Abstract
Aggregatibacter (Actinobacillus) actinomycetemcomitans is the causative organism of localized aggressive periodontitis, a rapidly progressing degenerative disease of the gingival and periodontal ligaments, and is also implicated in causing subacute infective endocarditis in humans. The bacterium produces a variety of virulence factors, including an exotoxic leukotoxin (LtxA) that is a member of the repeats-in-toxin (RTX) family of bacterial cytolysins. LtxA exhibits a unique specificity to macrophages and polymorphonuclear cells of humans and other primates. Human lymphocyte function-associated antigen 1 (LFA-1) has been implicated as the putative receptor for LtxA. Human LFA-1 comprises the CD11a and CD18 subunits. It is not clear, however, which of its subunits serves as the functional receptor that confers species-specific susceptibility to LtxA. Here we demonstrate that the human CD18 is the receptor for LtxA based on experiments performed with chimeric β2-integrins recombinantly expressed in a cell line that is resistant to LtxA effects. In addition, we show that the cysteine-rich tandem repeats encompassing integrin-epidermal growth factor-like domains 2, 3, and 4 of the extracellular region of human CD18 are critical for conferring susceptibility to LtxA-induced biological effects.
Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans is an oral bacterium that causes localized aggressive periodontitis, a rapidly progressing degenerative disease that occurs primarily in adolescent humans (38). The disease results in loss of alveolar bone if left untreated. A. actinomycetemcomitans is also implicated in subacute infective endocarditis (3, 7), brain abscess formation, urinary tract infections, and other systemic diseases in humans (15). The bacterium produces an array of virulence factors, including an exotoxic leukotoxin (LtxA) which is a member of the repeats-in-toxin (RTX) family of bacterial cytolysins. There is evidence to suggest that highly leukotoxic strains of A. actinomycetemcomitans are more often associated with aggressive forms of periodontitis in humans than the minimally leukotoxic variants (4, 13, 14). LtxA plays an important role in localized aggressive periodontitis pathogenesis by helping the bacterium destroy gingival crevice polymorphonuclear leukocytes (PMNs) and monocytes, resulting in the suppression of local immune defenses (1). It lyses leukocytes, induces degranulation of PMNs and apoptosis in T lymphocytes and PMNs, releases proinflammatory cytokines from human macrophages (21, 26), and lyses erythrocytes (2). There is also experimental evidence to support the hypothesis that LtxA directly perturbs mitochondrial function to induce cellular apoptosis (24). Recent work on LtxA described a caspase 1-dependent pathway in LtxA-induced apoptosis in human monocytes and suggested that it requires expression of lymphocyte function-associated antigen 1 (LFA-1) on the cell surface (22). Furthermore, tumor necrosis factor alpha (TNF-α) acts as a potent stimulator of LtxA-induced apoptosis in HL-60 cells via TNF receptor 1-mediated upregulation of LFA-1 expression (37). Despite the extensive DNA sequence homology shared by the RTX family, there is a marked dichotomy among the members of the family with respect to target cell specificity. For example, the hemolysins secreted by Escherichia coli and Actinobacillus pleuropneumoniae are toxic to a wide range of cell types from different species. The other category of the RTX family includes the leukotoxins from A. actinomycetemcomitans and Mannheimia haemolytica. While A. actinomycetemcomitans LtxA selectively interacts only with leukocytes from humans and primates (33, 35), the leukotoxin (LktA) from M. haemolytica interacts with only ruminant leukocytes (5, 6, 20). This restricted host cell specificity suggests that the species-specific effects of both leukotoxins are mediated through unique receptors on the target cells and the toxins possess precise regions that recognize and interact with these receptors. The principal feature of this species recognition region of the leukotoxins is that it contains a series of 14 tandemly repeated nonapeptides that have the consensus sequence GGXGXDX(L/I/V/W/Y/F)X, where X is any amino acid (25).
Lally et al. have provided compelling evidence to support the role of a β2-integrin family, namely, human LFA-1, as a receptor for A. actinomycetemcomitans LtxA (27). LFA-1 is made up of the CD11a and CD18 subunits, and CD18 is also part of other β2-integrins, including Mac1 and p150/95. However, it is not clear whether other β2-integrins and which of the subunits of the β2-integrins serve as the functional receptor for LtxA. Therefore, the objective of this study was to identify which subunit of β2-integrins served as the functional receptor for LtxA and to locate the domain within the subunit responsible for conferring species-specific susceptibility to LtxA.
MATERIALS AND METHODS
cDNAs.
Human CD18 cDNA in a pOTB7 vector obtained from Invitrogen (Carlsbad, CA) was subcloned into the MigR1 retroviral vector (32). Human CD11a (28) cDNA in the pAPRM8 vector was purchased from Addgene (Cambridge, MA) and subcloned into the pMSCVpuro retroviral vector (Clontech Laboratories Inc., Palo Alto, CA). Bovine CD18 cDNA in pBluescript SK(−) was a gift from Marcus Kehrli (NADC, Ames, IA) and was subcloned into the MigR1 vector. Bovine CD11a cDNA (11) in a pGEM-T vector, which was generated in our laboratory, was subcloned into the pMSCVpuro vector.
Generation of chimeric human/bovine CD18 constructs by domain swapping.
The various chimeric constructs generated using the bovine and human CD18 cDNAs are depicted in Fig. 1. The MEGAWHOP domain swapping technique of Miyazaki et al. (30, 31), described by us previously (10), was used to generate each construct. This is a two-step technique, and the primers used in this procedure were reported by us previously (9, 10). All 13 chimeric constructs were then sequenced at the Advanced Genetic Analysis Center, University of Minnesota, Minneapolis. Comparison of the sequence data from each construct with both human CD18 and bovine CD18 using MegAlign (DNAStar, Madison, WI) revealed that all constructs contained the appropriate swapped regions, with no nonspecific point mutations.
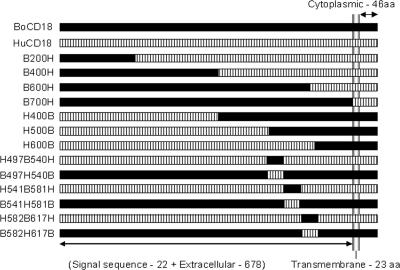
FIG. 1.
Schematic representation (not on scale) of the different chimeric CD18 constructs. B200H, the N-terminal 200 amino acids (aa) of human CD18 were replaced with bovine CD18; B400H, the N-terminal 400 amino acids of human CD18 were replaced with bovine CD18; B600H, the N-terminal 600 amino acids of human CD18 were replaced with bovine CD18; B700H, the entire extracellular domain of human CD18 was replaced with bovine CD18; H400B, the N-terminal 400 amino acids of bovine CD18 were replaced with human CD18; H500B, the N-terminal 500 amino acids of bovine CD18 were replaced with human CD18; H600B, the N-terminal 600 amino acids of bovine CD18 were replaced with human CD18; H497B540H, the EGF-2 domain (aa residues 497 to 540) of human CD18 was replaced with the corresponding sequence from bovine CD18; B497H540B, the EGF-2 domain (aa residues 497 to 540) of bovine CD18 was replaced with the corresponding sequence from human CD18; H541B581H, the EGF-3 domain (aa residues 541 to 581) of human CD18 was replaced with the corresponding sequence from bovine CD18; B541H581B, the EGF-3 domain (aa residues 541 to 581) of bovine CD18 was replaced with the corresponding sequence from human CD18; H582B617H, the EGF-3 domain (aa residues 582 to 617) of human CD18 was replaced with the corresponding sequence from bovine CD18; H582B617H, the EGF-3 domain (aa residues 582 to 617) of bovine CD18 was replaced with the corresponding sequence from human CD18. The figure also shows a schematic depiction of the BoCD18 and HuCD18 amino acids.
Recombinant expression of chimeric LFA-1.
The 13 different chimeric bovine × human CD18 cDNA constructs were recombinantly coexpressed with bovine CD11a cDNA in the human K562 cell line by transduction as described previously (10, 11). Stable cell clones were selected after growing in a selection medium containing 2 μg/ml puromycin. Seven of the 13 transductants derived from human × bovine CD18 cDNA constructs were designated with the same abbreviations as for the respective chimeric CD18 constructs (e.g., B200H, H400B, etc.) as described previously (10). The remaining six transductants derived from human × bovine chimeric integrin-epidermal growth factor-like domain 2 (I-EGF-2), I-EGF-3, and I-EGF-4 domain-switched CD18 constructs (9) were designated H497B540H, B497H540B, H541B581H, B541H581B, H582B617H, and B582H617B following the nomenclature adopted by Huang et al. (17). The designation H497B540H indicates that residues 497 to 540 are from the bovine CD18 and residues 1 to 496 and 541 to the C terminus are from the human CD18 (Fig. 1). Chimeric transductants were purified and enriched using a magnetically activated cell sorting column (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously (10). We used CD18-specific monoclonal antibody (MAb) BAQ30A in this procedure because it cross-reacts with both bovine and human CD18. Purity of these cells was confirmed by fluorescence imaging (not shown) and fluorescence-activated cell sorting (FACS) (Fig. 2). Transductants were thereafter used in functional studies to evaluate their susceptibility to LtxA-induced biological effects.
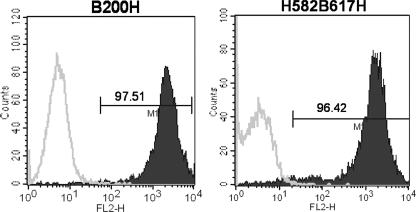
FIG. 2.
Demonstration of surface expression of bovine CD18 chimeric antigens in 2 of the 13 transductants, B200H and H582B617H, described in the study. Surface expression of chimeric CD18 antigens in the various transductants was confirmed by FACS using anti-CD18-specific MAb BAQ30A. The parent cell line K562 (open trace), which does not express CD18 antigen, was used as a negative control. The expression levels of chimeric CD18 antigens in the other 11 transductants were similar (data not shown). The x axis shows the fluorescence intensity (FL2-H), and the y axis shows cell number (counts); the numbers shown within the panels are the percentages of positive CD18 cells. Results show high levels of expression of CD18 antigen in chimeric transductants (solid traces). Data presented are representative of one of three experiments performed. FL2-H, height for phycoerythrin; M1, marker 1.
Flow cytometric analysis for the surface expression of bovine CD11a and chimeric CD18.
The transductants were tested for surface expression of bovine CD11a and chimeric CD18 using anti-CD11a-specific MAb 3.1 (data not shown) and CD18-specific MAb BAQ30A in a FACS assay as described previously (9-11). The transductant cells were analyzed for fluorescence on a FACSCalibur flow cytometry system using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA) and expressed as mean fluorescence intensity (Fig. 2). The parent cell line, K562, which does not express CD11a or CD18 antigen, was used as a negative control.
Preparation of purified A. actinomycetemcomitans leukotoxin (LtxA).
The LtxAs from strains JP2 (36) and NJ4500 (23) were produced, purified, verified for purity, and quantified as described previously (8, 19). Experiments done to assess the potency of the two purified LtxAs from strains JP2 and NJ4500 as measured by percent cytotoxicity and elevation of intracellular calcium ([Ca2+]i), using the HL-60 cell line, revealed that the LtxA of NJ4500 was more potent. Thus, all studies were done with the same batch of purified LtxA from strain NJ4500 of A. actinomycetemcomitans.
Determination of LtxA-induced intracellular calcium elevation.
We used a previously described (18) video fluorescence imaging technique to quantify the elevation of [Ca2+]i in transductant cells exposed to LtxA (concentration of 2 μg/ml). Briefly, cells were loaded with the fluorescent calcium indicator fura-2-acetoxymethyl ester (fura-2-AM) as described previously (10), processed, and attached to a coverslip, LtxA was added, and cells were viewed on a Diaphot inverted microscope (Nikon, Inc., Garden City, NY). Fluorescence signals were determined from regions of interest, and [Ca2+]i was calculated by the ratio method described by Grynkiewicz et al. (12). HL-60 and K562 cell lines served as controls.
Determination of LtxA-induced cytotoxicity.
The susceptibility of the different transductant cells to LtxA-induced cytolysis was determined by a previously described dye reduction assay (11) with 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT; Sigma). Fifty microliters of LtxA at a concentration of 2 μg/ml in XTT assay medium (RPMI 1640 without phenol red, supplemented with 1 mM Ca2+ and Mg2+) was used in the assay, and control wells contained only 50 μl of XTT medium. The percent cytotoxicity was calculated using the formula described previously (11). The HL-60 and K562 cell lines served as controls.
Reagents, cell lines, and antibodies.
RPMI 1640 medium with l-glutamine and Hanks' balanced salt solution were purchased from Celox Laboratories, Inc. (St. Paul, MN). All other reagents were obtained from Sigma Chemical Company (St. Louis, MO). The HL-60 cell line KL-4 expressing human LFA-1 (HuαLβ2-HuLFA-1) and P/5 cell line expressing human p150/95 (HuαXβ2-HuCR4) were obtained from Bruce Walcheck (University of Minnesota, St. Paul). The transductant cell lines BoLFA-1 (BoαLβ2), HuCD11a/BoCD18 (HuαLBoβ2), and BoCD11a/HuCD18 (BoαLHuβ2) were generated in our laboratory using previously described procedures (11). MAb BAQ30A was purchased from VMRD Inc. (Pullman, WA). MAb R3.1 was provided by R. Rothlein (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT).
Statistical analysis.
Data were analyzed statistically by the paired t test, and P values were determined using GraphPad Prism statistical analysis software (version 3.02; San Diego, CA). The term significant indicates a P value of less than 0.05.
RESULTS
Human CD18 is the receptor for LtxA.
To identify which of the subunits of the human β2-integrin functions as the receptor for A. actinomycetemcomitans LtxA, we compared the susceptibilities (as measured by elevation of [Ca2+]i and cytotoxicity) of HL-60, K562, KL-4, and P/5 cell lines with the BoLFA-1, HuCD11a/BoCD18, and BoCD11a/HuCD18 transductant cells to LtxA-induced biological effects. LtxA induced significant elevations of [Ca2+]i and cytotoxicity in HL-60, KL-4, P/5, and BoCD11a/HuCD18 cells, all of which express the human CD18. However, BoLFA-1 and HuCD11a/BoCD18 cell lines, which express bovine CD18, were not susceptible to the effects of LtxA (Fig. 3 and 4). These results suggest that the human CD18 is the LtxA functional receptor.
FIG. 3.
LtxA-induced cytolysis in different cell lines. Cytolysis of various cell lines exposed to LtxA (2 μg/ml) was measured by an XTT assay as described in the text. The parent K562 cells served as a negative control. Cell lines HL-60, KL-4, P/5, and BoCD11a/HuCD18 showed marked cytotoxicity upon exposure to LtxA. By contrast, cell lines BoLFA-1 (BoCD11a/BoCD18) and HuCD11a/BoCD18 showed no cytotoxicity. Results are expressed as means ± standard errors of the means of three separate experiments. Values that are significantly different from the negative control value (P < 0.05) are indicated by asterisks.
FIG. 4.
LtxA induces [Ca2+]i elevation in cell lines HL-60, KL-4, P/5, and BoCD11a/HuCD18, but not in BoLFA-1 (BoCD11a/BoCD18) and HuCD11a/BoCD18. Measurement of [Ca2+]i level was done using the cell-permeable fluorescent dye fura-2-AM. The net [Ca2+]i response (peak response subtracted from basal values) was measured as described in the text. Results are expressed as means ± standard errors of the means of three separate experiments. The parent K562 cells served as a negative control. Values that are significantly different from the negative control value (P < 0.05) are indicated by asterisks.
Cysteine-rich tandem repeats I-EGF-2, -3, and -4 of human CD18 are critical for species-specific susceptibility of human leukocytes to LtxA.
Results from FACS analysis indicated high levels of expression of CD18 antigen in all chimeric transductants, and expression levels were at comparable levels between different chimeric transductants (Fig. 2). Since preliminary experiments suggested that levels of expression markedly affected susceptibility to LtxA, all studies were done with transductants that expressed comparably high levels of expression of chimeric CD18 antigens. To locate the region within the human CD18 that is critical for susceptibility to the effects of LtxA, 13 human × bovine chimeric constructs were generated and coexpressed with bovine CD11a in K562 cells. We surmised that chimeric CD18 made with different regions of human and bovine CD18 should identify the region within the CD18 that is critical for conferring susceptibility to the biological effects of LtxA. If the insertion of a fragment of human CD18 into the corresponding region of bovine CD18 rendered the transductant-expressing chimeric CD18 susceptible to LtxA-induced effects, it would indicate that the human CD18 fragment that was inserted is critical for susceptibility. As shown in Fig. 5 and 6, the chimeric transductants B200H, B400H, and H600B showed marked susceptibility (as measured by elevation of [Ca2+]i and cytotoxicity) to LtxA-induced effects, but the transductants B600H, B700H, H400B, and H500B did not. The above results indicate that the region that is critical for conferring susceptibility to the biological effects of LtxA resides between 500 and 600 amino acid residues of the extracellular region of human CD18. In an attempt to identify a smaller functional domain within this 100-amino-acid-residue region of human CD18 that is responsible for conferring species-specific susceptibility to LtxA, we generated transductants in which individual I-EGF domains from human CD18 were switched with corresponding bovine sequences and vice versa. In transductants which had the human CD18 backbone with individual bovine I-EGF domains inserted (H497B540H, H541B581H, and H582B617H), LtxA elicited its biological effects (Fig. 5 and 6). By contrast, transductants which had the bovine CD18 backbone with individual human I-EGF domains inserted (B497H540B, B541H581B, and B582H617B) were not susceptible to the effects of LtxA. These results suggest that the entire, and not any individual, I-EGF domain in this region of the human CD18 is critical for conferring species-specific susceptibility to LtxA effects. In addition, these results suggest that susceptibility to LtxA cannot be attributed to individual I-EGF domains of human CD18 but rather to a larger region encompassing all three I-EGF domains.
FIG. 5.
LtxA-induced cytolysis in the chimeric tranductants. Cytolysis of various cell lines exposed to LtxA (2 μg/ml) was measured by an XTT assay as described in the text. Transductants B200H, B400H, H600B, H497B540H, H541B581H, and H582B617H showed marked cytotoxicity compared to the parent cell line. By contrast, transductants B600H, B700H, H400B, H500B, B497H540B, B541H581B, and B582H617B showed lower cytotoxicity upon exposure to LtxA that was comparable to that obtained with parent K562 cells. The HL-60 cells were used as a positive control. Results are expressed as means ± standard errors of the means of three separate experiments. Values that are significantly different from the negative control value (P < 0.05) are indicated by asterisks.
FIG. 6.
LtxA induces a [Ca2+]i elevation in B200H, B400H, H600B, H497B540H, H541B581H, and H582B617H transductants but not in B600H, B700H, H400B, H500B, B497H540B, B541H581B, and B582H617B transductants. Measurement of [Ca2+]i levels was done using the cell-permeable fluorescent dye fura-2-AM. The net [Ca2+]i response (peak response subtracted from basal values) was measured as described in the text. Results are expressed as means ± standard errors of the means of three separate experiments. HL-60 cells served as a positive control, and K562 cells served as a negative control. Values that are significantly different from the negative control value (P < 0.05) are indicated by asterisks.
DISCUSSION
In this study, we used cell lines expressing conventional β2-integrins (HuLFA-1, HuCR4, and BoLFA-1), chimeric LFA-1 (BoCD11a/HuCD18 and HuCD11a/BoCD18), and 13 different transductants derived from chimeric bovine × human CD18 cDNA constructs coexpressed with bovine CD11a cDNA in the human K562 cell line to identify the precise subunit that functions as the receptor for A. actinomycetemcomitans LtxA. The K562 cell line was chosen for transduction of chimeric bovine × human CD18 cDNA constructs and bovine CD11a cDNA because it is a cell line derived from an individual with chronic myelogenous leukemia and does not express any of the human leukocyte integrins (16). Most importantly, K562 cells are nonsusceptible (as measured by elevation of [Ca2+]i and cytotoxicity) to the biological effects of LtxA (34). We used two different assays, LtxA-induced [Ca2+]i elevation and cytolysis, to determine the susceptibility of the various cell lines to LtxA. Calcium is an intracellular second messenger which is widely used as an index of intracellular signaling, and cytolysis is the terminal event in the intoxication process.
All human β2-integrin-expressing cell lines and chimeric LFA-1 transductant cell lines that had human CD18 as their β subunit were susceptible to the effects of LtxA. By contrast, cell lines that express the bovine CD18 as the β subunit (BoLFA-1 and HuCD11a/BoCD18 cells) were not susceptible to LtxA effects. These results suggest that human CD18 is the functional receptor for A. actinomycetemcomitans LtxA. With the next set of data, we show that the region important for LtxA species-specific susceptibility resides within a 100-amino-acid-residue region (residues 500 to 600) of the extracellular region of the human CD18. This and adjoining regions of the human CD18 contain three cysteine-rich repeat amino acid regions which have an EGF-like structure; thus, these are referred to as integrin-EGF domains. I-EGF-2 spans from residues 497 to 540, I-EGF-3 is from 541 to 581, and I-EGF-4 is from 582 to 617. With these data in mind, we reasoned that studies with chimeric CD18 made with different EGF domains switched in human and bovine forms should reveal a smaller region responsible for imparting species-specific susceptibility to LtxA. Thus, we generated six human × bovine chimeric CD18 constructs with individual I-EGF-2, I-EGF-3, and I-EGF-4 domains switched and coexpressed with bovine CD11a. However, we failed to identify a smaller region within this 100-amino-acid sequence of human CD18 capable of conferring susceptibility to LtxA. Significant elevation of [Ca2+]i levels following exposure to LtxA was observed in the transductants H497B540H, H541B581H, and H582B617H but not in the B497H540B, B541H581B, and B582H617B transductants. This indicated that all of the chimeras with the human backbone were susceptible to the effects of LtxA and all the chimeras with the bovine backbone were not susceptible to LtxA effects, despite individual I-EGF domain switches.
The same pattern was seen with the cytotoxicity assay. These results indicated that replacement of any one of the I-EGF domains in the human CD18 backbone with corresponding sequence from the bovine CD18 does not influence susceptibility to LtxA, suggesting that the remaining two I-EGF domains are capable of supporting the LtxA interaction by providing the proper conformation. In support of this possibility is the finding from a complementary study by Lally et al., who used a panel of chimeric LktA/LtxA constructs for which they tested the ability of these chimeric toxins to kill either human or bovine cell lines (25). Their results demonstrated that the unique species recognition unit in LtxA is a 253-amino-acid fragment (residues 688 to 941) which contains the 14 glycine-rich nonapeptide repeat region (25). One of their chimeric toxin constructs, CH41, which was spliced within this repeat region, failed to kill the human target cells. CH41 is a chimeric toxin with only 9 glycine-rich repeats instead of the 14 repeats found in native LtxA. This suggests that all 14 glycine-rich repeats of LtxA are essential for optimal interaction with its receptor on target cells. Since LtxA utilizes a larger region (14 nonapeptide repeats) for binding to its receptor, it is tempting to speculate that there may be multiple interaction sites for LtxA scattered throughout the cysteine-rich region of human CD18 (encompassing regions I-EGF-2, -3, and -4). As an additional observation supporting our speculation, in the same study Lally et al. used a panel of monoclonal antibodies directed against CD11a and CD18 and tested their abilities to inhibit LtxA-mediated cytotoxicity in target cells (25). Of these monoclonal antibodies, MAbs KIM127 and KIM185 were potent in inhibiting the LtxA effects on HL-60 target cells, with KIM185 abrogating cytotoxicity almost completely. Both MAbs are known to activate β2-integrins and increase the binding capacity of β2-integrins to natural ligands, such as intercellular adhesion molecule 1. In a later study, Lu et al. (29) mapped the epitopes for these two monoclonal antibodies and found that MAb KIM127 mapped to the I-EGF-2 domain and KIM185 mapped to the I-EGF-4 domain of human CD18. These results further support our conclusions that the cysteine-rich region of CD18 encompassing I-EGF-2, -3, and -4 is required for conferring species-specific susceptibility to LtxA. By contrast, studies with another RTX toxin, M. haemolytica leukotoxin (LktA), revealed that the critical region within its receptor required for conferring species-specific susceptibility is a 40-amino-acid fragment (residues 541 to 581) in the I-EGF-3 domain of bovine CD18 (9). LktA has only 6 glycine-rich repeats, compared to the 14 for LtxA. In this context, results from Lally et al. (25) showed that the LkA/LtxA chimeric toxin construct CH41 was not cytotoxic to the LktA-susceptible BL-3 cell line (25). The CH41 construct had 769 amino acid residues out of a total of 953 residues and was constructed by splicing the glycine-rich repeat region at position 769 of LktA. Interestingly, the CH64 chimeric construct, which had an intact glycine-rich repeat region, was cytotoxic to BL-3 cells. These findings suggest that a 49-amino-acid fragment (residues 733 to 782) which encompasses the six glycine-rich repeat regions contains the domain for target cell recognition of M. haemolytica LktA.
To our knowledge, this is the first report to identify the human CD18 subunit of LFA-1 as the functional receptor for A. actinomycetemcomitans LtxA. In addition, we have shown that the region represented by I-EGF-2, -3, and -4 of the extracellular region of human CD18 is critical for conferring susceptibility to LtxA. Therapeutically, this region might serve as an effective target for agents that could specifically block the toxic effects of LtxA.
Acknowledgments
This work was supported, in part, by a grant from the National Institute of Dental and Craniofacial Research (RO1DE16133) to S.C.K.
We thank Mathur S. Kannan (Department of Veterinary and Biomedical Sciences, University of Minnesota) for reviewing the manuscript and helpful comments and Bruce Walcheck (Department of Veterinary and Biomedical Sciences, University of Minnesota) for providing technical assistance.
Editor: A. Camilli
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Baehni, P. C., C. C. Tsai, W. P. McArthur, B. F. Hammond, B. J. Shenker, and N. S. Taichman. 1981. Leukotoxic activity in different strains of the bacterium Actinobacillus actinomycetemcomitans isolated from juvenile periodontitis in man. Arch. Oral Biol. 26:671-676. [DOI] [PubMed] [Google Scholar]
- 2.Balashova, N. V., J. A. Crosby, L. Al Ghofaily, and S. C. Kachlany. 2006. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect. Immun. 74:2015-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berbari, E. F., F. R. Cockerill III, and J. M. Steckelberg. 1997. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin. Proc. 72:532-542. [DOI] [PubMed] [Google Scholar]
- 4.Bueno, L. C., M. P. Mayer, and J. M. DiRienzo. 1998. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J. Periodontol. 69:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinkenbeard, K. D., D. A. Mosier, and A. W. Confer. 1989. Effects of Pasteurella haemolytica leukotoxin on isolated bovine neutrophils. Toxicon 27:797-804. [DOI] [PubMed] [Google Scholar]
- 6.Confer, A. W., K. R. Simons, M. T. Barrie, and K. D. Clinkenbeard. 1990. Effects of Pasteurella haemolytica leukotoxin on neutrophils from white-tailed deer and several exotic ruminant species. Vet. Res. Commun. 14:175-180. [DOI] [PubMed] [Google Scholar]
- 7.Das, M., A. D. Badley, F. R. Cockerill, J. M. Steckelberg, and W. R. Wilson. 1997. Infective endocarditis caused by HACEK microorganisms. Annu. Rev. Med. 48:25-33. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, R., L. A. Ghofaily, J. Patel, N. V. Balashova, A. C. Freitas, I. Labib, and S. C. Kachlany. 2006. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb. Pathog. 40:48-55. [DOI] [PubMed] [Google Scholar]
- 9.Dileepan, T., M. S. Kannan, B. Walcheck, and S. K. Maheswaran. Integrin-EGF-3 domain of bovine CD18 is critical for Mannheimia haemolytica leukotoxin species specific susceptibility. FEMS Microbiol. Lett. 274:67-72. [DOI] [PubMed]
- 10.Dileepan, T., M. S. Kannan, B. Walcheck, P. Thumbikat, and S. K. Maheswaran. 2005. Mapping of the binding site for Mannheimia haemolytica leukotoxin within bovine CD18. Infect. Immun. 73:5233-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dileepan, T., P. Thumbikat, B. Walcheck, M. S. Kannan, and S. K. Maheswaran. 2005. Recombinant expression of bovine LFA-1 and characterization of its role as a receptor for Mannheimia haemolytica leukotoxin. Microb. Pathog. 38:249-257. [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 13.Haraszthy, V. I., G. Hariharan, E. M. Tinoco, J. R. Cortelli, E. T. Lally, E. Davis, and J. J. Zambon. 2000. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71:912-922. [DOI] [PubMed] [Google Scholar]
- 14.Haubek, D., and J. Westergaard. 2004. Detection of a highly toxic clone of Actinobacillus actinomycetemcomitans (JP2) in a Moroccan immigrant family with multiple cases of localized aggressive periodontitis. Int. J. Paediatr. Dent. 14:41-48. [DOI] [PubMed] [Google Scholar]
- 15.Henderson, B., S. P. Nair, J. M. Ward, and M. Wilson. 2003. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu. Rev. Microbiol. 57:29-55. [DOI] [PubMed] [Google Scholar]
- 16.Hickstein, D. D., E. Grunvald, G. Shumaker, D. M. Baker, A. L. Back, L. J. Embree, E. Yee, and K. A. Gollahon. 1993. Transfected leukocyte integrin CD11b/CD18 (Mac-1) mediates phorbol ester-activated, homotypic cell:cell adherence in the K562 cell line. Blood 82:2537-2545. [PubMed] [Google Scholar]
- 17.Huang, C., and T. A. Springer. 1995. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1). J. Biol. Chem. 270:19008-19016. [DOI] [PubMed] [Google Scholar]
- 18.Jeyaseelan, S., S. L. Hsuan, M. S. Kannan, B. Walcheck, J. F. Wang, M. E. Kehrli, E. T. Lally, G. C. Sieck, and S. K. Maheswaran. 2000. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect. Immun. 68:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachlany, S. C., D. H. Fine, and D. H. Figurski. 2002. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expr. Purif. 25:465-471. [DOI] [PubMed] [Google Scholar]
- 20.Kaehler, K. L., R. J. Markham, C. C. Muscoplat, and D. W. Johnson. 1980. Evidence of species specificity in the cytocidal effects of Pasteurella haemolytica. Infect. Immun. 30:615-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelk, P., R. Claesson, L. Hanstrom, U. H. Lerner, S. Kalfas, and A. Johansson. 2005. Abundant secretion of bioactive interleukin-1β by human macrophages induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 73:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelk, P., A. Johansson, R. Claesson, L. Hanstrom, and S. Kalfas. 2003. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 71:4448-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsuzawa, H., R. Asakawa, T. Kawai, K. Ochiai, T. Fujiwara, M. A. Taubman, M. Ohara, H. Kurihara, and M. Sugai. 2002. Identification of six major outer membrane proteins from Actinobacillus actinomycetemcomitans. Gene 288:195-201. [DOI] [PubMed] [Google Scholar]
- 24.Korostoff, J., N. Yamaguchi, M. Miller, I. Kieba, and E. T. Lally. 2000. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb. Pathog. 29:267-278. [DOI] [PubMed] [Google Scholar]
- 25.Lally, E. T., E. E. Golub, and I. R. Kieba. 1994. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 269:31289-31295. [PubMed] [Google Scholar]
- 26.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 27.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 28.Larson, R. S., A. L. Corbi, L. Berman, and T. Springer. 1989. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J. Cell Biol. 108:703-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, C., M. Ferzly, J. Takagi, and T. A. Springer. 2001. Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166:5629-5637. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki, K. 2003. Creating random mutagenesis libraries by megaprimer PCR of whole plasmid (MEGAWHOP). Methods Mol. Biol. 231:23-28. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki, K., and M. Takenouchi. 2002. Creating random mutagenesis libraries using megaprimer PCR of whole plasmid. BioTechniques 33:1033-1034, 1036-1038. [DOI] [PubMed] [Google Scholar]
- 32.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780-3792. [PubMed] [Google Scholar]
- 33.Taichman, N. S., R. T. Dean, and C. J. Sanderson. 1980. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect. Immun. 28:258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taichman, N. S., M. Iwase, E. T. Lally, S. J. Shattil, M. E. Cunningham, and H. E. Korchak. 1991. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J. Immunol. 147:3587-3594. [PubMed] [Google Scholar]
- 35.Taichman, N. S., D. L. Simpson, S. Sakurada, M. Cranfield, J. DiRienzo, and J. Slots. 1987. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol. Immunol. 2:97-104. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C. C., B. J. Shenker, J. M. DiRienzo, D. Malamud, and N. S. Taichman. 1984. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 43:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi, N., C. Kubo, Y. Masuhiro, E. T. Lally, T. Koga, and S. Hanazawa. 2004. Tumor necrosis factor alpha enhances Actinobacillus actinomycetemcomitans leukotoxin-induced HL-60 cell apoptosis by stimulating lymphocyte function-associated antigen 1 expression. Infect. Immun. 72:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]