Abstract
We previously demonstrated that mice of the I/St strain are extremely susceptible to Mycobacterium tuberculosis, as well as to the taxonomically distant intracellular bacteria Chlamydia pneumoniae and Salmonella enterica. To broaden our knowledge about the control of susceptibility to intracellular pathogens, we studied the infection caused by Mycobacterium avium virulent strain 724 in a panel of inbred mouse strains and found that I/St mice are resistant to M. avium. By comparing I/St mice with B6 mice, we demonstrated that (i) B6 mice are much more susceptible to infection caused by M. avium in terms of bacterial multiplication in the lung tissue and severity of lung pathology; (ii) in B6 mice but not in I/St mice infection leads to prolonged leukocyte infiltration of the lung tissue, development of necrotic lung granulomata, and lethality; and (iii) the unfavorable infectious course in B6 mice is accompanied by elevated production of gamma interferon, tumor necrosis factor alpha, and especially interleukin-12 in the lungs. Importantly, M. avium-resistant I/St mice carry a functional r allele of the Slc11a1 (formerly Nramp1) gene, while B6 mice have the Slc11a1s genotype. Segregation genetic analysis of (I/St × B6) F2 hybrids demonstrated that susceptibility or resistance to infection caused by M. avium largely depended upon the Slc11a1 genotype and that other genetic traits had a relatively weak influence. This close-to-monogenic pattern differs sharply from the host control of many other intracellular bacterial infections, for which the involvement of numerous quantitative trait loci has been ubiquitously observed.
Organisms of the Mycobacterium avium complex, which are widespread environmental mycobacteria, are intracellular human pathogens in the absence of normal T-cell immunity (14, 15). They are present in approximately 70% of patients with advanced untreated AIDS and are considered the major killer in this cohort (24). With a background of less severely impaired immunity (e.g., in older persons and in children), M. avium may cause chronic lung disease (4, 13, 27). In model systems based on infection of C57BL/6 (B6) inbred mice and their genetic derivatives bearing knockout mutations in genes important for immunity, it was demonstrated that the T-cell immune response to M. avium plays both protective and pathological roles in the course of infection. Thus, gamma interferon (IFN-γ) production by CD4+ T lymphocytes is required for protection against rapid death but also causes degenerative events (e.g., granuloma necrosis) in lung tissue (6). Similarly, the presence of intact tumor necrosis factor alpha (TNF-α) receptors is required to maintain the integrity of M. avium-induced granulomas, but granuloma necrosis is likely triggered by TNF-α (3, 7). These features of M. avium infection are very similar to those that reflect the balance between immune defense and pathogenesis in tuberculosis (TB) (35, 37). Given the apparent similarities between TB granulomas in patients and M. avium-induced granulomas in B6 mice (3), the underlying immune and genetic mechanisms are worthy of investigation.
Initial studies of the genetics of susceptibility to M. avium-induced infection were based on traditional comparisons of bacterial loads in organs following intravenous challenge between mice of various inbred strains (1, 29, 40). It was shown that susceptible and resistant phenotypes of individual strains followed the distribution pattern of susceptible (s) and resistant (r) alleles of the Bcg (later Nramp1; now Slc11a1) chromosome 1 gene (38, 41), which encodes a proton-coupled divalent metal ion transporter associated with bacterial phagosomes in macrophages (12). Subsequently, the dependence of susceptibility to infection on expression of r and s alleles of this gene was confirmed using Bcg congenic mouse strains and the model consisting of in vitro infection of macrophages with M. avium (5, 22). However, it is still not known whether Slc11a1 is a major gene predominantly determining the outcome of M. avium-caused disease or accounts for only a small to moderate proportion of the total genetic variation. A priori, the latter possibility seems more realistic, given the polygenic nature of the control of infections caused by Mycobacterium tuberculosis and other intracellular parasites in mouse models (16, 21, 33, 34, 36; for a review, see reference 19) and the marginal to nonexistent role of Slc11a1 in TB susceptibility (20, 25). However, a direct segregation genetic analysis to prove or refute this hypothesis has not been performed so far. Also, a “natural” model of M. avium infection via the respiratory tract was characterized in considerable detail with respect to the host immunity (6) but has never been applied to genetic studies.
Previously, we demonstrated that mice of the I/St inbred strain are exceptionally susceptible to M. tuberculosis infection and develop severe pulmonary disease in various experimental models of acute, chronic, and reactivation TB (9, 26, 32). Susceptibility did not depend upon the Slc11a1 gene, since I/St mice carry the resistant allele (18), but was controlled by a few interacting quantitative trait loci mapped to chromosomes 3, 9, 17, and X (18, 36). Interestingly, when TB-susceptible I/St mice were infected with virulent strains of the taxonomically distant intracellular bacteria Chlamydia pneumoniae and Salmonella enterica serovar Typhimurium, they also displayed a susceptible phenotype characterized by significantly shortened survival times, higher levels of proinflammatory interleukin-6 (IL-6) and TNF-α, and rapid development of lung pathology after C. pneumoniae infection (23). Since in other model systems the severity of infection caused by S. enterica substantially depended on the expression of the r and s alleles of Slc11a1 (42), it was reasonable to assume that I/St mice represent an interesting example of Slc11a1-independent susceptibility to a wide spectrum of intracellular infections. Previously, these mice have not been studied with regard to their immune response to M. avium infection or as a partner for segregation genetic analysis of this infection in a polygenic setting.
In the present study we compared the susceptibilities to the M. avium 724 virulent chicken isolate (31) of several inbred mouse strains. After establishing that I/St mice expressed a surprisingly resistant phenotype with respect to the bacterial loads in organs, the degree of lung pathology, and mortality, we performed segregation genetic and comparative immunologic analyses using B6 mice as a susceptible partner.
MATERIALS AND METHODS
Animals.
Mice of inbred strains A/SnEgYCit (A/Sn), BALB/cJCit (BALB/c), C3H/SnYCit (C3H), I/StSnEgYCit (I/St), and C57BL/6YCit (B6) and (I/St × B6) F1 and F2 hybrids were bred and maintained under conventional, non-specific-pathogen-free conditions at the Animal Facilities of the Central Institute for Tuberculosis (Moscow, Russia) in accordance with guidelines from the Russian Ministry of Health (guideline 755) and the NIH Office of Laboratory Animal Welfare (assurance A5502-06). Water and food were provided ad libitum. Female mice that were 2.5 to 3.0 months old at the beginning of experiments were used. All experimental procedures were approved by the Central Institute for Tuberculosis institutional animal care committee.
Infection.
Mice were infected with M. avium strain 724, which has been characterized previously (31) and was a kind gift from T. Ulrichs, Max Planck Institute for Infectious Biology, Berlin, Germany. Following 3 weeks of growth in Dubos broth at 37°C, mycobacteria were suspended in sterile saline containing 0.05% Tween 20 and kept at −80°C until they were used. For intravenous and intratracheal infections, 107 and 104 CFU, respectively, were inoculated into the tail lateral vein or cannulated trachea as previously described for M. tuberculosis infection (9, 26). Mice were also infected by the respiratory route with 2.5 × 103 viable CFU using an inhalation exposure system (Glas-Col, Terre Haute, IN). Animals were exposed for 40 min to an aerosol produced by nebulizing 8 ml of a bacterial suspension in a phosphate-buffered saline solution with 0.05% Tween 80 containing 1 × 107 bacilli/ml.
CFU counting.
At indicated time points following infection, lungs from individual mice were homogenized in 2.0 ml of sterile saline, and 0.2-ml samples of 10-fold serial dilutions were plated on Dubos agar (Difco) and incubated at 37°C for 20 to 22 days before M. avium CFU were counted.
Lung cell immunophenotyping by flow cytometry.
Lung cell suspensions obtained by enzymatic disruption of lung tissue as described previously (8, 9) were washed twice in phosphate-buffered saline containing 0.01% NaN3 and 0.5% bovine serum albumin and incubated for 15 min at 4°C in the presence of CD16/CD32 monoclonal antibody (clone 2.2G2; PharmMingen, San Diego, CA) to block Fc receptors. Cells were then stained with the following directly conjugated antibodies according to the manufacturer's instructions: fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (clone H129.19; PharMingen), FITC-conjugated anti-CD8a (clone 53-6.7; PharMingen), phycoerythrin-conjugated anti-CD19 (clone 1D3; PharMingen), FITC-conjugated MAC-3 (clone M3/84; PharMingen), and FITC-conjugated anti-Ly6G (clone RB6-8C5; Caltag, Burlingame, CA). Stained cells (104 cells per sample) were washed twice, fixed with 1% paraformaldehyde, and analyzed by flow cytometry using a FACSCalibur cytometer (BD, San Diego, CA) and BD-CellQuestPro (Becton Dickinson) and FlowJo 4.5.9 (Tree Star, Inc., San Carlos, CA) software.
Histopathology.
Three, eight, and sixteen weeks after infection with M. avium, lung tissue was examined for pathology. Mice were euthanized by a thiopental overdose. Lung tissue (the middle right lobe) was frozen using a −60 to −20°C temperature gradient in an electronic Cryotome (ThermoShandon, United Kingdom), and serial 6- to 8-μm-thick sections were obtained across the widest area of the lobe. Sections were stained with hematoxylin and eosin and examined by an experienced pathologist without knowledge of the experimental group.
Cytokine assays.
At weeks 4 and 8 postinfection, whole lungs from individual mice were homogenized in 2 ml of sterile saline, and suspensions were frozen at −30°C until they were used. Immediately before assaying, thawed samples were centrifuged at 800 × g to remove debris, and IFN-γ, IL-6, IL-10, IL-12, and TNF-α were measured in supernatants using sandwich enzyme-linked immunosorbent assays (ELISA). The following ELISA kits were purchased from PharMingen and used according to the manufacturer's instructions: OptEIA mouse IFN-γ set (sensitivity, 21 pg/ml), OptEIA mouse TNF-α set (31 pg/ml), OptEIA mouse IL-12 set (31 pg/ml), OptEIA mouse IL-10 set (63 pg/ml), and OptEIA mouse IL-6 set (15.6 pg/ml).
Slc11a1 genotyping.
Slc11a1 alleles in (I/St × B6) F2 mice were determined by PCR-restriction fragment length polymorphism analysis using BcmFI restriction nuclease (NEB, Beverly, MA). The PCR product of a portion of the Slc11a1 gene containing a G169D mutation in the s allelic variant was amplified from genomic DNA using native Pfu polymerase (Stratagene, La Jolla, CA) under the following conditions: forward primer 5′-CCCACCCCCATCTATGTTATCA-3′, reverse primer 3′-CCCTGCCTACTTTTATCCCCCAA-5′, and 2 min of denaturation at 94°C followed by 30 cycles of 94°C for 15 s, 66°C for 20 s, and 72°C for 45 s. Length variants were resolved on 4% agarose gels.
Statistical analysis.
Differences between experimental groups were considered to be statistically significant at a P value of <0.05. A one-way analysis of variance, construction of Gohan's survival curves, and Fisher's processing of the data were performed using BIOSTAT (Practica, Moscow, Russia) and GraphPad Prism 4 software.
RESULTS AND DISCUSSION
Susceptibility to infection with M. avium follows the Slc11a1 allelic pattern irrespective of the dose and route of challenge.
In previous studies it has been demonstrated that the capacity of mice to control multiplication of M. avium in livers and spleens follows the distribution pattern of the r and s alleles of the Slc11a1 (formerly Bcg) gene (1, 29). However, it was worthwhile to perform a small-scale screening experiment in order to (i) include lung CFU counts in the analysis; (ii) confirm that non-specific-pathogen-free mice follow the Slc11a1 pattern of susceptibility; (iii) add the TB-supersusceptible I/St strain to the panel; and (iv) use the “natural,” respiratory route of infection.
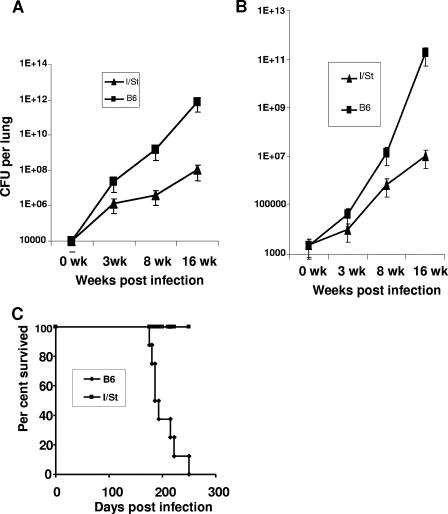
We first infected mice of the B6 (Slc11a1s), BALB/c (Slc11a1s), C3H (Slc11a1r), A/Sn (Slc11a1r), and I/St (Slc11a1r) strains in groups of 10 with 107 M. avium strain 724 CFU/mouse intravenously and evaluated the mycobacterial loads in their lungs at weeks 3 and 8 postchallenge. In agreement with earlier findings, approximately 1.5- to 2-log differences between Slc11a1r and Slc11a1s strains were universally observed (data not shown). To our surprise, I/St mice were resistant to M. avium infection despite their high susceptibility not only to M. tuberculosis but also to taxonomically distant intracellular bacteria (see above). We then performed experiments in which infection was initiated by introducing M. avium via the respiratory tract and multiplication of mycobacteria was followed for a substantially longer period. Delivery of mycobacteria by either intratracheal instillation (Fig. 1A) or aerosol exposure (Fig. 1B) in B6 and I/St mice provided phenotypes consistent with the phenotypes observed after intravenous challenge, and the interstrain differences increased up to 4 logs during the course of the disease. Following dissemination at week 3 postinfection, the difference in mycobacterial multiplication in spleens of B6 and I/St mice reached approximately 3 logs by week 16 (data not shown). Moreover, the resistant and susceptible phenotypes of I/St and B6 mice, respectively, were readily confirmed in a survival experiment using the aerosol route of infection; all B6 mice died by 7 months postinfection, whereas all I/St mice survived at least 11 months, when the experiment was terminated (Fig. 1C). Thus, the disease caused by M. avium infection is effectively controlled by I/St mice, which is profoundly different from infections caused by M. tuberculosis and other intracellular bacteria (23).
FIG. 1.
I/St mice are resistant and B6 mice are susceptible to M. avium infection administered via the respiratory tract. I/St and B6 mice were infected with either 104 CFU given as an intratracheal inoculum (A) or ∼2 × 103 CFU given in aerosol (B and C). The size of the initially inhaled mycobacterial dose was estimated by plating lung homogenates from mice of the two strains at 24 h postinfection in groups of three in two independent experiments. No statistical difference in the mean size of the challenge dose between I/St mice (2.5 × 103 CFU per lung) and B6 mice (2.2 × 103 CFU per lung) was observed (P = 0.24, n = 6). At the indicated time points, the numbers of lung CFU in four individual mice were estimated as described in Materials and Methods (A and B). The results of one of four similar experiments are expressed as the means ± standard deviations (n = 4 for each point). For panel C, the mortality of B6 and I/St mice in groups of eight animals was monitored until all B6 mice died. All I/St mice survived >10 months.
Lung pathology.
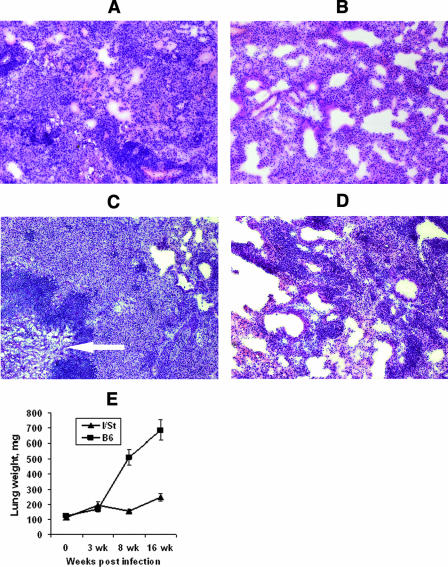
M. avium 724 infection in mice was suggested as a model of TB lung pathology in humans (3, 6). Therefore, we determined if the differences in mycobacterial multiplication in the lungs and survival time were reflected by the dynamics of lung pathology. After 3 weeks of infection, lung tissue from both B6 and I/St mice showed slight hyperemia, greater thickness of alveolar septa, and low-grade mononuclear infiltration (data not shown). By week 8, lungs of B6 mice displayed numerous granulomas of different sizes, infiltration of peribroncheal and perivascular zones, severe hyperemia, and very thick alveolar septum walls (Fig. 2A). Granulomas contained macrophages and epithelioid cells surrounded by lymphocytes. The lung pathology in I/St mice was substantially milder, represented mainly by moderate infiltration and increased thickness of alveolar septum walls in the absence of condensed inflammatory foci (Fig. 2B). By week 16 of infection, the differences between B6 and I/St lungs became striking. B6 mice developed severe lung pathology characterized by large atelectasis, hyperemia, edema, and necrotic foci. Both diffuse and compact cell infiltrations of the lung, as well as large foci with central caseous necrosis surrounded by a well-established zone of degenerating neutrophils, demarcated from the remaining breathing tissue by a wide layer of mononuclear cells, were readily observed (Fig. 2C). This is in full agreement with the results obtained by Ehlers et al. (6) after aerogenic challenge of B6 mice with a 10-fold-higher dose of M. avium 724. Lung pathology in I/St mice was significantly less prominent and was represented by small and medium-size granulomas with central zones containing live macrophages and epithelioid cells without signs of necrosis (Fig. 2D). Interstrain differences in the degree of lung pathology were reflected by the lung weights, which were manyfold higher for B6 mice than for I/St mice at weeks 8 and 16 postinfection (Fig. 2E).
FIG. 2.
B6 mice develop substantially more severe lung pathology than I/St mice develop following infection with M. avium. At weeks 8 (A and B) and 16 (C and D) after aerosol challenge, cryosections of lung tissue from B6 (A and C) and I/St (B and D) mice were stained with hematoxylin-eosin and examined microscopically (magnification, ×125). The results of one of two similar experiments are displayed. At week 8, numerous granulomas were present in the lungs of B6 mice (A), whereas the lung pathology of I/St mice was represented by moderate infiltration and an increased thickness of alveolar septa (B). At week 16, B6 mice developed severe lung pathology (C) with necrotic foci losing their vascular appearance (arrow). Lung pathology in I/St mice was significantly less prominent, represented by small and medium-size granulomas (D). The weight of lungs estimated in two independent experiments with four mice each (n = 8) was significantly (P < 0.001) greater in B6 mice than in I/St mice at weeks 8 and 16 of infection (E).
Thus, the conclusion that lung pathology triggered by M. avium in mice and lung pathology triggered by M. tuberculosis in humans have similar features (3, 6) is correct, as far as susceptible B6 mice are concerned, but the similarity is not universal. In resistant I/St mice, the hallmark of “human-type TB lung pathology,” i.e., central caseous necrosis of granulomas, never developed following M. avium infection, whereas the opposite was true for the M. tuberculosis-triggered disease (A. S. Apt, E. V. Kondratieva, and M. M. Averbakh, Jr., unpublished data). Furthermore, in our model system there was an obvious correlation between two parameters of susceptibility to infection: M. avium grew significantly better in the lungs, and the degree of lung pathology was significantly greater in susceptible B6 mice than in resistant I/St mice (Fig. 1 and 2). In contrast, it was reported recently that granuloma necrosis developed in the lungs of B6 mice but not in the lungs of BALB/c and DBA/1 mice despite similar levels of M. avium multiplication in all three strains (10). The most likely explanation for this difference is that in our system interstrain phenotypic differences were determined largely by the alleles of the Slc11a1 gene (see below), whereas in the study of Florido and Appelberg (10) all mouse strains had the Slc11a1s genotype and apparently another genetic polymorphism(s) was operating.
Dynamics of cellular infiltration of the lung.
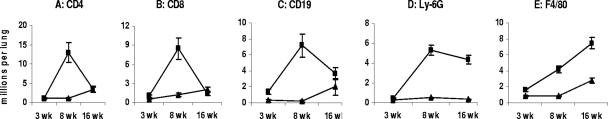
To characterize the dynamics of lung inflammation in more detail, we performed a fluorescence-activated cell sorting analysis of the enzyme-digested lung tissue at different stages of the disease (Fig. 3). In agreement with histopathology data, there was little difference between B6 and I/St lungs at week 3 of infection in terms of the lung content of CD4+ and CD8+ T cells, CD19+ B cells, Mac-3+ macrophages, and Ly-6G+ neutrophil granulocytes. However, by week 8 postchallenge the numbers of T cells, B cells, and neutrophils had increased 10- to 20-fold in B6 mice (Fig. 3A-D), reflecting the rapid development of lung inflammation. The increase in macrophage content was less prominent (∼2.5-fold) but also statistically significant (P < 0.01) (Fig. 3E). In contrast, no statistically significant changes in lung cell content between weeks 3 and 8 of infection were observed in I/St mice. By week 16 of infection, the numbers of T lymphocytes in the lungs of B6 mice had dropped dramatically (Fig. 3A and B), whereas abundant infiltration with macrophages, neutrophils, and, to a somewhat lesser extent, B lymphocytes continued (Fig. 3C to E). In I/St mice, the sizes of the lymphocyte and macrophage populations in the lungs slowly increased between weeks 8 and 16 of infection, which is in a good agreement with slowly progressing lung pathology. Importantly, in sharp contrast to the inflammatory response induced by M. tuberculosis infection (8), following M. avium challenge lung neutrophil influx in I/St mice remained very low throughout the infectious course. The lack of necrosis and a low level of lung neutrophil influx seem to be characteristic features of mycobacterial infections efficiently controlled by a resistant host, as demonstrated previously for M. tuberculosis (8, 30) and here for M. avium infections.
FIG. 3.
Differences in the cell content in the lungs of B6 and I/St mice during infection with M. avium. By week 8 postchallenge, the numbers of CD4 T cells (A), CD8 T cells (B), B cells (C), neutrophils (D), and macrophages (E) had increased significantly (P < 0.01) in B6 mice compared with the numbers at week 3, but the numbers had not changed in I/St mice. At week 16, the numbers of T lymphocytes in the lungs of B6 mice had dropped, whereas abundant infiltration with macrophages and neutrophils continued. In I/St mice, the sizes of the lymphocyte and macrophage populations in the lungs slowly increased between weeks 8 and 16 of infection. The results of one of two similar experiments obtained with three individual mice per time point are expressed as means ± standard errors of the means.
Cytokine response in the lungs.
The balance between different cytokines produced locally by cells of the immune system is thought to be critical in determining protection versus immune pathology in the course of infections caused by M. avium (6) and M. tuberculosis (28). Thus, we compared mice of the two strains with regard to their capacity to produce key effector and inflammatory cytokines in the lungs shortly after initiation (week 4) and at the peak (week 8) of the inflammatory response, using whole-organ homogenates for the analysis. As shown in Table 1, the levels of IFN-γ, TNF-α, and IL-12 were strikingly higher in the lungs of B6 infected mice than in the lungs of I/St infected mice. At week 8 postinfection interstrain differences in cytokine contents may be explained by underlying differences in the numbers of cytokine-producing cells per organ (Table 1 and Fig. 3). However, at the earlier stage of the disease, when the cellularity of I/St lungs and the cellularity of B6 lungs were similar, differences in cytokine production may have been due to the different sizes of the mycobacterial loads (i.e., antigenic stimuli) in the two mouse strains (Fig. 1) or to the genetically distinct capacities to produce proinflammatory cytokines in response to M. avium or both. Whatever the reason, these differences may have had a prominent influence on lung pathology. Whereas an intact IL-12-IFN-γ cytokine axis is required for protective immunity against M. avium (2, 10, 11), the hyperproduction of these mediators by B6 mice observed in our experiments (Table 1) likely led to severe lung inflammation and necrosis (Fig. 2), which is in agreement with the results and concept of Ehlers et al. (6).
TABLE 1.
Cytokine production by lung cells from B6 and I/St mice infected with M. aviuma
| Time postinfection (wk) | Mouse strain | Total lung cellularity (106) | Cytokine concn (pg/ml)
|
|||
|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-12 | IL-6 | |||
| 4 | B6 | 26 ± 4 | 1,688 ± 162b | 432 ± 58b | 6,481 ± 550b | 128 ± 22b |
| I/St | 17 ± 3 | 25 ± 4 | <31c | 278 ± 31 | <16c | |
| 8 | B6 | 92 ± 12b | 2,541 ± 275b | 793 ± 114b | >12,000b,d | <16c |
| I/St | 20 ± 5 | 38 ± 6 | 51 ± 20 | 1,105 ± 528 | <16c | |
Mice were infected aerogenically with ∼2 × 103 CFU of M. avium as described in Materials and Methods, and at the indicated time points lungs were removed, homogenized in 2 ml of sterile saline, and used both for immediate plating to estimate CFU counts and for evaluation of the cytokine content. To this end, homogenates were frozen at −30°C until they were used. After thawing, tubes were centrifuged to remove cell debris, and the supernatant was used for evaluation of cytokines with the ELISA format as described in Materials and Methods. The results obtained in one of two similar experiments, each involving three mice evaluated individually, are expressed as means ± standard deviations. The IL-10 levels were always below the sensitivity of the test.
P < 0.001 for a comparison with the I/St counterparts.
Below the sensitivity of the kit used.
Above the plateau level of the calibration curve.
Slc11a1 is a major gene determining susceptibility to M. avium.
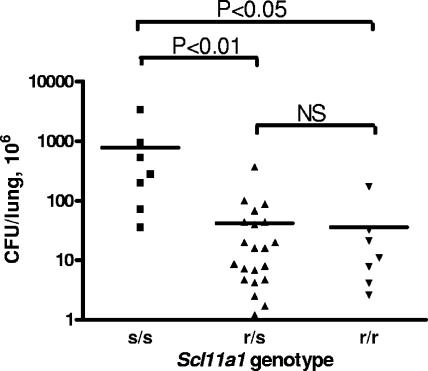
Despite their Slc11a1r genotype, I/St mice proved to be extremely susceptible to three different intracellular bacteria whose interactions with the mouse host are under polygenic control (23). In order to directly assess the role of the Slc11a1 gene in M. avium infection, we performed a segregation genetic analysis with (B6s/s × I/Str/r) F2 mice, using restriction polymorphism of an Slc11a1 PCR product for genotyping and counting lung CFU in week 5 of infection as a reliable parameter of susceptibility. We anticipated that segregation of numerous genetic loci involved in the control of infection would provide a continuum of phenotypes without obvious linkage with Slc11a1 alleles, at least in small samples, especially since F1 hybrids between the two mouse strains expressed an intermediate susceptibility phenotype, although it was biased towards resistance (data not shown). To our surprise, a simple segregation picture for F2 mice was obtained, in that the lung CFU counts were significantly (P < 0.01 to P < 0.05) higher in animals having the Slc11a1s/s genotype (Fig. 4). Susceptibility to M. avium infection was inherited as a single recessive Mendelian trait (χ2 = 1.39; n′ = 2). No difference between groups of resistant mice having the Slc11a1r/r and Slc11a1r/s genotypes was found, which confirms the dominant nature of the r allele (38). High deviation values for both resistant groups suggest that “resistance” might be a more complex trait than “susceptibility,” and non-Slc11a1 loci may modify the expression level of the former. In this regard, the recent finding of Florido and Appelberg (10) that the Hc locus encoding complement component C5 is involved in the control of M. avium granuloma necrosis is of particular interest. I/St mice carry the Hc0 allele and have a nondetectable level of complement (39), which opens the possibility that Hc segregation in an Slc11a1-resistant background may modify lung pathology and thus the conditions of M. avium growth, leading to variable CFU counts, while with a background of a “major” defect in the Slc11a1 gene these minor variations remain invisible.
FIG. 4.
Susceptibility to infection is largely determined by the Sc11a1 gene. Individual (I/St × B6) F2 mice were genotyped to determine the r and s alleles of the Sc11a1 gene and infected aerogenically with ∼2 × 103 M. avium CFU, and the lung CFU were assessed at week 5 postinfection. The significance of the differences between groups of mice having the s/s, r/s, and r/r genotypes is indicated. NS, not significant.
Overall, our results indicate that, unlike many other intracellular bacterial infections, including TB, infection caused by M. avium is predominantly controlled by the Slc11a1 gene. A putative functional role of this gene as an endosome efflux pump that sequesters iron and possibly other divalent cations from the endosomal system (12, 17) suggests a difference between M. avium and M. tuberculosis in intraendosomal, 2+-cation-dependent metabolism. Recent studies by Wagner et al. (43) with an in vitro macrophage infection system demonstrated a similar ability of the two species to accumulate and retain iron in the phagosome using transferrin receptor and siderophore production. Although nickel and zinc concentrations were significantly higher in M. avium-infected phagosomes than in M. tuberculosis-infected phagosomes at 1 h postinfection, the differences disappeared shortly thereafter, leaving open the question about possible physiological consequences of these temporary shifts. Presently, it is not clear whether a functional r allele deprives M. avium of the host sources of 2+ cations more efficiently than it deprives M. tuberculosis.
Acknowledgments
This work was financially supported by grants from the Russian Foundation for Basic Research to M.M.A. and A.S.A.
We are grateful to David McMurray for critically reading the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Appelberg, R., and A. M. Sarmento. 1990. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin. Exp. Immunol. 80:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R. 2006. Pathogenesis of Mycobacterium avium infection: typical responses to an atypical mycobacterium? Immunol. Res. 35:179-190. [DOI] [PubMed] [Google Scholar]
- 3.Benini, J., E. M. Ehlers, and S. Ehlers. 1999. Different types of pulmonary granuloma necrosis in immunocompetent vs. TNFRp55-gene-deficient mice aerogenically infected with highly virulent Mycobacterium avium. J. Pathol. 189:127-137. [DOI] [PubMed] [Google Scholar]
- 4.Benson, C. A. 1994. Disease due to the Mycobacterium avium complex in patients with AIDS: epidemiology and clinical syndrome. Clin. Infect. Dis. 3:S218-S222. [DOI] [PubMed] [Google Scholar]
- 5.de Chastellier, C., C. Frehel, C. Offredo, and E. Skamene. 1993. Implication of phagosome-lysosome fusion in restriction of Mycobacterium avium growth in bone marrow macrophages from genetically resistant mice. Infect. Immun. 61:3775-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlers, S., J. Benini, H.-D. Held, C. Roeck, G. Alber, and S. Uhlig. 2001. γδ T cell receptor-positive cells and interferon-γ, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 194:1847-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers, S., S. Kutsch, E. M. Ehlers, J. Benini, and K. Pfeffer. 2000. Lethal granuloma disintegration in mycobacteria-infected TNFRp55−/− mice is dependent on T cells and IL-12. J. Immunol. 165:483-492. [DOI] [PubMed] [Google Scholar]
- 8.Eruslanov, E. B., I. V. Lyadova, T. K. Kondratieva, K. B. Majorov, I. V. Scheglov, M. O. Orlova, and A. S. Apt. 2005. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect. Immun. 73:1744-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eruslanov, E. B., K. B. Majorov, M. O. Orlova, V. V. Mischenko, T. K. Kondratieva, A. S. Apt, and I. V. Lyadova. 2004. Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge. Clin. Exp. Immunol. 135:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florido, M., and R. Appelberg. 2006. Genetic control of immune-mediated necrosis of Mycobacterum avium granulomas. Immunology 118:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florido, M., A. S. Goncalves, M. S. Gomes, and R. Appelberg. 2004. CD40 is required for the optimal induction of protective immunity to Mycobacterium avium. Immunology 111:323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-405. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, D. E. 1997. Nontuberculous mycobacteria. Curr. Opin. Pulm. Med. 3:139-145. [DOI] [PubMed] [Google Scholar]
- 14.Horsburgh, C. R., Jr. 1991. Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS). N. Engl. J. Med. 324:1332-1338. [DOI] [PubMed] [Google Scholar]
- 15.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramnik, I., W. F. Dietrich, P. Demant, and B. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn, D., W. P. Lafuse, and B. S. Zwilling. 2001. Iron transport into Mycobacterium avium-containing phagosomes from an Nramp1(Gly169)-transfected RAW264.7 macrophage cell line. J. Leukoc. Biol. 69:43-50. [PubMed] [Google Scholar]
- 18.Lavebratt, C., A. S. Apt, B. V. Nikonenko, M. Schalling, and E. Schurr. 1999. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J. Infect. Dis. 180:150-156. [DOI] [PubMed] [Google Scholar]
- 19.Lengeling, A., K. Pfeffer, and R. Balling. 2001. The battle of two genomes: genetics of bacterial/host pathogen interactions in mice. Mamm. Genome 12:261-271. [DOI] [PubMed] [Google Scholar]
- 20.Medina, E., and R. J. North. 1996. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J. Exp. Med. 183:1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsos, L.-M., L. R. Cardon, A. Fortin, L. Ryan, R. LaCrouse, R. J. North, and P. Gros. 2000. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 1:467-477. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, R. M. 1992. Effect of natural resistance gene on the immune response against Mycobacterium avium complex infection. Kekkaku 67:41-46. [PubMed] [Google Scholar]
- 23.Nesterenko, L. N., D. V. Balunets, A. S. Tomova, J. M. Romanova, J. S. Alyapkina, N. A. Zigangirova, M. A. Kapina, E. V. Kondratieva, A. V. Pichugin, K. B. Majorov, and A. S. Apt. 2006. Mycobacterium tuberculosis-susceptible I/St mice develop severe disease following infection with taxonomically distant bacteria, Salmonella enterica and Chlamydia pneumoniae. Clin. Exp. Immunol. 146:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nightingale, S. D., L. T. Byrd, P. M. Southern, J. D. Jockusch, S. X. Cal, and B. A. Wynne. 1992. Incidence of Mycobactereium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J. Infect. Dis. 165:1082-1085. [DOI] [PubMed] [Google Scholar]
- 25.Nikonenko, B. V., A. S. Apt, M. B. Mezhlumova, V. G. Avdienko, V. V. Yeremeev, and A. M. Moroz. 1996. Influence of the mouse Bcg, Tbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of BCG vaccination. Clin. Exp. Immunol. 104:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikonenko, B. V., M. M. Averbakh, Jr., C. Lavebratt, E. Schurr, and A. S. Apt. 2000. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuber. Lung Dis. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 27.Nolt, D., M. G. Michaels, and E. R. Wald. 2003. Intrathoracic disease from nontuberculous mycobacteria in children: two cases and a review of the literature. Pediatrics 112:e434. [DOI] [PubMed] [Google Scholar]
- 28.North, R. J., and Y.-J. Jung. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599-623. [DOI] [PubMed] [Google Scholar]
- 29.Orme, I. M., R. W. Stokes, and F. M. Collins. 1986. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect. Immun. 54:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan, H., B. S. Yan, Y. V. Shebzukhov, H. Zhou, L. Kobzik, D. E. Higgins, M. J. Daly, B. R. Bloom, and I. Kramnik. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedrosa, J., M. Florido, Z. M. Kunze, A. G. Castro, F. Portaels, J. McFadden, M. T. Silva, and R. Appelberg. 1994. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin. Exp. Immunol. 98:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radaeva, T. V., B. V. Nikonenko, V. V. Mischenko, M. M. Averbakh, Jr., and A. S. Apt. 2005. Direct comparison of low-dose and Cornell-like models of chronic and reactivation tuberculosis in genetically susceptible I/St and resistant B6 mice. Tuberculosis 85:65-72. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, L. J., T. M. Baldwin, J. M. Curtis, E. Handman, and S. J. Foote. 1997. Resistance to Leishmania major is linked to the H2 region on chromosome 17 and to chromosome 9. J. Exp. Med. 185:1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts, L. J., T. M. Baldwin, T. P. Speed, E. Handman, and S. J. Foote. 1999. Chromosomes X, 9, and the H2 locus interact epistatically to control Leishmania major infection. Eur. J. Immunol. 29:3047-3050. [DOI] [PubMed] [Google Scholar]
- 35.Rook, G. A. W., and R. Hernandez-Pando. 1996. The pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-282. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez, F., T. V. Radaeva, B. V. Nikonenko, A.-S. Persson, S. Sengul, M. Schalling, E. Schurr, A. S. Apt, and C. Lavebratt. 2003. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infect. Immun. 71:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 38.Skamene, E., P. Gros, A. Forget, P. A. Kongshavn, C. Charles, and B. A. Taylor. 1982. Genetic regulation of resistance to intracellular pathogens. Nature 297:506-508. [DOI] [PubMed] [Google Scholar]
- 39.Staats, J. 1976. Standardized nomenclature for inbred strains of mice: sixth listing. Cancer Res. 36:4333-4377. [PubMed] [Google Scholar]
- 40.Stokes, R. W., I. M. Orme, and F. M. Collins. 1986. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect. Immun. 54:811-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-479. [DOI] [PubMed] [Google Scholar]
- 42.Vidal, S. M., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner, D., J. Maser, B. Lai, Z. Cai, C. E. Barry III, K. H. zu Bentrup, D. G. Russel, and L. E. Bermudez. 2005. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J. Immunol. 174:1491-1500. [DOI] [PubMed] [Google Scholar]