 6 h after infection did not differ between mice inoculated with hemolysin-expressing strains and those infected with non-hemolysin-expressing strains. Although accessory toxins were linked to virulence, a strain defective in the production of accessory toxins was still immunogenic since mice immunized with a multitoxin-deficient strain were protected from a subsequent lethal challenge with the wild type. These data suggest that hemolysin and MARTX toxin contribute to vaccine reactogenicity but that the genes for these toxins can be deleted from vaccine strains without affecting vaccine efficacy.
The diarrheal disease cholera is caused by the ingestion of food or water contaminated with the gram-negative bacterium Vibrio cholerae. Nonhemolytic O1 strains of the classical biotype were responsible for the six pandemics of cholera that occurred from the 18th century to the early 20th century. After nearly 40 years of absence, cholera reemerged in 1961, with El Tor O1 being the most prevalent biotype (11). Upon the colonization of the upper intestine, both El Tor and classical strains secrete cholera toxin (CT). This ADP-ribosylating toxin is responsible for massive fluid secretion by intestinal epithelial cells and watery diarrhea, the main characteristic of cholera disease (27). However, the loss or absence of the ctx genes from El Tor strains does not eliminate all disease symptoms. Naturally occurring CT-negative strains both of the El Tor biotype and of non-O1 types have been associated with enterocolitis, as well as septicemia and extraintestinal infections (2, 4, 35). Additionally, studies with CT-negative vaccine strains resulted in mild to severe diarrhea and evidence of inflammation in human volunteers (40, 41, 43, 44). All these findings strongly suggest the existence of additional virulence factors associated with El Tor and non-O1 V. cholerae pathogenesis.
V. cholerae El Tor strains secrete at least three other proteins that may contribute to pathogenesis. These accessory toxins include the V. cholerae multifunctional autoprocessing RTX (MARTXVc) toxin, hemolysin, and hemagglutinin (HA)/protease. Although the cytopathic effects of these accessory toxins in vitro have been well characterized, the role of the toxins in vivo is presently not well understood (16, 38). Hemolysin causes vacuolation and pore formation in many cell types (9, 14, 16, 25, 34), and the purified toxin is rapidly lethal for mice after intravenous administration (23). The deletion of the hemolysin gene reduces virulence in infant mice (1); however, it does not notably diminish the rate of mild diarrhea in human volunteers (30, 42). HA/protease is a soluble Zn metalloprotease that can proteolytically degrade several physiologically important host proteins, including mucin (15) and occludin in tight junctions (33, 46). So far, little evidence that HA/protease is an essential virulence factor has been provided. The ΔhapA vaccine candidate strain 638 still causes mild diarrhea (19). The MARTXVc toxin causes depolymerization and the cross-linking of actin (18, 39); however, the extent to which these activities influence pathogenesis in vivo is mostly unknown. Vaccine strains that do not produce MARTXVc are still reactogenic (44), but when combined with a mutation affecting motility, the loss of MARTXVc reduces reactogenicity (28). In lung infection studies, the MARTXVc toxin has been associated with increased inflammation and tissue damage (17, 22). Even though the three accessory toxins have been shown to have an influence on pathogenesis, these results have all been observed by studying the toxins individually. Therefore, it may be interesting to investigate how the toxins influence virulence in combination.
In this study, we infected adult C57BL/6 mice with V. cholerae according to methods established in our lab that will be detailed elsewhere (36). We observed dose-dependent virulence. At high doses, the infection is shown to be lethal depending on the repertoire of accessory toxins, most importantly hemolysin. Furthermore, we established that this animal model can also be used to test vaccine efficacy. We showed that the multitoxin-deficient mutant strain KFV101 can induce protective immunity.
Hence, this research used a new model for the study of cholera pathogenesis and vaccine efficacy and revealed that hemolysin and the MARTXVc toxin are virulence factors carried by El Tor strains.
MATERIALS AND METHODS
Bacterial strains.
Strains were derived from the streptomycin-resistant isolates of El Tor O1 Inaba strains P27459 and N16961, originally isolated in Bangladesh (18, 32), and Bah1, a vaccine derivative of the O1 Ogawa strain E7946 (44). Strain P4 has a kanamycin resistance cassette replacing the ctx genes in P27459 (20). Strains were further modified to delete toxin genes as previously described (17). For complementation of the hlyA deletion, the entire hlyA gene was introduced into the V. cholerae lacZ loci of ΔhlyA strains KFV103 and KFV101. For this procedure, the 3.3-kb DNA segment containing the entire hlyA gene and flanking DNA including the hlyA promoter was amplified from N16961 by PCR using primers hlyA6215 and hlyA2980 (17). The amplified product was cloned into the EcoRV site of pBluescript II. The 3.3-kb insert was then excised from the resulting plasmid by SpeI and XhoI digestion and ligated into the StuI site of pJL1. This vector, constructed by D. Beattie and J. Letterman, contains a 2.2-kb HpaI internal fragment of the V. cholerae lacZ gene from p6891MCS (6) cloned into the SmaI site of pCVD442 (12). The insert was oriented in reverse of lacZ.
All mutants were analyzed by PCR to ensure the loss or gain of the gene, and toxin activities were determined by assaying for HEp-2 cell rounding (31), for protease activity on milk agar plates (18), and for hemolytic activity by using the CAMP assay with Staphylococcus aureus as the indicator strain (37). For the experiments, strains were grown in Luria-Bertani medium, supplemented with streptomycin (100 μg/ml) and kanamycin (50 μg/ml) when needed, at 30°C with shaking until the cells reached the mid-log phase, when they were pelleted and washed twice with phosphate-buffered saline (PBS), and then the cell suspensions were adjusted to the desired number of CFU per milliliter.
Mouse inoculation.
All experiments were done according to protocols approved by the Northwestern University institutional animal care and use committee. Unless otherwise noted, 4- to 5-week-old female specific-pathogen-free C57BL/6 mice (Harlan, Indianapolis, IN) were treated with streptomycin (1 mg/ml in water) for 4 to 7 days. The evening before the experiments, food but not water was removed from the cage. Mice were anesthetized with 60 to 70 mg of ketamine/kg of body weight and 12.5 mg of xylazine/kg and were fed 50 μl of 8.5% (wt/vol) NaHCO3 intragastrically (i.g.), immediately followed by 50 μl of the bacterial suspension in PBS, by using a 22-gauge animal feeding needle (Popper & Sons, Inc., New Hyde Park, NY). After inoculation, mice had free access to food and sterile water without streptomycin.
For survival experiments, mice were weighed and their health statuses were monitored as previously described (17). When possible, fecal samples were collected, weighed, suspended in PBS, and plated to determine the number of CFU per gram of fecal matter. Mice were included in the study if (i) weight loss exceeded 5% of the total body weight at the time of inoculation, (ii) the health score dropped more than 2 points, and/or (iii) bacteria were shed in the stools. Mice that did not meet any one of these three criteria were excluded from the analysis as inoculation failures. In total, 5 of 124 mice were excluded. Mice were euthanized when severely moribund, and survivors were euthanized 7 days after infection.
For colonization experiments, mice were inoculated and sacrificed at 6 h postinfection (hpi). Small intestines, spleens, and livers were dissected, weighed, and then homogenized in PBS. Serial dilutions were plated onto Luria-Bertani agar with streptomycin and kanamycin for CFU counts.
Histopathology.
Sections from the ileocecal junctions, jejuna, and duodena of the small intestines were fixed in formalin, embedded, sliced, mounted, and stained with hematoxylin and eosin (H&E) at the Mouse Phenotyping Facility at the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL. The slides were initially viewed by a single pathologist (G. K. Haines) blinded to the mouse groupings and were photographed using an Olympus BX40 microscope with an Olympus DP12 digital camera and a Zeiss Axioscope with a Nuance spectral imaging camera.
Bacterial challenge.
Four-week-old mice were treated with streptomycin for 4 days prior to the initial inoculation and each subsequent booster inoculation. Mice were inoculated with 5 × 106 CFU of the multitoxin-deficient mutant strain KFV101 or with PBS as a control. Booster inoculations were performed on days 7 and 14 with doses of 1 × 107 and 5 × 107 CFU, respectively. On day 21, both groups of mice were inoculated i.g. with 108 CFU of P27459. Mice were monitored daily for changes in body weight and health status as described above.
Statistical analysis.
Statistical analyses were performed using InStat software (GraphPad Software, Inc., San Diego, CA) for the analysis of variance or Student's t tests, assuming unequal variances, and GraphPad Prism software for the analysis of survival curves. P values of <0.05 were determined to be statistically significant.
RESULTS
V. cholerae induces lethal infection at high doses.
Conditions for the colonization and susceptibility of streptomycin-fed adult mice to V. cholerae infection have been defined and detailed elsewhere (36). For this study, the role of secreted toxins in virulence in adult mice was investigated using standard survival assays. The dose-survival curve for infection with P27459 showed that the virulence of V. cholerae in mice was dose dependent, with a difference of only 2 log units between doses yielding 100% survival and 100% lethality (Fig. 1). We calculated a sublethal dose of 106 CFU, a 50% lethal dose of 107 CFU, and a 100% lethal dose of 108 CFU and used these calculated doses for these studies.
FIG. 1.
Rates of survival after infection with wild-type V. cholerae were dose dependent. C57BL/6 mice were inoculated with increasing doses of wild-type V. cholerae P27459, and survival was monitored over 7 days.
High levels of lethality after oral infection with V. cholerae are triggered by accessory toxins.
To determine if CT was the cause of animal death, the CT deletion mutant P4 was used in parallel with the wild-type strain P27459. Inoculation with the lethal dose of P27459 (producing CT, hemolysin, HA/protease, and MARTXVc) resulted in 100% lethality (Table 1). This death was rapid, with 100% of mice dying within 24 hpi (Fig. 2A). When mice were inoculated with a similar dose of P4 (producing hemolysin, HA/protease, and MARTXVc), only a slight but not statistically significant difference (P = 0.29) in survival was observed (Table 1). Here, death also occurred rapidly, with 13 of 15 mice dying during the first 24 h and mouse 14 dying within 48 h (Fig. 2A). Thus, in this model, CT surprisingly was not the cause of rapid death, indicating that other factors contribute to virulence in adult mice.
TABLE 1.
Toxin repertoires of V. cholerae strains play an important role during infection
| Strain | Description or genotype | Toxin(s) expresseda | Inoculum (CFU/50 μl) | No. of surviving mice/no. of infected miceb | Peak wt change (%)c | Lowest health scorec,d |
|---|---|---|---|---|---|---|
| P27459 | V. cholerae O1 El Tor | CT, Y, P, R | 0.8 × 108 | 0/8 | NA | 0 |
| P4 | P27459 ΔctxAB | Y, P, R | 0.7 × 108 | 1/15 | +31 | 2.0 |
| KFV101 | P4 ΔhapA ΔrtxA ΔhlyA | None | 1.1 × 108 | 11/13 | −7.9 ± 14 | 1.7 ± 0.5 |
| KFV70 | P4 ΔhapA | Y, R | 1.2 × 108 | 2/15 | −12 ± 9.8 | 1.5 ± 0.7 |
| KFV103 | P4 ΔhlyA | P, R | 0.9 × 108 | 8/13 | −19 ± 7.3 | 1.5 ± 0.5 |
| KFV105 | P4 ΔrtxA | Y, P | 1.0 × 108 | 3/14 | −9.5 ± 6.3 | 1.7 ± 0.6 |
| KFV98 | P4 ΔhapA ΔrtxA | Y | 0.7 × 108 | 0/14 | NA | NA |
| KFV102 | P4 ΔhapA ΔhlyA | R | 1.3 × 108 | 4/13 | −19 ± 4.6 | 1.3 ± 0.3 |
| VOV3 | P4 ΔrtxA ΔhlyA | P | 1.3 × 108 | 8/14 | −17 ± 6.0 | 1.4 ± 0.5 |
Y, hemolysin; P, HA/protease; R, MARTXVc.
The time to death for these mice is shown graphically in Fig. 2.
Values are means ± standard deviations of results for all surviving mice in each group. NA, not applicable.
Health was scored on the following scale as described previously (17): 0, dead; 1, near death; 2, poor health; 3, moderate illness; 4, some malaise; or 5, normal. The mean lowest scores recorded on days 1 to 3 pi are shown.
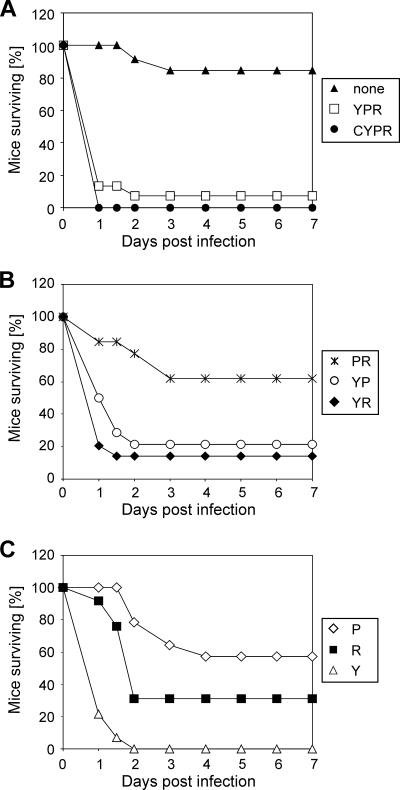
FIG. 2.
Survival after infection with V. cholerae. Mice were inoculated with various V. cholerae mutant strains expressing hemolysin (Y), MARTXVc (R), or HA/protease (P). Survival rates for mice inoculated with either wild-type P27459 (expressing CT, hemolysin, HA/protease, and MARTXVc; CYPR), CT mutant P4 (expressing hemolysin, HA/protease, and MARTXVc; YPR), or the multitoxin-deficient strain KFV101 (none) (A) or with strains expressing two accessory toxins (B) or only one accessory toxin as indicated (C) are shown. Quantitative data on survival and health are shown in Table 1.
To investigate the influence of accessory toxins on lethality, mice were inoculated with KFV101, a mutant with deletions of all three of the accessory toxin genes in a CT-negative background. This strain was previously shown to be avirulent in a lung infection model (17). In contrast to the rapidly dying P27459- and P4-inoculated mice, 11 of 13 mice inoculated with the multitoxin-deficient strain KFV101 at a dose similar to those of P27459 and P4 survived (Table 1). The two nonsurvivors died more slowly than the P27459- and P4-inoculated mice, at 2 and 3 days pi, and these results generated significantly different (P < 0.0001) survival curves (Fig. 2B). As evidence that the mice were infected, all surviving mice inoculated with KFV101 showed signs of illness observed as a drop in the health score by an average of 3.3 points and an average weight loss of 8%. In all, these data show that mice inoculated with V. cholerae at high doses succumb to rapid death due to the action of one or more accessory toxins, independent of CT.
Hemolysin is the primary cause of rapid lethality in mice.
To further assess which accessory toxins contributed to rapid lethality, additional deletion mutants expressing only one or two of the accessory toxins were constructed. Infection studies with strains expressing two accessory toxins showed that hemolysin plays a major role with regard to lethality (Fig. 2B and Table 1). Both strains expressing hemolysin in combination with either MARTXVc (KFV70) or HA/protease (KFV105) caused rapid death and high levels of lethality, 87 and 79%, respectively, while the deletion of hemolysin (KFV103) increased the survival rate to 62%. Statistical analysis showed significantly higher survival rates among mice inoculated with KFV103 than among KFV105- and KFV70-inoculated mice (P, 0.014 and 0.0008, respectively).
The deletion of two accessory toxin genes provided additional evidence that hemolysin is responsible for rapid death in adult mice, as infection with a strain deficient in both MARTXVc and HA/protease but expressing hemolysin caused 100% lethality (Fig. 2C). Overall, the survival rate was only 6% (4 of 64 mice) among all mice that were inoculated with a hemolysin-expressing strain, compared to 56% (28 of 50) among mice infected with any ΔhlyA strain. Therefore, hemolysin was the primary cause of rapid death in adult mice.
Although hemolysin has a major role in virulence, the MARTXVc toxin also plays a role in lethality, as shown by the survival of only 31% of mice inoculated with the mutant KFV102, expressing only MARTXVc, a significant difference from the rate of survival of KFV101-inoculated mice (P = 0.004) (Fig. 2C; Table 1). The deaths of KFV102-infected mice occurred somewhat more slowly than those of mice infected with a hemolysin-expressing strain, with half taking place between 24 and 48 hpi. (Fig. 2C). This result demonstrates that MARTXVc contributes to virulence but induces death more slowly than hemolysin.
However, the expression of the MARTXVc toxin was not highly lethal when HA/protease was also expressed, and the corresponding survival rates were widely variable and ranged from 62% (Fig. 2B; Table 1) to 100% (see Fig. 5B). Similarly, mutant VOV3 expressing only HA/protease yielded survival rates not significantly different from those associated with KFV101 (P = 0.13). Together, these results indicate not only that the expression of HA/protease had no influence on lethality but also that the coexpression of HA/protease decreased the lethality of MARTXVc. Indeed, survival rates of mice inoculated with VOV3 (57%) expressing only HA/protease and those inoculated with KFV103 (62 to 100%) coexpressing MARTXVc and HA/protease were almost identical (P = 0.95). These observations indicate that HA/protease may proteolyze MARTXVc in vivo, a result similar to that reported by Boardman et al. (5), who observed that proteases present in culture supernatant fluids, such as HA/protease, inactivate MARTXVc in vitro.
FIG. 5.
Survival after infection with V. cholerae. (A) Mice were inoculated with 108 CFU of either N16961 and Bah1 or the respective ΔhlyA mutants. (B) The entire hlyA gene was introduced into the lacZ loci of ΔhlyA strains KFV103 (PR) and KFV101 (none). Mice were inoculated with both ΔhlyA mutants and the complemented strains (PR::hlyA and none::hlyA). Rates of survival over 7 days are shown.
Hemolysin does not promote bacterial dissemination.
The data presented above show that hemolysin is a primary virulence factor in adult mice. However, the cause of rapid lethality mediated by hemolysin is presently unknown. Some strains of V. cholerae, particularly strains that do not express functional CT, are known to cause septicemia (2, 35). In addition, hemolysin has been shown to be lethal when injected intravenously (23). In vitro data showed that hemolysin has cytolytic activity, and therefore, we considered that hemolysin might increase bacteremia and that mice might die from septic shock. To determine if V. cholerae causes severe tissue damage, mice were inoculated with a high but not lethal dose (107 CFU) of either the wild-type strain P27459 (expressing CT, hemolysin, HA/protease, and MARTXVc), the CT deletion mutant P4 (expressing hemolysin, HA/protease, and MARTXVc), or the multitoxin-deficient strain (expressing no toxins) or mock inoculated with PBS. After 12 h, the small intestines from three independently inoculated mice from each group were collected and sections from the proximal, middle, and distal regions were fixed in formalin, embedded, sliced, and stained with H&E. In all sections, the damage to the intestine was minimal and the intestinal epithelial cells did not appear to be swollen, necrotic, or apoptotic. In addition, inflammation was minimal or absent.
The only observable pathology in wild-type V. cholerae-infected mice was an accumulation of sloughed cells in the distal intestinal lumina of the mice (Fig. 3A and B). These cells were also detected in mice infected with the CT deletion mutant (expressing hemolysin, HA/protease, and MARTXVc) but not in PBS- or multitoxin-deficient mutant-inoculated mice (Fig. 3C and D). These sloughed cells appeared to be necrotic or apoptotic upon the examination (by G. K. Haines) of H&E-stained sections on slides but were negative for immunostaining for CD45, indicating that they were not likely to be innate immune cells undergoing apoptosis (data not shown). These cells were also negative for immunostaining for keratin, although it is possible that these cells were necrotic epithelial cells that had shed the keratin marker. Sections from the proximal and middle intestinal regions also showed sloughed cells in the lumina at a lower density (data not shown). Taken with the other data, the presence of the unidentifiable sloughed cells in the lumina suggested that localized cell death may have been occurring in the small intestines during infection due to accessory toxins but that it was not sufficiently severe to cause extensive tissue damage.
FIG. 3.
Accumulations of sloughed cells (S) are observed in the lumina of mice inoculated with 107 CFU of wild-type V. cholerae (A and B) but not in those of mice inoculated with the multitoxin-deficient mutant (C and D). No damage of the villus epithelia (V) in mice of any group is observed. Shown are representative H&E-stained areas of the distal small intestine 12 hpi. The scale bar represents 50 μm.
To further investigate whether hemolysin leads to increased bacteremia, mice were inoculated with a lethal dose of P4 (expressing hemolysin, HA/protease, and MARTXVc) or the hemolysin deletion mutant KFV103 (expressing HA/protease and MARTXVc). At 6 hpi, bacterial CFU recovered from the small intestines showed that hemolysin did not promote more rapid early growth within the small intestine (Fig. 4A). Bacterial numbers recovered from the spleens and livers of mice inoculated with the hemolysin deletion mutant KFV103 were slightly lower than those recovered from the spleens and livers of P4-inoculated mice, but these differences were not statistically significant (Fig. 4B and C). Also, no significant differences were found by comparing the weights of spleens, livers, and small intestines.
FIG. 4.
Hemolysin was not responsible for bacterial dissemination into the spleen and liver. Four mice were inoculated i.g. with 108 CFU of CT mutant P4 (YPR) or KFV103 (P4 ΔhylA; PR). Small intestines (A), spleens (B), and livers (C) were collected 6 hpi, weighed, homogenized in 5 ml of PBS, and plated for CFU counting. Bacterial numbers per gram of organ for individual mice are shown, along with the medians for the groups.
Overall, we concluded that the high levels of lethality observed after the infection of adult mice with hemolysin-expressing strains were not due to rapid bacterial dissemination that resulted in more severe systemic infections. Thus, hemolysin was contributing to lethality by a presently unknown mechanism that will require further investigation.
Deletion of hemolysin from other El Tor strains also decreases lethality.
To determine whether the high degree of lethality caused by hemolysin was confined to the wild-type strain P27459 or would apply to other El Tor strains, mice were inoculated with 108 CFU of either wild-type N16961, a V. cholerae El Tor O1 Inaba strain (18); Bah1, a vaccine derivative of V. cholerae El Tor O1 Ogawa strain E7946 (44); or their respective hemolysin deletion mutants. Both wild-type strains showed high degrees of lethality in mice, with only one and two of five mice surviving the first 24 h after infection, respectively (Fig. 5A). However, when mice were inoculated with the ΔhlyA mutants, survival was 100% at 24 hpi and 100% and 60% after 7 days. Therefore, hemolysins from other El Tor strains are associated with rapid lethality, further indicating that hemolysin is a major virulence factor of the El Tor biotype.
Reintroduction of hemolysin into ΔhlyA mutants restores lethality.
To find additional evidence that hemolysin is the cause of rapid death, the entire hlyA gene was introduced into the lacZ loci of two ΔhlyA mutants, KFV103 (expressing HA/protease and MARTXVc) and KFV101 (expressing no toxins). Mice were inoculated with 108 CFU of either ΔhlyA strain, KFV103 or KFV101, or one of the two respective strains in which hemolysin had been reintroduced. While survival was 100% after inoculation with either ΔhlyA strain, only 20% of the mice survived after inoculation with the hemolysin-complemented strains (Fig. 5B). Thus, the reintroduction of the hemolysin gene into V. cholerae strains restores a high degree of lethality.
Oral vaccination with the multitoxin-deficient mutant protects mice from lethal infection.
Our data have demonstrated that hemolysin and MARTXVc are linked to virulence during intestinal infection, supporting our previous suggestion that a multitoxin-deficient strain may be an effective vaccine strain as long as it remains sufficiently virulent to stimulate immunity (22). In two separate experiments, mice were inoculated i.g. with a sublethal dose of KFV101 or mock inoculated with PBS and then given booster doses after 7 and 14 days. On day 21, mice were challenged with a lethal dose of wild-type P27459. While 10 (83%) of 12 control mice died rapidly due to P27459, 10 (77%) of 13 KFV101-immunized mice were protected and survived the challenge (Fig. 6). All of the surviving mice developed disease as the health scores dropped to a median ± standard deviation of 2.5 ± 0.4 points for the KFV101-immunized group and 2.3 ± 1.1 for the control group. Weight loss of more than 5% of the body weight at the time of challenge was observed in 8 of the 10 surviving KFV101-immunized mice, compared to 0 of 2 surviving PBS-inoculated mice. These data show that a multitoxin-deficient strain can stimulate protective immunity, suggesting that these reduced-virulence strains may be effective for use as human vaccines against cholera.
FIG. 6.
Prior vaccination with multitoxin-deficient mutant strain KFV101 protected mice from lethal challenge with wild-type P27459. Mice were either immunized with KFV101 (diamonds) or mock inoculated with PBS (triangles). On day 21, all mice were challenged with wild-type P27459, and survival was monitored.
DISCUSSION
Extensive vaccine research with human volunteers has been conducted to elucidate the factors produced by El Tor O1 strains that are responsible for the clinical symptoms of disease in the absence of CT production (26). Although it has been proposed that hemolysin (30), MARTXVc (31), and HA/protease (3) all contribute to this underlying disease syndrome, no adequate models existed to understand the role of these secreted factors in pathogenesis. Researchers from our lab have presented evidence that the accessory toxins are important in the lung model (17, 22) and that accessory toxins are necessary for the prolonged colonization of the adult intestinal tract (36). Here, we used the adult mouse model to study the contribution of accessory toxins to virulence and vaccine efficacy.
We identified the accessory toxins hemolysin and MARTXVc as virulence factors associated with lethality. Previous studies have also associated hemolysin with virulence. In human studies, the deletion of the hemolysin gene in conjunction with the CT gene reduced disease symptoms, although this deletion strain was reactogenic (30). In addition, hemolysin is suggested to be the major virulence factor of Amazonian strains that do not produce CT (9). The deletion of either the hemolysin gene hlyA or the regulator for hemolysin gene expression, hlyU, decreases virulence in infant mice by 100-fold (1, 45). Purified hemolysin was previously shown to cause fluid accumulation in the rabbit ileal loop and the infant mouse (24) and is rapidly lethal for mice upon intravenous administration (23). The MARTXVc toxin was previously linked to pathogenesis in the lung infection model (17).
The quick action of hemolysin was surprising, although the hemolysin gene was previously identified as one of the most highly expressed genes just 9 h after the introduction of El Tor strains into the rabbit duodenum (13). Indeed, this rapid action may have been sufficient to block the effects of CT, which has a pattern of delayed expression in the infant mouse (29). Further evidence for a role of CT in this model will require a demonstration of the timing of CT expression in vivo and the redefinition of inoculation conditions for hlyA mutant strains to facilitate the detection of CT-dependent phenotypes. It is notable that mice inoculated with CT-expressing wild-type strains had more fluid in their upper intestines after sublethal-dose infection than mice infected with non-CT-expressing strains, but this observation has not yet been quantified.
The mechanism of hemolysin-mediated death remains elusive. Extensive research into the toxin structure and function has shown that hemolysin is a cholesterol-dependent pore-forming toxin that damages nucleated cells (25). Indeed, hemolysin is known to cause the necrosis and cytolysis of various cell types (16). If this tissue damage occurred in the small intestinal epithelial layer, it could enhance bacterial spreading and septicemia and cause rapid death. However, the numbers of bacteria recovered from the spleens and livers of mice inoculated with the ΔhlyA strain KFV103 were not significantly lower than the numbers recovered from those of P4-inoculated mice. It is also possible that the high lipopolysaccharide concentrations present in the high doses in these experiments caused the mice to die from endotoxic shock. However, 85% of mice inoculated with the multitoxin-deficient mutant strain KFV101 survived a dose that was lethal when hemolysin-expressing strains were inoculated, which would negate this hypothesis. Furthermore, we found experimental evidence that hemolysin does not enhance initial colonization or early growth to result in death from an increased bacterial load. Therefore, further research using low-dose inoculation and the detection of more subtle phenotypes such as those involving colonization and innate immune responses will be necessary to fully elucidate the action of hemolysin in mice.
A final major impact of this model is the ability to test proposed vaccine strains for the assessment of protective immunity. As V. cholerae is known to be a human pathogen previously thought to be unable to colonize the intestinal tracts of most animal species, vaccine efficacy studies were usually performed with human volunteers. Some immunization studies were performed with rabbits (10), but the use of rabbits is expensive, labor-intensive, and time-consuming. Infant mice have been used to study passive immunity (21), but studies of the immune response are impracticable due to the immaturity of the immune system. Butterton et al. (7) developed a germfree mouse model and used this model to test candidate vaccine strains by comparing immunoglobulin A levels. However, due to the missing intestinal flora during development, the immune responses can differ from those of conventional mice (8). Therefore, this mouse model may become an important tool to test candidate vaccine strains and to understand the mechanism for the reactogenicity of CT-negative strains, as it is possible to test greater numbers of different mutant strains with this model than with human volunteer studies.
Acknowledgments
We thank Amanda Bonebrake, Sarah Antinone, and Brian Meehan for technical assistance, Susan Crawford of the Mouse Phenotyping Facility of the Robert H. Lurie Cancer Center for advice on histopathology, and Paul Cheresh of the Northwestern University Cell Imaging Facility for assistance with microscopic images.
Development of the mouse model was supported by a Biomedical Research Support Program award from the Howard Hughes Medical Institute, and this work was funded by an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund and by the National Institutes of Health grant AI051490 (to K.J.F.S.).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Alm, R. A., G. Mayrhofer, I. Kotlarski, and P. A. Manning. 1991. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine 9:588-594. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, A. M., J. B. Varkey, C. A. Petti, R. A. Liddle, R. Frothingham, and C. W. Woods. 2004. Non-O1 Vibrio cholerae septicemia: case report, discussion of literature, and relevance to bioterrorism. Diagn. Microbiol. Infect. Dis. 49:295-297. [DOI] [PubMed] [Google Scholar]
- 3.Benitez, J. A., L. Garcia, A. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, M. Ramirez, T. Ledon, M. D. Jidy, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXφ-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, P. A., R. E. Weaver, and D. G. Hollis. 1980. Diseases of humans (other than cholera) caused by vibrios. Annu. Rev. Microbiol. 34:341-367. [DOI] [PubMed] [Google Scholar]
- 5.Boardman, B. K., B. M. Meehan, and K. J. F. Satchell. 2007. Growth phase regulation of Vibrio cholerae RTX toxin export. J. Bacteriol. 189:1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterton, J. R., D. T. Beattie, C. L. Gardel, P. A. Carroll, T. Hyman, K. P. Killeen, J. J. Mekalanos, and S. B. Calderwood. 1995. Heterologous antigen expression in Vibrio cholerae vector strains. Infect. Immun. 63:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterton, J. R., E. T. Ryan, R. A. Shahin, and S. B. Calderwood. 1996. Development of a germfree mouse model of Vibrio cholerae infection. Infect. Immun. 64:4373-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 9.Coelho, A., J. R. Andrade, A. C. Vicente, and V. J. Dirita. 2000. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect. Immun. 68:1700-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cray, W. C., Jr., E. Tokunaga, and N. F. Pierce. 1983. Successful colonization and immunization of adult rabbits by oral inoculation with Vibrio cholerae O1. Infect. Immun. 41:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvjetanovic, B., and D. Barua. 1972. The seventh pandemic of cholera. Nature 239:137-138. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faruque, S. M., N. Chowdhury, M. Kamruzzaman, M. Dziejman, M. H. Rahman, D. A. Sack, G. B. Nair, and J. J. Mekalanos. 2004. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. USA 101:2123-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa-Arredondo, P., J. E. Heuser, N. S. Akopyants, J. H. Morisaki, S. Giono-Cerezo, F. Enriquez-Rincon, and D. E. Berg. 2001. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 69:1613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein, R. A., M. Boesman-Finkelstein, and P. Holt. 1983. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F. M. Burnet revisited. Proc. Natl. Acad. Sci. USA 80:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fullner, K. J. 2003. Toxins of Vibrio cholerae: consensus and controversy, p.481-502. In G. Hecht (ed.), Microbial pathogenesis and the intestinal epithelial cell,vol. 26. ASM Press, Washington, DC. [Google Scholar]
- 17.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines III, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia, L., M. D. Jidy, H. Garcia, B. L. Rodriguez, R. Fernandez, G. Ano, B. Cedre, T. Valmaseda, E. Suzarte, M. Ramirez, Y. Pino, J. Campos, J. Menendez, R. Valera, D. Gonzalez, I. Gonzalez, O. Perez, T. Serrano, M. Lastre, F. Miralles, J. Del Campo, J. L. Maestre, J. L. Perez, A. Talavera, A. Perez, K. Marrero, T. Ledon, and R. Fando. 2005. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect. Immun. 73:3018-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg, I., and J. J. Mekalanos. 1986. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J. Bacteriol. 165:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guentzel, M. N., and L. J. Berry. 1974. Protection of suckling mice from experimental cholera by maternal immunization: comparison of the efficacy of whole-cell, ribosomal-derived, and enterotoxin immunogens. Infect. Immun. 10:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haines, G. K., III, B. A. Sayed, M. S. Rohrer, V. Olivier, and K. J. Satchell. 2005. Role of Toll-like receptor 4 in the proinflammatory response to Vibrio cholerae O1 El Tor strains deficient in production of cholera toxin and accessory toxins. Infect. Immun. 73:6157-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda, T., and R. A. Finkelstein. 1979. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect. Immun. 26:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichinose, Y., K. Yamamoto, N. Nakasone, M. J. Tanabe, T. Takeda, T. Miwatani, and M. Iwanaga. 1987. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect. Immun. 55:1090-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikigai, H., A. Akatsuka, H. Tsujiyama, T. Nakae, and T. Shimamura. 1996. Mechanism of membrane damage by El Tor hemolysin of Vibrio cholerae O1. Infect. Immun. 64:2968-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabir, S. 2005. Cholera vaccines: the current status and problems. Rev. Med. Microbiol. 16:101-116. [Google Scholar]
- 27.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenner, J. R., T. S. Coster, D. N. Taylor, A. F. Trofa, M. Barrera-Oro, T. Hyman, J. M. Adams, D. T. Beattie, K. P. Killeen, D. R. Spriggs, et al. 1995. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J. Infect. Dis. 172:1126-1129. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 30.Levine, M. M., J. B. Kaper, D. Herrington, G. Losonsky, J. G. Morris, M. L. Clements, R. E. Black, B. Tall, and R. Hall. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mekalanos, J. J., D. J. Swartz, G. D. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 33.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra, R., P. Figueroa, A. K. Mukhopadhyay, T. Shimada, Y. Takeda, D. E. Berg, and G. B. Nair. 2000. Cell vacuolation, a manifestation of the El Tor hemolysin of Vibrio cholerae. Infect. Immun. 68:1928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ninin, E., N. Caroff, D. El Kouri, E. Espaze, H. Richet, M. L. Quilici, and J. M. Fournier. 2000. Nontoxigenic Vibrio cholerae O1 bacteremia: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 19:489-491. [DOI] [PubMed] [Google Scholar]
- 36.Olivier, V., N. H. Salzman, and K. J. F. Satchell. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75:5043-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockhill, R. C., M. Lesmana, and A. Moechtar. 1983. Improved method, using staphylococcal beta-hemolysin, for detection of hemolysin(s) produced by Vibrio cholerae biotype El Tor. Southeast Asian J. Trop. Med. Public Health 14:181-185. [PubMed] [Google Scholar]
- 38.Satchell, K. J. 2003. Activation and suppression of the proinflammatory immune response by Vibrio cholerae toxins. Microbes Infect. 5:1241-1247. [DOI] [PubMed] [Google Scholar]
- 39.Sheahan, K. L., C. L. Cordero, and K. J. Satchell. 2004. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc. Natl. Acad. Sci. USA 101:9798-9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. Guerrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 64:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tacket, C. O., K. L. Kotloff, G. Losonsky, J. P. Nataro, J. Michalski, J. B. Kaper, R. Edelman, and M. M. Levine. 1997. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD111. Am. J. Trop. Med. Hyg. 56:533-537. [DOI] [PubMed] [Google Scholar]
- 42.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 43.Tacket, C. O., G. Losonsky, J. P. Nataro, S. J. Cryz, R. Edelman, A. Fasano, J. Michalski, J. B. Kaper, and M. M. Levine. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD110, a ΔctxA Δzot Δace derivative of El Tor Ogawa Vibrio cholerae. J. Infect. Dis. 168:1536-1540. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, D. N., K. P. Killeen, D. C. Hack, J. R. Kenner, T. S. Coster, D. T. Beattie, J. Ezzell, T. Hyman, A. Trofa, M. H. Sjogren, et al. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J. Infect. Dis. 170:1518-1523. [DOI] [PubMed] [Google Scholar]
- 45.Williams, S. G., S. R. Attridge, and P. A. Manning. 1993. The transcriptional activator HlyU of Vibrio cholerae: nucleotide sequence and role in virulence gene expression. Mol. Microbiol. 9:751-760. [DOI] [PubMed] [Google Scholar]
- 46.Wu, Z., P. Nybom, and K. E. Magnusson. 2000. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell. Microbiol. 2:11-17. [DOI] [PubMed] [Google Scholar]