Abstract
The bundle-forming pilus (BFP) of enteropathogenic Escherichia coli (EPEC) is an important virulence factor. We examined the role of divergent alleles of bfpA encoding bundlin, the BFP pilin protein, in pilus biogenesis, pilus interactions, and immune responses. We found that the BFP biogenesis machine from an EPEC strain that expresses one bundlin type is capable of assembling all other bundlin types. Furthermore, we found that EPEC strains expressing divergent bundlin types are capable of forming mixed autoaggregates, suggesting that different pilin types can intertwine. However, we found that there was a marked difference between alleles in immunogenicity in both rabbits and mice of a peptide derived from a region of bundlin undergoing apparent diversifying selection. In addition, despite a high degree of cross-reactivity between divergent bundlin proteins, in both mice and rabbits responses appeared to be stronger against the homologous pilin protein than against the heterologous protein. This result was verified using sera from a volunteer study, which demonstrated that the human antibody responses after an initial challenge with live EPEC were stronger against the homologous bundlin protein than against a divergent bundlin protein. However, a repeat challenge induced equivalent responses. These results are consistent with the hypothesis that human immune responses against bundlin exert selective pressure on bfpA sequence divergence.
Type IV pili (Tfps) are surface appendages that are expressed by diverse gram-negative species. Tfps play numerous roles in pathogenesis, including roles in colonization, adherence, autoaggregation, biofilm formation, horizontal gene transfer, motility, and virulence (5, 8, 21, 22, 24, 28, 32, 36, 38, 41-43). The bundle-forming pilus (BFP) of enteropathogenic Escherichia coli (EPEC) is an excellent model for the study of Tfps as expression of the 14-gene bfp cluster in a laboratory strain of E. coli is sufficient for BFP biogenesis and function (30, 40). EPEC is an important cause of serious diarrhea in infants in developing countries (1, 12, 16, 19). Volunteer studies have confirmed the importance of BFP expression for full virulence of EPEC (5). BFP are composed of repeating subunits of the pilin protein bundlin, the product of the bfpA gene. Antibodies against bundlin are found in volunteers convalescing from experimental EPEC infection and in breast milk of mothers and serum of infants in developing countries (15, 25, 33). Whether these antibodies confer protection against subsequent infection is not known.
The Tfps expressed by different strains in a species may vary in sequence. In Neisseria gonorrhoeae, transformation and recombination from multiple silent pilus loci encoding variant pilin proteins result in abundant antigenic variation in pilin expression (29). This extremely dynamic process can lead to the expression of several pilin variants during infection of a single host and may help the bacteria avoid the immune response and cause persistent infection (39). Pilin sequence variation has also been noted in other species that produce Tfps, including Pseudomonas aeruginosa and Vibrio cholerae (10, 31). We found that EPEC bfpA genes are also variable and defined nine bfpA alleles produced by diverse EPEC strains (6, 7). These alleles could be grouped into two categories based on sequence similarity. The three α alleles are highly similar to one other, resulting in proteins that are 97% identical, while the six β alleles are more divergent, encoding proteins that are 89% identical to one another. In all bundlin proteins 80% of the amino acids are identical (6). The bundlin sequence diversity is concentrated near the carboxyl terminus of the 180- to 182-amino-acid mature proteins, and in particular, the region encoding amino acids 137 to 155 has an excess of nonsynonymous substitutions over synonymous substitutions (Fig. 1) (7). Excess nonsynonymous substitutions imply that evolutionary forces provided a selective advantage to strains that expressed novel amino acids in this region, a phenomenon known as diversifying selection. In contrast, the rest of the protein shows evidence of sequence constraints with an excess of synonymous substitutions. The purpose of this study was to test the hypothesis that variations in bundlin amino acid sequences result in differences in BFP expression, function, and immunogenicity.
FIG. 1.
Sequence comparison of α1 and β6 bundlins. An alignment of the sequences of the mature bundlin proteins is shown. Identical residues are connected with lines, and similar residues are connected with two dots. The black background indicates residues that were replaced by a histidine tag to purify the recombinant soluble bundlin proteins. The shaded area indicates the peptides used in this study.
MATERIALS AND METHODS
Strains, plasmids, and media.
The strains and plasmids used in this study are listed in Table 1. We employed allelic exchange using suicide vector pCVD442cys1 as described previously (44) to construct strain UMD949, a bfpA null mutant of EPEC strain RN587/1, which, like bfpA mutant strain UMD901, has a mutation that changes cysteine 129 to serine. This mutation renders the protein unstable and subject to rapid degradation (44). For complementation of bfpA null mutant strain UMD901, we amplified plasmid pRPA100, which contains bfpA from strain E2348/69 under control of its native promoter in a low-copy-number vector, by inverse PCR using primers Donne-362 (5′-AGGTCTGTCTTTGATTGAA-3′) and Donne-535 (5′-CGCATCCGGACCATGGAAACTGTTTTCCTTATA-3′). This resulted in insertion of an NcoI restriction endonuclease site at the start codon of bfpA. We then amplified each bfpA allele from genomic DNA using conserved primers Donne-536 (5′-GCTCGGCCATGGTTTCTAAAATCA-3′) and Donne-537 (5′-CGTCGAATTCACAGGGCGTATTATGTAGATTA-3′), which add an NcoI site at the start codon and an EcoRI site after the stop codon of bfpA, and replaced the bfpA gene of pRPA100 with the alleles to create plasmids pXLW10 through pXLW17. After confirmation by sequencing, these plasmids were introduced into bfpA mutant strain UMD901 by electroporation. Plasmid pBluescript was modified by insertion between the SacI and XbaI sites of an oligonucleotide composed of complementary oligonucleotides Donne-200 (5′-CTGCGACTTGACAGGATCCACGCGTCTAGTTATAAT-3′) and Donne-201 (5′-CTAGATTATAACTAGACGCGTGGATCCTGTCAAGTCGACGAGCT-3′) specifying a consensus sigma-70 promoter, to create pTR102. A gfp gene encoding enhanced green fluorescence protein (eGFP) was cloned from plasmid pKEN into the XbaI site to create pTR103 for constitutive expression of eGFP.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description or genotype | Reference or source |
|---|---|---|
| Strains | ||
| E2348/69 | Wild-type EPEC strain, serotype O127:H6, produces α1 bundlin | 7 |
| UMD901 | E2348/69 bfpA C129S | 44 |
| RN587/1 | Wild-type EPEC strain, serotype O157:H+, produces β6 bundlin | 6 |
| UMD949 | RN587/1 bfpA C129S | This study |
| RN410/1 | Wild-type EPEC strain, serotype O119:H+, produces α3 bundlin | 7 |
| RN191/1 | Wild-type EPEC strain, serotype O111:H+, produces α2 bundlin | 7 |
| B171 | Wild-type EPEC strain, serotype O111:H+, produces α2 bundlin | 18 |
| E56/54 | Wild-type EPEC strain, serotype O128ab:H2, produces β4 bundlin | 7 |
| 012-050982 | Wild-type EPEC strain, serotype O142:H+, produces β1 bundlin | 7 |
| E851/71 | Wild-type EPEC strain, serotype O142:H6, produces β5 bundlin | 7 |
| Z188-93 | Wild-type avian EPEC strain, serotype O110:H6, produces β2 bundlin | 7 |
| CA89-4221 | Wild-type canine EPEC strain, serotype NT:H+, produces β3 bundlin | 7 |
| BL21(AI) slyD | F−ompT hsdSB (rB− mB−) gal dcm araB::T7RNAP-tetA ΔslyD::cat | 13 |
| Plasmids | ||
| pRPA100 | bfpA gene with native promoter cloned into low-copy-number vector pWKS30 | 3 |
| pXLW10 | pRPA100 with bfpA alpha-3 allele from strain RN410/1 | This study |
| pXLW11 | pRPA100 with bfpA alpha-2 allele from strain RN191/1 | This study |
| pXLW12 | pRPA100 with bfpA beta-4 allele from strain E56/54 | This study |
| pXLW13 | pRPA100 with bfpA beta-1 allele from strain 012-050982 | This study |
| pXLW14 | pRPA100 with bfpA beta-5 allele from strain E851/71 | This study |
| pXLW15 | pRPA100 with bfpA beta-6 allele from strain RN587/1 | This study |
| pXLW16 | pRPA100 with bfpA beta-2 allele from strain Z188-93 | This study |
| pXLW17 | pRPA100 with bfpA beta-3 allele from strain CA89-4221 | This study |
| pIL14 | AFA-1 afimbrial adhesin genes cloned in a pBR322 vector | 23 |
| pPF301 | Expression vector for soluble α1 bundlin under control of T7 promoter | 34 |
| pPF302 | Expression vector for soluble β6 bundlin under control of T7 promoter | This study |
| pKEN | pUC derivative encoding eGFP | 11 |
| pTR103 | pBluescript derivative with eGFP under control of consensus sigma-70 promoter | This study |
| pDsRed | Red fluorescent protein under control of lac promoter in pUC19 backbone | Clonetech |
Bacteria were stored at −80°C in 50% (vol/vol) Luria broth-50% (vol/vol) glycerol and grown on Luria plates or in Luria broth with antibiotics at the following concentrations as needed to maintain plasmids: ampicillin, 200 μg/ml; nalidixic acid, 50 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 25 μg/ml. To induce expression of BFP, overnight cultures of bacteria were diluted 100-fold in Dulbecco modified Eagle medium/F10 and grown at 37°C with shaking (225 rpm) for 3 h.
BFP phenotypic assays.
Autoaggregation and disaggregation and localized adherence assays were performed as previously described (2).
Bundlin purification and immunization.
Soluble α1 bundlin with an amino-terminal hexahistidine tag was expressed in the periplasm and purified as previously described (34). Similarly, soluble β6 bundlin was expressed by amplifying the sequences encoding the amino acid glycine 25 of the mature protein through the stop codon from strain RN587/0 with primers Donne-596 and Donne-597. This strategy deleted the hydrophobic amino terminus from the protein, thereby making the protein soluble. The PCR product was cloned as an NcoI-BamHI fragment into plasmid pPF301, replacing the α1 allele from this plasmid to form a fusion with the signal sequence of dsbA followed by an amino-terminal hexahistidine tag. The resulting plasmid, pPF302, was transferred to strain BL21(AI) slyD for purification as described previously (34). Protein concentrations were calculated from extinction coefficients (17).
Bundlin peptides and bundlin peptides conjugated to keyhole limpet hemocyanin (KLH) were purchased from Research Genetics (Huntsville, AL). Female 6- to 8-week-old CBA/J mice (Harlan Sprague Dawley, Indianapolis, IN) were immunized subcutaneously with 100 μg purified bundlin protein or bundlin peptide-KLH conjugate in complete Freund's adjuvant (1:1 [vol/vol] emulsion; Difco Laboratories, Detroit, MI). Booster immunizations of 50 μg in incomplete Freund's adjuvant were given on days 21 and 42. Male 1.8- to 2.2-kg New Zealand White rabbits (Covance, Denver, PA) were immunized subcutaneously with 100 μg purified bundlin protein or 200 μg bundlin peptide-KLH conjugate in complete Freund's adjuvant and boosted on days 30 and 60 with 30 μg purified bundlin protein or 200 μg bundlin peptide-KLH conjugate in incomplete Freund's adjuvant. Serum was harvested on day 72.
Enzyme-linked immunosorbent assays (ELISA).
Nunc-Immuno 96-well MaxiSorp plates (Nalge Nunc International, Rochester, NY) were coated with 100 μl/well of purified soluble α1 or β6 bundlin, unconjugated bundlin peptides, or KLH (2 μg ml−1 in phosphate-buffered saline [PBS], pH 7.2) and incubated at 4°C overnight. After washing in PBS plus 0.1% Tween 20, plates were blocked in 10% goat serum (Gibco, Paisley, Scotland, United Kingdom) in PBS plus 0.1% Tween 20 (blocking buffer) and incubated at 37°C for 1 h. After washing, six samples of control sera or duplicate samples of twofold serial dilutions of test sera in blocking buffer were added and incubated at 37°C for 1 h. After washing, 100 μl horseradish peroxidase-conjugated secondary antibody (anti-mouse immunoglobulin G [IgG], anti-rabbit IgG, or anti-human IgG, as appropriate) was added to each well at a dilution of 1:5,000 in blocking buffer, and plates were incubated at 37°C for 1 h. After washing, 100 μl of SureBlue 3,3′,5,5′-tetra-methylbenzidine peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added to each well, and plates were incubated in the dark for 20 min. The reaction was stopped by addition of 1 M sulfuric acid to each plate, and absorbance at 450 nm was recorded. The mean absorbance of four blank wells was subtracted from all other values. Values greater than the mean plus two standard deviations of the mean of six negative control samples were considered positive. Pooled preimmune sera from each group of mice or preimmune rabbit sera at a dilution of 1:1,000 for protein samples or at a dilution 1:2,000 for peptide samples served as negative controls. Pooled serum obtained in a serological study from 20 infants who were 10 to 14 months old from Mali was used at a dilution of 1:5,000 as a positive control for human antibundlin antibodies, and serum pooled from 10 infants who were 2 to 4 months old from a Rochester, NY, serological study was used at a dilution of 1:1,200 as a negative control. The use of human sera was approved by the institutional review board of the University of Maryland School of Medicine. Responses were compared using analysis of variance in Microsoft Excel.
Antibodies.
Antisera were affinity purified after conjugation of purified soluble α1 or β6 bundlin using AminoLink Plus immobilization kits as described by the manufacturer (Pierce Biotechnology, Rockford, IL). The concentration of the antibodies was adjusted according to the protein concentration, as estimated by absorbance at 280 nm. Fab antibody fragments were prepared and purified using an ImmunoPure Fab preparation kit according to the manufacturer's instructions (Pierce). For immunoblotting, whole-cell lysates or purified proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% (wt/vol) nonfat dry milk in PBS (pH 7.4) plus 0.5% (vol/vol) Tween 20. Primary, affinity-purified rabbit α1 or β6 bundlin antisera were used at a dilution of 1:100,000 in PBS plus 5% nonfat dry milk and 0.1% Tween 20. Bands were detected with horseradish peroxidase-conjugated anti-rabbit serum at a dilution of 1:20,000 and enhanced chemiluminescence (ECL Plus) reagents (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom).
Fluorescence microscopy.
HeLa cells were grown in chamber slides and infected with bacteria under blinded conditions as previously described (14). After 3 h, the slides were washed three times with PBS and fixed for 25 min with 4% (vol/vol) formaldehyde. The slides were then blocked for 1 h at 37°C with 10% normal goat serum (Gibco) and left overnight at 4°C. Next, the slides were washed three times with PBS, incubated for 1 h at 37°C with affinity-purified rabbit anti-α1 or anti-β6 bundlin sera at a dilution of 1:1,000 in PBS plus 10% goat serum, washed three times, incubated for 1 h at 37°C with fluorescein isothiocyanate-conjugated goat anti-rabbit sera (Sigma Chemical Co., St. Louis, MO) at a dilution of 1:320 in PBS plus 10% goat serum, and washed three times. The slides were covered with antifade reagent (Invitrogen, Carlsbad, CA) and examined with a Zeiss Axioskop microscope. Images were captured with an Axiocam digital 20 camera and analyzed using AxioVision 3.1 software (Zeiss). For mixed autoaggregation assays, overnight cultures of strain E2348/69 carrying plasmid pDsRed and strain RN587/1 carrying pTR103 were diluted 1:10, and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the E2348/69(pDsRed) culture to induce expression of DsRed. After 3 h of incubation at 37°C, the cultures were mixed at a 1:3 ratio and grown under BFP-inducing conditions. This ratio was necessary to compensate for poorer autoaggregation by strain RN587/1. After 2 to 3 h, 5 μl of the culture was placed on a glass slide under a coverslip, examined for red and green fluorescence, and analyzed as described above.
Quantification of bundlin expression.
Wild-type strains E2348/69 and RN587/1 were grown under BFP-expressing conditions. The number of CFU present in one half of each culture was determined after serial 10-fold dilution. The other half of each culture was centrifuged, and the pellet was resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. Immunoblotting was performed with serial twofold dilutions of the whole-cell lysates along with serial twofold dilutions of purified homologous pilin protein. The densitometry of bands corresponding to bundlin was determined using Kodak ID image analysis software, and a standard curve for known bundlin samples was constructed to determine the concentration of bundlin in each culture using the average density of two dilutions that fell within the range of the known sample densities. The experiment was conducted three times, and the mean and standard deviation of the number of molecules per CFU were calculated and compared using Student's t test.
RESULTS
Bundlin proteins are functionally interchangeable.
Prior studies have demonstrated a considerable degree of sequence variation in the bfpA gene encoding prebundlin, the BFP pilin protein precursor (6, 7). Although some microorganisms, such as P. aeruginosa, are capable of assembling type IV pili upon introduction of highly divergent pilin genes from distantly related species (4, 26, 37), EPEC is unable to assemble heterologous pili when it is transformed with the tcpA pilin gene for the closely related toxin-coregulated pilus of V. cholerae or with gene fusions encoding hybrid bundlin-TcpA pilins (27). To determine whether bfpA sequence variation affects pilus biogenesis, we constructed an expression vector containing the native bfpA promoter from EPEC strain E2348/69 followed by restriction sites that would allow cloning of the bfpA gene from any source strain. Amplification of bfpA from strains that express each of the known bundlin variants and subsequent cloning resulted in a series of eight plasmids encoding each alternative bundlin variant. After introduction into strain UMD901, a bfpA null mutant of E2348/69, we monitored pilus biogenesis and function using the autoaggregation/disaggregation assay (2). This assay corresponds with the ability to produce BFP as assessed by electron microscopy (3). We found that each of eight known alternative bfpA alleles was able to complement strain UMD901 to restore both aggregation and disaggregation (data not shown). Thus, the BFP machinery encoded by the bfp operon in strain E2348/69 is able to assemble all known bundlin variants into functional BFP.
EPEC strains expressing divergent bundlin proteins are capable of forming mixed autoaggregates.
BFP filaments aggregate longitudinally to form dense bundles (18), an effect that appears to require complementary binding between filaments. As the filaments from many bacteria intertwine, autoaggregation of the bacteria is observed. To determine whether the sequence variation among bfpA alleles affects the ability of bacteria to form heterologous autoaggregates, we used immunofluorescence to follow autoaggregation in mixed cultures of EPEC bacteria after transformation with plasmids that direct the expression of either eGFP or DsRed. We then allowed the bacteria to autoaggregate and examined the aggregates by immunofluorescence to determine whether they were uniform or mixed. We first examined mixtures of homologous strains expressing each fluorescent protein. We found that for both prototypic α1 bundlin strain E2348/69 and β6 bundlin strain RN587/1 autoaggregates displayed both green and red fluorescence with strong colocalization of the fluorescence signals (not shown). This result indicates that autoaggregates are not clonal in origin but are derived primarily from the association of individual or small groups of bacteria. This result is also consistent with the kinetics of autoaggregation, which results in the formation of aggregates containing thousands of bacteria within 3 h, a rate that exceeds the generation time of E. coli. We next examined autoaggregates derived from a mixture of prototype α1 bundlin strain E2348/69 expressing DsRed and prototype β6 bundlin strain RN587/1 expressing eGFP. Once again, we found that autoaggregates were composed of bacteria that expressed both fluorescent proteins and were therefore composed of both strains (Fig. 2). To determine whether these results can be generalized to other bundlin types, we repeated the studies with strains that express α2 bundlin and β1 bundlin. We found that each strain was capable of forming mixed autoaggregates with the other three strains (data not shown). We concluded, therefore, that pili composed of divergent bundlin proteins are capable of bundling together.
FIG. 2.
Mixed autoaggregation of strains expressing α1 bundlin and β6 bundlin alleles. Strain E2348/69 expressing α1 bundlin and DsRed and strain RN587/1 expressing β6 bundlin and eGFP were incubated together and examined by immunofluorescence microscopy to detect red (left panel) or green (middle panel) fluorescent bacteria in microcolonies. The right panel shows the merged red and green images. Bar = 20 μm.
Epitopes of divergent bundlin proteins are conserved and cross-reactive.
An analysis of the sequences of bfpA alleles revealed that the region of greatest sequence variation is near the 3′ end of the gene. Furthermore, a region encompassing the codons for amino acids residues 137 to 155 near the C terminus of the mature protein (Fig. 1) has an excess of nonsynonymous substitutions, an effect indicating the influence of diversifying selection (7). As this effect may have been exerted by the selective pressure of the immune response from humans infected with EPEC, we developed the hypothesis that this region of bundlin is immunodominant and surface exposed. To test this hypothesis, we raised antisera in rabbits against peptides representing this region. We found that the peptide derived from strain E2348/69, which expresses α1 bundlin, was highly immunogenic, inducing antibody responses in two rabbits at a titer of 1:250,000 as determined by ELISA and recognizing bundlin as a single band on an immunoblot of whole-cell lysates at a titer of 1:40,000 (data not shown). To our surprise, however, the corresponding peptide from β5 bundlin was not immunogenic, yielding a titer of 1:50 or less in two different rabbits and failing to recognize specific proteins by Western blotting. Furthermore, despite its high level of reactivity against denatured protein, the antiserum raised against the α1 peptide was unable to recognize intact BFP by immunofluorescence, suggesting that the epitopes that it recognizes are either nonconformational or not surface exposed (data not shown). In addition, neither the α1 peptide antiserum nor Fab fragments of this antiserum inhibited autoaggregation (data not shown). These results are consistent with the immunofluorescence data showing that antibodies raised against this highly variable region of bundlin do not recognize intact pili and fail to support (but do not refute) our hypothesis that this region of the pilus is surface exposed.
We conducted further experiments to explore the apparent difference in immunogenicity between cognate regions of divergent bundlin proteins. We decided to raise additional antisera in mice rather than in rabbits, because mice would provide the opportunity to test a greater number of individual animals and to determine whether the observed difference in immunogenicity was limited to a single species. We also elected to test a different β bundlin sequence to determine whether the observed effect was limited to a single bundlin type. Therefore, we raised antisera in 10 mice each to peptides representing amino acids 137 to 155 of α1 bundlin and the corresponding region of β6 bundlin. We also raised antisera in 10 mice each to purified soluble (lacking the conserved N-terminal hydrophobic 24 amino acids) α1 and β6 bundlin proteins. The results of ELISA experiments testing the immunogenicity of these peptides and proteins are shown in Fig. 3 and summarized in Table 2. We found that both bundlin proteins were highly immunogenic, eliciting immune responses in all mice tested with geometric mean titers of >1:200,000 against both the homologous and heterologous pilin proteins. For mice immunized with α1 bundlin, the response against the homologous protein was greater than that against the heterologous protein (geometric mean titers, 879,000 and 422,000, respectively), but not significantly greater (P = 0.09). However, in mice immunized with β6 bundlin the responses against the homologous and heterologous proteins were equivalent. Given the high-titer responses against heterologous proteins, it appears that immunodominant epitopes are conserved among divergent bundlin proteins.
FIG. 3.
Immune responses to bundlin proteins and peptides in mice. The antibody titers of individual mice immunized with purified α1 bundlin protein (A), purified β6 bundlin protein (B), α1 bundlin peptide conjugated to KLH (C), and β6 bundlin peptide conjugated to KLH (D) are shown. The results for preimmune serum pooled from the mice in each group are also shown. The responses against α1 bundlin peptide are indicated by open bars, the responses against β6 bundlin peptide are indicated by filled bars, the responses against α1 bundlin protein are indicated by bars with diagonal strips, the responses against β6 bundlin protein are indicated by bars with horizontal stripes, and the responses against KLH are indicated by cross-hatched bars.
TABLE 2.
Summary of antibody responses in mice to immunization with bundlin proteins and peptidesa
| Immunogen | α1 protein antigen
|
β6 protein antigen
|
α1 peptide antigen
|
No. with response/total no. with β6 peptide antigen | KLH antigen
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. with response/total no. | Geometric mean titer | Geometric mean titer for responding animals | No. with response/total no. | Geometric mean titer | Geometric mean titer for responding animals | No. with response/total no. | Geometric mean titer | Geometric mean titer for responding animals | No. with response/total no. | Geometric mean titer | Geometric mean titer for responding animals | ||
| α1 protein | 10/10 | 879,000 | 879,000 | 10/10 | 422,000 | 422,000 | 8/10 | 4,850 | 9,870 | 0/10 | |||
| β6 protein | 10/10 | 368,000 | 368,000 | 10/10 | 211,000 | 211,000 | 5/10 | 696 | 2,425 | 0/10 | |||
| α1 peptide | 7/10 | 1,299 | 2,378 | 0/10 | 6/10 | 527 | 1,425 | 0/10 | 10/10 | 139,000 | 139,000 | ||
| β6 peptide | 0/10 | 0/10 | 0/10 | 0/10 | 9/10 | 52,900 | 98,300 | ||||||
A response was defined as a fourfold increase in the titer compared with pooled preimmune sera.
Similar to the responses to the proteins, the α1 peptide was recognized by the majority of mice immunized with either the homologous peptide or the homologous bundlin protein. In stark contrast, the β6 peptide was not immunogenic, as no mice immunized with either β6 bundlin or β6 peptide developed antibodies against the peptide. The fact that these mice received the immunization was confirmed by the finding that all mice immunized with the α1 peptide and 9 of 10 mice immunized with the β6 peptide had significant responses to the KLH carrier protein to which the peptide was conjugated. Curiously, 5 of the 10 mice immunized with the β6 bundlin protein reacted against the α1 peptide, suggesting a degree of conformational conservation in this region of the pilin proteins that was not expected based on the primary amino acid sequences (Fig. 1). However, the fact that the same sera did not react against the β6 peptide suggests that there might be differences in the folding of the two peptides used to measure the response. Overall, these results obtained using a larger number of animals, a different species, and a different β bundlin variant are consistent with those obtained in the pilot study with rabbits, indicating that the region of β bundlin representing an area under diversifying selection is poorly immunogenic in comparison to the corresponding region of α bundlin. These results also demonstrate that the bundlin proteins themselves share a considerable degree of immunogenic epitopes.
Surface-exposed epitopes of bundlin are cross-reactive.
To determine whether surface-exposed epitopes of bundlin are cross-reactive, given the small quantity of antisera available from mice, it was necessary to raise additional antisera in rabbits against the purified soluble α1 and β6 bundlin proteins. We affinity purified these antisera by passage over columns to which the purified bundlin proteins were conjugated. Once again, these antisera were highly reactive as determined by ELISA against both the homologous and heterologous bundlin proteins (Fig. 4). In addition, each affinity-purified antiserum reacted more strongly against the homologous bundlin protein than against the heterologous bundlin protein, again indicating that immunization with bundlin elicits both cross-reactive and type-specific antibodies. However, in contrast to the poor reactivity of the β6 bundlin antisera raised in mice against the corresponding peptide, the antiserum raised in rabbits against the soluble β6 bundlin protein reacted strongly with the homologous peptide. Thus, we concluded that in the context of the entire protein, the β6 peptide is capable of eliciting an antibody response, at least in rabbits. Furthermore, in keeping with the results obtained with mice, the serum raised against the soluble β6 bundlin protein recognized the α1 peptide at low titer. Similarly, the serum raised against the soluble α1 protein recognized the β6 peptide at low titer. These results confirm that there is a degree of similarity in the conformation of cognate regions of divergent bundlin proteins even though only 8 of 20 residues are identical.
FIG. 4.
Reactivity of affinity-purified rabbit antisera against bundlin proteins and peptides. The titers of affinity-purified rabbit sera raised against soluble α1 bundlin (open columns) and β6 bundlin (filled columns) against α1 and β6 bundlin proteins and peptides are shown.
To determine whether the cross-reactive antibundlin antibodies can recognize whole pili and react with surface-exposed epitopes, we performed immunofluorescence assays with EPEC bacteria infecting HeLa cells and exhibiting localized adherence. As controls, we infected cells with bfpA mutant strains transformed with a plasmid encoding an afimbrial adhesin to compensate for the otherwise poor adherence of the mutants. We found that affinity-purified antisera raised against α1 bundlin recognized BFP expressed by both the prototypic α1 strain E2348/69 and the prototypic β6 strain RN587/1 (Fig. 5). The specificity of this result was demonstrated by the absence of staining of the corresponding bfpA mutant strains. Similarly, affinity-purified antisera raised against β6 bundlin specifically recognized BFP expressed by both the homologous and heterologous strains. However, the fluorescence intensity observed using both antisera was greater in samples containing the α1 bundlin strain than in samples containing the β6 bundlin strain, suggesting either that strain E2348/69 produces more pili than strain RN587/1 or that both antisera reacted more strongly with α1 pili than with β6 pili.
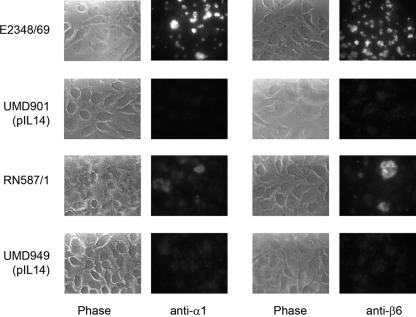
FIG. 5.
Immunofluorescence of BFP. HeLa cells were infected with wild-type strain E2348/69, which expresses α1 bundlin; with wild-type strain RN587/1, which expresses β6 bundlin; or with corresponding bfpA mutants UMD901 and UMD949, each transformed with plasmid pIL14 encoding an afimbrial adhesin to compensate for poor adherence. Infected cells were examined by immunofluorescence microscopy using affinity-purified antisera against α1 and β6 bundlins, as indicated. Corresponding phase-contrast and fluorescence micrographs are shown.
Differences in bundlin expression by prototype strains expressing different bundlin alleles.
Despite the fact that affinity-purified antisera against soluble α1 and β6 bundlins had high ELISA titers against both the homologous and heterologous bundlin proteins, we consistently observed that the rabbit antiserum raised against β6 bundlin produced a band in immunoblots containing whole-cell lysates of the prototype β6 bundlin strain RN587/1 that was less intense than the band produced by the α1 bundlin antiserum in immunoblots against the prototype α1 bundlin strain E2348/69 (data no shown). Furthermore, we observed a marked difference in the abilities of these two prototype strains to exhibit autoaggregation (Fig. 6), which correlates with pilin expression. To determine whether these effects were due to differences in pilin expression levels, we determined the number of bundlin molecules per bacterial cell expressed by each wild-type strain. To do this, we grew each strain under BFP-expressing conditions, prepared serial dilutions of the cultures, and subjected these dilutions both to colony counting and to immunoblotting using homologous sera. Serial dilutions of known quantities of the homologous purified bundlin proteins were included in the immunoblots to construct standard curves. In three experiments, we found that strain E2348/69 expressed 9.99 × 105 ± 0.43 × 105 bundlin molecules per CFU (mean ± standard deviation). In contrast, strain RN587/1 expressed 1.73 × 105 ± 0.56 × 105 molecules per CFU (P = 0.01). Thus, the differences that we observed between these prototype strains in BFP immunofluorescence (Fig. 5), autoaggregation (Fig. 6), and immunoblotting were due to significant differences in BFP expression levels rather than to differences in recognition of surface epitopes by antisera raised against the divergent bundlin proteins.
FIG. 6.
Aggregation indices of prototype strains. Strain E2348/69 expressing α1 bundlin (filled circles) and strain RN587/1 expressing β6 bundlin (open squares) were grown under BFP-expressing conditions, and the aggregation indices were determined at the indicated times as described in Materials and Methods. The symbols and error bars indicate the means and standard errors of three experiments. The indices were significantly different (P < 0.05) at 2 and 3 h.
Human responses to homologous and heterologous bundlin proteins after experimental EPEC infection.
In mice and rabbits immunized with α1 bundlin we consistently found stronger responses against the homologous bundlin protein than against the heterologous (β6) bundlin protein. To determine whether such differences are also found in humans infected with EPEC, we examined archived sera from a volunteer challenge study (15). This study involved a comparison of volunteers who had received wild-type EPEC strain E2348/69 (n = 6) or isogenic eae mutant strain CVD206 (n = 5) 70 days earlier or who were naïve prior to EPEC challenge (n = 6). All volunteers were then challenged with an oral dose of live E2348/69 bacteria. We tested prechallenge sera and sera from 27 days postchallenge for antibodies against purified α1 and β6 bundlin proteins using ELISA. As in the earlier study using a less-highly-purified prebundlin molecule with an uncertain degree of proper folding (15), we found that the responses to bundlin were modest (Fig. 7). As there were no detectable differences in titers between veteran volunteers previously challenged with wild-type bacteria and veteran volunteers previously challenged with eae mutant bacteria, we pooled these groups for further analysis. We found that the day 27 geometric mean titer against α1 bundlin was significantly higher than the prechallenge titer in naïve volunteers (P = 0.025). In contrast, the day 27 titer against β6 bundlin was not significantly higher than the prechallenge titer in this group. These results are consistent with the results obtained with mice and rabbits, indicating that the antibody responses after exposure to bundlin are greater against the homologous protein than against a divergent bundlin protein. Furthermore, these results extend the findings obtained with animals immunized with purified protein to humans infected with wild-type EPEC. Consistent with these results is the finding that the veterans had significantly higher prechallenge titers against α1 bundlin than against β6 bundlin (P = 0.006). These higher titers reflect the exposure of the subjects to α1 bundlin 70 days earlier. In contrast, there was no difference among the naïve veterans in the prechallenge titers against α1 and β6 bundlins. However, there was no evidence of an anamnestic response in the veteran volunteers, as there was no significant rise in titers against either α1 or β6 bundlin in these volunteers. Nevertheless, the day 27 titers against β6 bundlin in the veterans were significantly higher than those in the naïve group (P = 0.027), possibly reflecting a broadening of the immune response with repeated exposure to favor conserved epitopes.
FIG. 7.
Antibody responses to bundlin in volunteers challenged with wild-type EPEC strain E2348/69. The geometric mean prechallenge (Pre) and day 27 postchallenge titers of veteran volunteers (open columns) or naïve volunteers (filled columns) against α1 and β6 bundlin proteins are shown. The error bars indicate the standard errors of the geometric means. Asterisks indicate significant differences (P < 0.05) between pairs of data.
DISCUSSION
The EPEC BFP is an important virulence factor required for full pathogenicity in an experimental human model of disease (5). Previous studies have demonstrated a considerable degree of sequence variation in bfpA alleles specifying bundlin, the major subunit of the BFP (6, 7). In particular, a region encompassing the codons for amino acid residues 137 to 155 near the C terminus of the mature protein (Fig. 1) has an excess of nonsynonymous substitutions, indicating the influence of diversifying selection (7). As this effect may have been exerted by the selective pressure of the immune response from humans infected with EPEC, we developed the hypothesis that this region of bundlin is recognized by host antibodies and is surface exposed. As an initial step in testing this hypothesis, we examined antibody responses in mice, rabbits, and humans against divergent bundlin peptides, proteins, and pili. Our results supported some aspects of the hypothesis and failed to support others. In particular, we could not verify that the divergent region is surface exposed. In addition, we found that antibodies raised against one bundlin type reacted strongly with a divergent type. However, we found dramatic differences in immunogenicity between cognate peptides from divergent types, and we determined that responses were stronger against the homologous proteins than against the heterologous proteins. Given the high degree of overall sequence conservation between divergent pilin proteins, the ability of sera from volunteers to distinguish between two types suggests an important degree of type-specific responses that may be relevant to subsequent susceptibility and may provide the basis for bfpA sequence divergence. It is possible that alternative forces contribute to bundlin sequence variation as well. For example, pressure due to pilus-specific bacteriophages could lead to sequence diversity, although BFP phages have not yet been described.
The structure of soluble α1 bundlin has been determined by nuclear magnetic resonance, and a model that fits the bundlin monomers into the BFP has been proposed (35). According to this model, amino acids corresponding to the amino acids under the influence of diversifying selection are predicted to be surface exposed. However, we could not verify this prediction, as antiserum raised in rabbits against a peptide from this region of α1 bundlin did not recognize intact pili and did not block autoaggregation, despite the high titer of this serum when it was used in ELISA or immunoblotting assays. Failure to interact with intact pili should not be interpreted as an indication that this region of the protein is not surface exposed. The relevant epitopes may not be recognized in the context of the intact pilus due to differences in three-dimensional conformation. For example, the solution structure of the isolated receptor binding peptide from the type IV pilin of P. aeruginosa is not identical to that of the intact pilin (9, 20).
In addition to an effect on immunogenicity, we examined whether bfpA sequence diversity could influence pilus biogenesis or function. To determine whether the biogenesis machine from a strain that produces α1 bundlin can assemble divergent BFP, we complemented a bfpA null mutant of this strain with plasmids encoding each alternative bfpA allele. We found that each plasmid restored the ability to produce functional BFP, demonstrating that BFP biogenesis machines are capable of assembling pili composed of all bundlin types. Thus, the sequence divergence among these alleles is not critical to BFP biogenesis.
BFP are so named because of their tendency to interact longitudinally to form rope-like structures (18). This property, combined with the ability of the bacteria to retract the pili, endows EPEC with the autoaggregation and disaggregation phenotypes. To determine whether BFP composed of divergent bundlin proteins are capable of forming mixed bundles, we examined autoaggregation using pairs of strains expressing four different bundlin types and two different fluorescent proteins. We found that aggregates were composed of mixtures of both strains of bacteria with colocalization of the fluorescence signals. Thus, the sequence divergence does not preclude interpilus bundle formation. These experiments also provide further evidence that autoaggregates result from recruitment of planktonic bacteria rather than from proliferation of a progenitor (5).
One of the striking findings of this study was the marked difference in immunogenicity of cognate peptides from α1 and β6 bundlin molecules representing the region under apparent diversifying selection. Whereas the α1 bundlin peptide was immunogenic in mice and rabbits, the corresponding β6 bundlin peptide was not. However, rabbits immunized with soluble β6 bundlin produced antibodies against the β6 bundlin peptide, indicating that in the context of the full protein, epitopes from this region can be recognized. We were also surprised to find that 5 of 10 mice immunized with the soluble β6 bundlin protein were able to recognize the α1 bundlin peptide, despite conservation of only 8 of 20 amino acids. This result was verified in rabbits. Thus, it appears that cross-reactive epitopes exist even in this highly divergent region, indicating perhaps that similar three-dimensional conformations are adopted by the divergent proteins. This result may help explain how the α1 strain is able to assemble all other pilin types.
To determine whether there are cross-reactive surface-exposed epitopes in BFP composed of divergent bundlin molecules, we tested affinity-purified rabbit anti-α1 bundlin and affinity-purified rabbit anti-β6 bundlin sera by fluorescence microscopy against strains expressing homologous and heterologous BFP. We found that both sera were able to recognize BFP from both strains. Any apparent differences in signal intensity were accounted for by the fact that the strain expressing α1 bundlin produced almost sixfold more bundlin protein than the strain expressing β6 bundlin. Thus, animals immunized with one bundlin protein can recognize pili as well as pilin from a strain expressing a divergent bundlin type.
We took advantage of archived sera from a human EPEC rechallenge study to determine whether people infected with one EPEC strain develop antibodies against homologous and heterologous bundlin types. The results were enlightening. Although the responses of individual volunteers against bundlin were modest, the responses of veteran volunteers undergoing rechallenge were significantly different than those of naïve volunteers infected with EPEC for the first time. First, the veterans had higher initial titers of serum IgG against α1 bundlin, to which they were previously exposed, than against β6 bundlin, to which they were not previously exposed. Similarly, the naïve volunteers developed titers against the homologous α1 bundlin that were significantly higher than their prechallenge titers. Despite these differences, the pre- and postchallenge titers against both bundlin types were similar for the veteran and naïve groups with one exception: the postchallenge titers against β6 bundlin were significantly higher in the veterans than in the naïve group. This result may indicate that repeated exposure to EPEC can lead to an increase in the production of cross-reactive antibodies.
It has long been known that EPEC disease almost exclusively afflicts children under 2 years of age, particularly the very youngest infants (1, 12, 16, 19). Whether this unique predilection is the result of differences in exposure to the pathogen, inherent susceptibility to its effects, or acquired protective immunity from repeated infection has never been ascertained. In the current study we determined that initial exposure to a strain of EPEC expressing one type of bundlin results in stronger responses against the homologous bundlin protein than against a divergent bundlin type. These results are consistent with our hypothesis that the human immune response may exert selective pressure that favors bfpA sequence variation. According to this hypothesis, individuals exposed to one EPEC strain are more likely to be infected and transmit the bacteria to other individuals upon exposure to a subsequent strain if the latter strain has a bfpA allele specifying a different sequence in this region. Whether the difference in initial response correlates with differences in subsequent susceptibility to infection with strains expressing homologous and heterologous bundlin types cannot be determined from the current study. A longitudinal birth cohort study that examines infection with EPEC, development of antibodies against EPEC antigens, and signs and symptoms of EPEC infection may help unravel the relative roles of exposure, age, and immunity in susceptibility to EPEC disease.
Acknowledgments
We thank Xiaolin Wang, Virginia Lockatell, Leon De Masi, and Thomas J. Reilly for technical assistance.
This work was supported by Public Health Service award R01 AI-37606 from the National Institutes of Health.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Albert, M. J., A. S. Faruque, S. M. Faruque, R. B. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 1998. The role of BfpF, a member of the PilT family of putative nucleotide-binding proteins, in type IV pilus biogenesis and in interactions between enteropathogenic Escherichia coli and host cells. Infect. Immun. 66:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 2000. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J. Bacteriol. 182:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard, M. K., J. S. Mattick, L. J. Moore, M. R. Mott, C. F. Marrs, and J. R. Egerton. 1990. Morphogenetic expression of Moraxella bovis fimbriae (pili) in Pseudomonas. aeruginosa. J. Bacteriol. 172:2601-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia. coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 6.Blank, T. E., D. W. Lacher, I. C. A. Scaletsky, H. L. Zhong, T. S. Whittam, and M. S. Donnenberg. 2003. Enteropathogenic Escherichia coli O157 strains from Brazil. Emerg. Infect. Dis. 9:113-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blank, T. E., H. Zhong, A. L. Bell, T. S. Whittam, and M. S. Donnenberg. 2000. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect. Immun. 68:7028-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley, D. E. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol. 26:146-154. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, A. P., D. L. Bautista, B. Tripet, W. Y. Wong, R. T. Irvin, R. S. Hodges, and B. D. Sykes. 1997. Solution secondary structure of a bacterially expressed peptide from the receptor binding domain of Pseudomonas aeruginosa pili strain PAK: a heteronuclear multidimensional NMR study. Biochemistry 36:12791-12801. [DOI] [PubMed] [Google Scholar]
- 10.Castric, P. A., and C. D. Deal. 1994. Differentiation of Pseudomonas aeruginosa pili based on sequence and B-cell epitope analyses. Infect. Immun. 62:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 12.Cravioto, A., R. Reyes, R. Ortega, G. Fernández, R. Hernández, and D. López. 1988. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol. Infect. 101:123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel, A., A. Singh, L. J. Crowther, P. J. Fernandes, W. Schreiber, and M. S. Donnenberg. 2006. Interaction and localization studies of enteropathogenic Escherichia coli type IV bundle-forming pilus outer membrane components. Microbiology 152:2405-2420. [DOI] [PubMed] [Google Scholar]
- 14.Donnenberg, M. S., and J. P. Nataro. 1995. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods Enzymol. 253:324-336. [DOI] [PubMed] [Google Scholar]
- 15.Donnenberg, M. S., C. O. Tacket, G. Losonsky, G. Frankel, J. P. Nataro, G. Dougan, and M. M. Levine. 1998. Effect of prior experimental human enteropathogenic Escherichia coli infection on illness following homologous and heterologous rechallenge. Infect. Immun. 66:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germani, Y., E. Begaud, P. Duval, and C. Le Bouguenec. 1996. Prevalence of enteropathogenic, enteroaggregative, and diffusely adherent Escherichia coli among isolates from children with diarrhea in New Caledonia. J. Infect. Dis. 174:1124-1126. [DOI] [PubMed] [Google Scholar]
- 17.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. (Erratum, 189:283, 1990.) [DOI] [PubMed] [Google Scholar]
- 18.Girón, J. A., A. S. Y. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 19.Gomes, T. A. T., V. Rassi, K. L. Macdonald, S. R. T. S. Ramos, L. R. Trabulsi, M. A. M. Vieira, B. E. C. Guth, J. A. N. Candeias, C. Ivey, M. R. F. Toledo, and P. A. Blake. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J. Infect. Dis. 164:331-337. [DOI] [PubMed] [Google Scholar]
- 20.Hazes, B., P. A. Sastry, K. Hayakawa, R. J. Read, and R. T. Irvin. 2000. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J. Mol. Biol. 299:1005-1017. [DOI] [PubMed] [Google Scholar]
- 21.Henrichsen, J. 1983. Twitching motility. Annu. Rev. Microbiol. 37:81-93. [DOI] [PubMed] [Google Scholar]
- 22.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labigne-Roussel, A. F., D. Lark, G. Schoolnik, and S. Falkow. 1984. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 46:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, K. K., H. B. Sheth, W. Y. Wong, R. Sherburne, W. Paranchych, R. S. Hodges, C. A. Lingwood, H. Krivan, and R. T. Irvin. 1994. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11:705-713. [DOI] [PubMed] [Google Scholar]
- 25.Loureiro, I., G. Frankel, J. Adu-Bobie, G. Dougan, L. R. Trabulsi, and M. M. Carneiro-Sampaio. 1998. Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia coli virulence-associated proteins: intimin, BfpA, EspA, and EspB. J. Pediatr. Gastroenterol. Nutr. 27:166-171. [DOI] [PubMed] [Google Scholar]
- 26.Mattick, J. S., M. M. Bills, B. J. Anderson, B. Dalrymple, M. R. Mott, and J. R. Egerton. 1987. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J. Bacteriol. 169:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara, B. P., and M. S. Donnenberg. 2000. Evidence for specificity in type 4 pilus biogenesis by enteropathogenic Escherichia coli. Microbiology 146:719-729. [DOI] [PubMed] [Google Scholar]
- 28.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98-102. [DOI] [PubMed] [Google Scholar]
- 29.Meyer, T. F., C. P. Gibbs, and R. Haas. 1990. Variation and control of protein expression in Neisseria. Annu. Rev. Microbiol. 44:451-477. [DOI] [PubMed] [Google Scholar]
- 30.Nevesinjac, A. Z., and T. L. Raivio. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J. Bacteriol. 187:672-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novais, R. C., A. Coelho, C. A. Salles, and A. C. Vicente. 1999. Toxin-co-regulated pilus cluster in non-O1, non-toxigenic Vibrio cholerae: evidence of a third allele of pilin gene. FEMS Microbiol. Lett. 171:49-55. [DOI] [PubMed] [Google Scholar]
- 32.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 33.Parissi-Crivelli, A., J. M. Parissi-Crivelli, and J. A. Girón. 2000. Recognition of enteropathogenic Escherichia coli virulence determinants by human colostrum and serum antibodies. J. Clin. Microbiol. 38:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramboarina, S., P. Fernandes, P. Simpson, G. Frankel, M. Donnenberg, and S. Matthews. 2004. Complete resonance assignments of bundlin (BfpA) from the bundle-forming pilus of enteropathogenic Escherichia coli. J. Biomol. NMR 29:427-428. [DOI] [PubMed] [Google Scholar]
- 35.Ramboarina, S., P. J. Fernandes, S. Daniell, S. Islam, P. Simpson, G. Frankel, F. Booy, M. S. Donnenberg, and S. Matthews. 2005. Structure of the bundle-forming pilus from enteropathogenic Escherichia coli. J. Biol. Chem. 280:40252-40260. [DOI] [PubMed] [Google Scholar]
- 36.Rudel, T., I. Scheuerpflug, and T. F. Meyer. 1995. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 373:357-359. [DOI] [PubMed] [Google Scholar]
- 37.Sauvonnet, N., P. Gounon, and A. P. Pugsley. 2000. PpdD type IV pilin of Escherichia coli K-12 can be assembled into pili in Pseudomonas aeruginosa. J. Bacteriol. 182:848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seifert, H. S., R. S. Ajioka, C. Marchal, P. F. Sparling, and M. So. 1988. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature 336:392-395. [DOI] [PubMed] [Google Scholar]
- 39.Seifert, H. S., C. J. Wright, A. E. Jerse, M. S. Cohen, and J. G. Cannon. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 93:2744-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone, K. D., H.-Z. Zhang, L. K. Carlson, and M. S. Donnenberg. 1996. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for biogenesis of a type IV pilus. Mol. Microbiol. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 41.Tacket, C. O., R. K. Taylor, G. Losonsky, Y. Lim, J. P. Nataro, J. B. Kaper, and M. M. Levine. 1998. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 32:1-10. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida, T., S. R. Kim, and T. Komano. 1999. Twelve pil genes are required for biogenesis of the R64 thin pilus. J. Bacteriol. 181:2038-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, H.-Z., and M. S. Donnenberg. 1996. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol. Microbiol. 21:787-797. [DOI] [PubMed] [Google Scholar]