Abstract
Although humoral immunity has been shown to contribute to host defense during intracellular bacterial infections, its role has generally been ancillary. Instead, CD4 T cells are often considered to play the dominant role in protective immunity via their production of type I cytokines. Our studies of highly pathogenic Ehrlichia bacteria isolated from Ixodes ovatus (IOE) reveal, however, that this paradigm is not always correct. Immunity to IOE infection can be induced by infection with a closely related weakly pathogenic ehrlichia, Ehrlichia muris. Type I cytokines (i.e., gamma interferon, tumor necrosis factor alpha, and interleukin-12) were not necessary for E. muris-induced immunity. In contrast, humoral immunity was essential, as shown by the fact that E. muris-infected B-cell-deficient mice were not protected from IOE challenge and because E. muris immunization was effective in CD4-, CD8-, and major histocompatibility complex (MHC) class II-deficient mice. Immunity was unlikely due to nonspecific inflammation, as prior infection with Listeria monocytogenes did not induce immunity to IOE. Antisera from both wild-type and MHC-II-deficient mice provided at least partial resistance to challenge infection, and protection could also be achieved following transfer of total, but not B-cell-depleted, splenocytes obtained from E. muris-immunized mice. The titers of class-switched antibodies in immunized CD4 T-cell- and MHC class II-deficient mice, although lower than those observed in immunized wild-type mice, were significant, indicating that E. muris can induce class switch recombination in the absence of classical T-cell-mediated help. These studies highlight a major protective role for classical T-cell-independent humoral immunity during an intracellular bacterial infection.
Despite increasing evidence that humoral immunity can play a role in protection against intracellular bacterial pathogens, it is often generally accepted that cellular immunity is the dominant form of protection during intracellular bacterial infections. We have drawn similar conclusions on the basis of our previous studies of protective immunity during ehrlichial infections, where we have shown that CD4 T-cell production of gamma interferon (IFN-γ) is essential (4). However, work from several laboratories, using a variety of infection models, has demonstrated that antibodies can also play an important role in immunity (6, 8, 10, 26, 33, 35, 44, 51). In our own studies, for example, we found that immune serum or outer membrane protein (OMP)-specific monoclonal antibodies could protect susceptible SCID mice from fatal monocytotropic ehrlichia infection (24, 26). Although these and other studies indicated that antibodies could control ehrlichia infections in immunodeficient mice, they did not reveal the extent to which antibodies mediate protection in immunocompetent mice.
More recent studies of immunity to highly pathogenic Ehrlichia bacteria isolated from Ixodes ovatus (IOE) revealed that B cells were essential for protection in immunocompetent mice following a low-dose sublethal infection (51). However, low-dose IOE-infected wild-type mice generated relatively poor antibody responses and were not protected from a subsequent fatal high-dose IOE challenge infection (5, 51). In contrast, infection with a closely related low-pathogenicity ehrlichia, Ehrlichia muris (19), was shown to generate effective immunity to IOE challenge (17). In these latter studies, infection was associated with production of IFN-γ by CD4 T cells, although the requirement(s) for CD4 T cells, B cells, and inflammatory cytokines in protective immunity was not fully resolved.
Here we have addressed the underlying mechanisms of protective immunity induced by E. muris infection. In contrast to our expectations that CD4 Th1 cells would play an important and essential role in immunity, we found instead that B cells and antibodies were required for protection. Moreover, B-cell-dependent protective immunity was generated in the absence of CD4 T cells. These findings indicate that B cells and antibodies can play not only auxiliary but also central roles in host defense during an intracellular bacterial infection in immunocompetent mice.
MATERIALS AND METHODS
Mice.
The mice used in these studies were obtained from Jackson Laboratories, Bar Harbor, ME, or were bred in the Animal Care Facility at the Wadsworth Center under microisolator conditions, in accordance with institutional guidelines for animal welfare. The inbred strains were C57BL/6 and C57BL/6-scid (B6.CB17-Prkdcscid/SzJ). The following gene-targeted strains were utilized: CD4 deficient (B6.129S2-Cd4tm1Mak), CD8 deficient (B6.129S2-Cd8atm1Ma), interleukin-12 p40 (IL-12 p40) deficient (B6.129S1-Il12atm1Jm), IFN-γ deficient (C.129S7(B6)-Ifngtm1s), tumor necrosis factor alpha (TNF-α) deficient (B6.129S6-Tnfftm1Gk1), B-cell deficient (B6.129S2-Igh-6tm1Cgn/J; also known as μMT), and major histocompatibility complex (MHC) class II deficient (B6.129-H2dIAb1-Ea/J). Mice were gender matched for each experiment and were 6 to 12 weeks in age.
Antibodies.
The anti-CD4 antibody (GK1.5) used for flow cytometry was obtained from BD Biosciences (Franklin Lakes, NJ). The same antibody was used to neutralize CD4 T cells in vivo. IFN-γ was neutralized using rat immunoglobulin G1 (IgG1), which was produced by the hybridoma XMG1.2; the antibody was purified by protein A affinity chromatography.
Bacterial infections and immunizations.
Infections were performed as described previously (4). Mice were inoculated via the peritoneum. Institutional standards for animal welfare did not permit the use of death as an end point in the infection experiments, and so mice were routinely sacrificed when deemed moribund and judged to be incapable of surviving infection. Morbidity was indicated by ruffled coat, immobility, and hunched posture. Quantitative PCR was used to determine the bacterial copy number in the frozen aliquots, as described previously (4). We have made the simplifying assumption that copy number and numbers of viable bacteria were equivalent in our experimental model. For immunizations, mice were challenged with IOE >4 weeks after E. muris immunization. Listeria monocytogenes infection was performed by intraperitoneal inoculation of 7.2 × 104 CFU. CFU were determined on blood agar.
CD4 T-cell purification.
CD4 T cells were purified from mouse spleen cell homogenates using a CD4 T-cell isolation kit (BD Biosciences) following the instructions of the manufacturer. For further CD4 T-cell enrichment, the samples were sorted by flow cytometry using a FACSVantage flow cytometric cell sorter (BD Biosciences), which yielded cells of a purity of >99%. For T-cell adoptive transfers, CD4 T cells were purified by negative magnetic bead selection (Miltenyi Biotec) and were resuspended in Hanks’ balanced salt solution at a concentration of 2 × 106/ml prior to transfer (0.5 ml) to recipient mice by tail vein injection.
B-cell depletion.
B cells were depleted from whole splenocyte suspensions using goat anti-mouse polyclonal IgG microbeads (Polysciences Inc.). The beads were washed and mixed with the splenocytes (4 ml/spleen), and the suspension was incubated at 4°C on a rocker for 30 min prior to binding to the magnet. The supernatant containing unbound cells was used in cell transfer experiments. Fluorescence-activated cell sorter (FACS) analysis revealed that the depleted cell suspensions contained fewer than 2% B220-positive cells.
Cell and cytokine neutralization.
For neutralization of CD4 T cells, mice were administered anti-CD4 (GK1.5; 200 μg/dose) 1 day prior to IOE challenge. For IFN-γ neutralization, mice were administered two doses of anti-IFN-γ (XMG1.2) on days 1 and 4 post-IOE challenge.
Transfer of polyclonal sera and monoclonal antibodies.
E. muris immune serum was obtained from C57BL/6 or MHC class II-deficient mice 2 to 4 weeks after infection, and normal serum was obtained from uninfected C57BL/6 mice. The serum titer was determined by enzyme-linked immunosorbent assay (ELISA) using purified recombinant E. muris OMP-19, as described previously (51). The E. muris immune and normal sera (100 μl/injection) were transferred into C57BL/6 mice via the peritoneum 1 day prior and 3 and 7 days post-IOE infection. The monoclonal antibody Ec56.5 (26) and the isotype control antibody KJ1-26 (200 μg/injection) were injected in a similar fashion.
Statistical analyses.
Statistical analyses of survival/morbidity in the challenge studies were performed using a log rank test. Other data were analyzed using a one-tailed Mann-Whitney test with a confidence interval of 95%. Data were analyzed using Prism software (GraphPad Software, Inc.).
RESULTS
E. muris infection protects against high-dose IOE challenge.
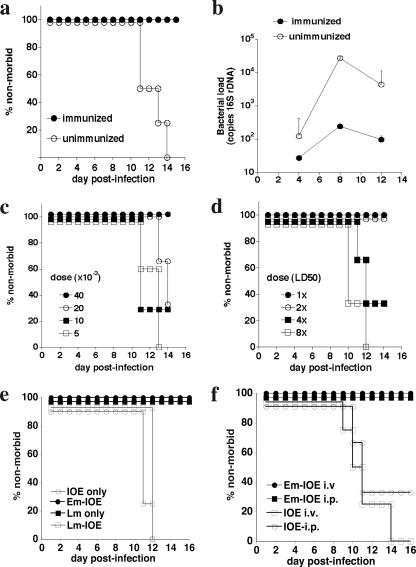
Previous studies demonstrated that E. muris infection induces immunity to fatal high-dose IOE challenge infection (17). To begin to identify the cells and factors required for immunity in this model, we first determined the dose of E. muris required to generate protective immunity and the degree of protection obtained. C57BL/6 mice were infected with 4 × 104 bacteria, via the peritoneum, and were challenged 32 days later with an inoculum of IOE (2× the 50% lethal dose [LD50]) known to cause fatal infection. As expected, unimmunized mice became moribund within 12 to 14 days following IOE challenge (Fig. 1a). E. muris infection, in contrast, protected susceptible mice from fatal IOE challenge, and protection was associated on day 8 with an approximately 2-log reduction in bacterial infection in the spleen (Fig. 1b). Moreover, E. muris immunization induced long-lasting immunity: similar challenge infections performed as long as 110 days following E. muris infection were successfully resolved (data not shown). Effective immunization required infection with a minimum of 4 × 104 E. muris (Fig. 1c), and this dose protected mice against a 2× LD50 dose (approximately 500 bacteria), but not a higher IOE challenge dose (Fig. 1d). These data confirm and extend previous observations that heterologous immunization can protect mice against fatal IOE challenge. Although protection was only achieved at moderate challenge doses, immunity was not due to nonspecific inflammation induced at the site of infection. First, intraperitoneal infection with Listeria monocytogenes 7 days prior to high-dose IOE challenge did not result in any protection against IOE (Fig. 1e). Furthermore, protection against fatal IOE infection was achieved following intravenous challenge, demonstrating that immunity was systemic and was not a consequence of persistent E. muris infection in the peritoneal cavity (Fig. 1f).
FIG. 1.
E. muris infection generates dose-dependent immunity to IOE challenge infection. a. C57BL/6 mice were infected with 4 × 104 E. muris, and 30 days later infected and uninfected control mice were challenged with high-dose IOE (2× LD50). Morbidity was monitored using the criteria described in Materials and Methods. Four mice were used per group. b. Bacterial infection in the spleens of immunized and control mice was determined on the indicated days postinfection. Standard deviations of the means are indicated. c. Mice were immunized with the indicated doses of E. muris and challenged with high-dose IOE. d. IOE challenge infections of immunized mice were performed using from 1 to 8 times the LD50, as indicated. In all experiments at least four mice were used in each group. e. Mice were infected via the peritoneum with L. monocytogenes (Lm; 7.2 × 104 CFU) 7 days prior to challenge with IOE. Control mice received only Lm or IOE, or were immunized prior to IOE challenge with E. muris (Em-IOE). f. Mice were immunized via the peritoneum with E. muris and challenged with IOE via the peritoneum (i.p.) or intravenously (i.v.). The protection observed following either route of injection was statistically significant (P < 0.04).
CD4 or CD8 T cells are not required for immunity during IOE challenge.
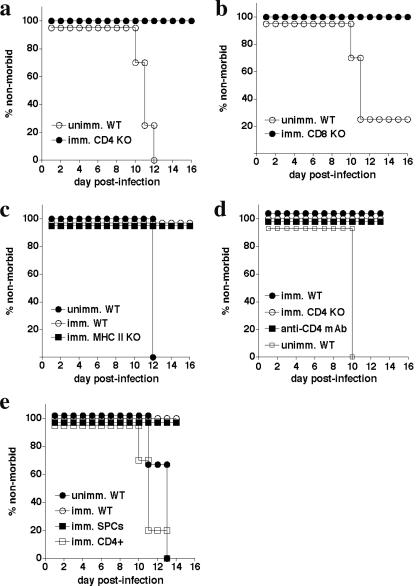
To test the requirement for particular cells and factors in E. muris-induced immunity, we first examined the susceptibilities of a panel of immune-deficient mice to E. muris infection. E. muris causes relatively mild, nonfatal disease in immunocompetent mice (20, 38), and previous studies suggested that the CD8 coreceptor was required in C3H/HeN mice (9). In our infection studies in C57BL/6 mice, neither CD4-, CD8-, B-cell-, IFN-γ-, nor TNF-α-deficient mice were susceptible to fatal E. muris infection, although SCID and RAG-deficient mice succumbed within 16 to 20 days postinfection (data not shown). Our data indicated that either T or B cells are sufficient for host defense against E. muris, as has been reported previously for Ehrlichia chaffeensis (50). Since most of the immune-deficient mouse strains examined were resistant to primary E. muris infection, we next tested whether these strains could be immunized against high-dose IOE challenge. Both CD4- and CD8-deficient E. muris-immunized mice were found to be protected (Fig. 2a and b), and bacterial burden in the CD4-deficient mice was similar to E. muris-immunized wild-type controls (see Fig. 3b, below). These data, which indicate that neither CD4 nor CD8 T cells are required for resistance to IOE challenge, contrast with our previous finding that the CD4 coreceptor was required for protection against a low-dose (nonfatal) IOE infection in unimmunized mice (4).
FIG. 2.
T cells are not essential for E. muris-induced immunity following IOE challenge. a. CD4-deficient mice were immunized with E. muris and then challenged with IOE (2× LD50) approximately 30 days later. Similar studies were performed with CD8-deficient (b) and class II MHC-deficient (c) mice. Unimmunized (unimm) and immunized (imm) mice were used as controls. Data in panel b were statistically significant (P = 0.043). d. Wild-type and CD4-deficient mice were immunized with E. muris and then challenged with IOE. One group of wild-type mice was administered anti-CD4 1 day prior to IOE challenge (GK1.5; 200 μg/injection). e. Naive mice were administered 2 × 106 splenocytes (imm. SPCs) or flow cytometry-purified CD4 T cells (imm. CD4+; 99.2% purity) that had been obtained from E. muris-immunized mice at least 30 days after immunization. All experiments utilized three to five mice per group.
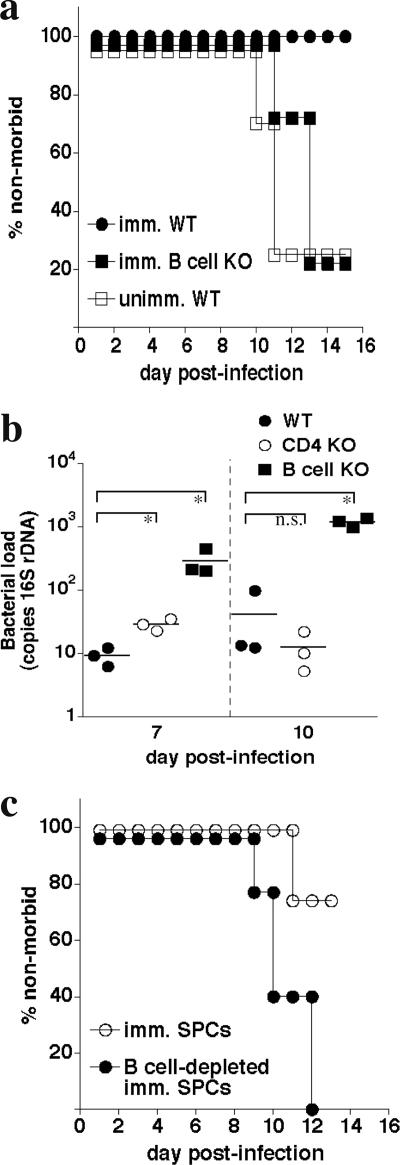
FIG. 3.
B cells are essential for immunity. a. B-cell-deficient (μMT) mice were immunized with E. muris and challenged with IOE (2× LD50), and morbidity was monitored. The data were statistically significant (P = 0.043; analysis of the immunized groups). b. Bacterial infection was quantitated in spleens of wild-type, B-cell-deficient (B cell KO), and CD4-deficient (CD4 KO) mice. Each data point represents a single mouse. Brackets indicate data used for statistical analysis. *, P = 0.05. n.s., not significant. c. One day prior to IOE challenge, naive mice were administered 1 × 106 total (imm. SPCs) or B-cell-depleted splenocytes obtained from E. muris-immunized donor mice. Each group contained four mice. The data were statistically significant (P = 0.017).
To address whether the survival of the CD4-deficient mice was due to the presence of class II MHC-restricted T cells that can develop in the absence of the CD4 coreceptor (40, 47), we immunized MHC class II-deficient mice with E. muris. The MHC class II-deficient mice, which lack CD4 T cells, were protected against IOE challenge, further supporting the finding that CD4 T cells are dispensable for protection (Fig. 2c). Moreover, CD4 T-cell neutralization in E. muris-immunized mice 1 day prior to high-dose IOE challenge infection did not affect the animals’ survival following IOE challenge (Fig. 2d). We also addressed whether lymphocytes and/or CD4 T cells could transfer protective immunity to naive mice. For these studies, total splenocytes, or FACS-purified CD4 T cells (99% purity) obtained from E. muris-immunized mice, were transferred to naive mice prior to IOE challenge. The splenocytes, but not the purified CD4 T cells, transferred protection (Fig. 2e), providing further evidence that CD4 T cells are not essential for protection following IOE challenge.
B cells are essential for protection.
Since we have previously shown that antibodies and/or B cells can play potentially important roles during ehrlichia infection, we next addressed whether B cells are essential during IOE challenge. E. muris immunization of B-cell-deficient mice did not generate protective immunity (Fig. 3a). Morbidity in the B-cell-deficient mice was accompanied by bacterial loads that were at least 30-fold higher than wild-type mice on days 7 and 10 postinfection (Fig. 3b). No or only nominal differences were observed between the wild-type and CD4-deficient mice. Moreover, in transfer studies similar to those described above, depletion of B cells from the spleen cell suspensions resulted in loss of protection following IOE challenge (Fig. 3c). These data indicated that B cells, but not CD4 T cells, are essential for E. muris-induced immunity during fatal IOE challenge.
Type I cytokines are not required for immunity.
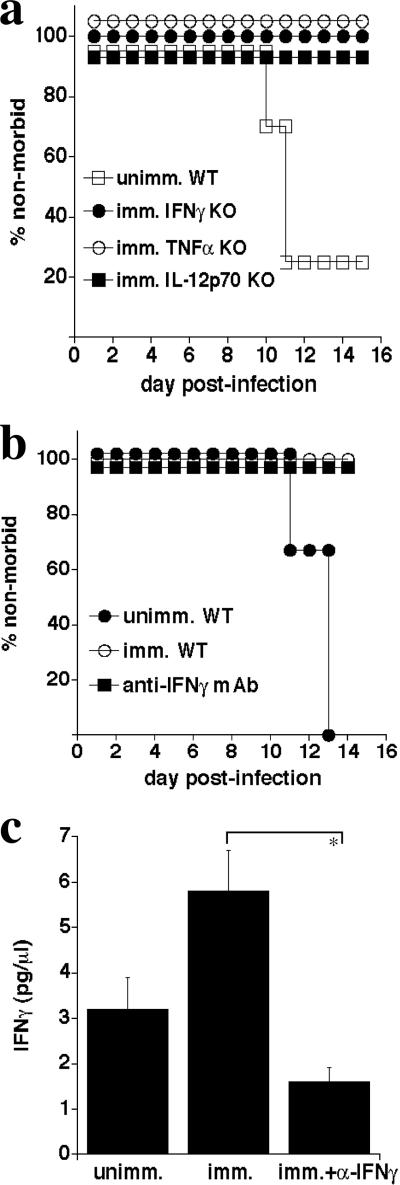
Although we found B cells to be required for immunity, we did not know whether, in addition, type I cytokines play an important role. Type I cytokines have been found to be important in resolving primary low-dose IOE infection (4), and so it is possible that B cells mediate protection in part via the secretion of these cytokines, as has been observed in other studies (14, 22). Given that several of our cytokine-deficient strains survived E. muris infection (Fig. 2), IFN-γ-, TNF-α-, and IL-12 p40-deficient mice were immunized with E. muris and challenged at least 4 weeks later with IOE. All of the cytokine-deficient mice examined survived an IOE challenge that was fatal to unimmunized mice (Fig. 4a), suggesting either that the cytokines were nonessential or that these cytokine functions were redundant in the genetically deficient mice.
FIG. 4.
Type I cytokines are not required for protective immunity. a. Wild-type and IFN-γ-, TNF-α-, and IL-12 p40-deficient mice were immunized with E. muris and later challenged with 2× LD50 of IOE. In each case the groups of gene-targeted mice were statistically different from the wild-type control mice (P = 0.043). b. E. muris-immunized mice were treated with two doses of anti-IFN-γ neutralizing antibody (clone XMG1.2; 200 μg/dose) 1 and 4 days after IOE challenge. Naïve and immunized mice were used as controls. c. Serum concentrations of IFN-γ were measured on day 16 post-IOE challenge in the antibody-treated and nontreated immunized mice. Standard deviations are indicated. *, P < 0.05.
The above findings were unexpected, since IFN-γ is well known to be an essential cytokine during many intracellular bacterial infections and because our previous studies had demonstrated that this cytokine is essential during low-dose IOE infection (4). We therefore used an alternative approach to address whether IFN-γ is required in the E. muris-immunized mice. IFN-γ neutralization on days 1 and 4 postinfection did not affect protective immunity (Fig. 4b), indicating that the lack of a requirement for IFN-γ in the genetically deficient mice was unlikely due to compensatory mechanisms operative in the genetically deficient mice. To confirm that antibody-mediated IFN-γ neutralization was effective, we measured IFN-γ concentrations in the sera of treated and untreated mice. Serum IFN-γ concentrations in treated IOE-challenged immunized mice were approximately threefold lower than in nontreated IOE-challenged immunized mice, and they were twofold lower relative to susceptible unimmunized naive mice (Fig. 4c). Thus, our data collectively demonstrate that type I immunity is not required for E. muris-induced immunity to IOE challenge infection.
Antibodies are sufficient to mediate immunity.
E. muris infection generates robust antibody responses (9, 17, 51), so we next considered whether protective immunity could be generated following passive transfer of immune serum. Polyclonal sera obtained from E. muris-immunized mice protected naive mice from fatal IOE challenge (Fig. 5a). Data from three separate experiments revealed that immune sera-treated mice were significantly protected relative to normal serum-treated mice (P < 0.0001), indicating that serum antibodies were likely responsible. ELISA confirmed that the immune serum from the E. muris-immunized mice contained high titers of IOE OMP-19-specific antibodies of various Ig subclasses (data not shown). For the ELISA, OMP-19 was used as a representative ehrlichia antigen, since we have previously demonstrated that OMPs are immunodominant during ehrlichia infections (26). The data suggest that antibodies can provide at least a component of immunity, although these studies do not exclude an additional role(s) for B cells.
FIG. 5.
Immune serum can transfer protection against IOE challenge. a. Serum was pooled from immunized mice at least 30 days after infection and was administered to naïve mice 1 day prior and 3 and 7 days post-IOE challenge (100 μl/dose). Normal serum was administered as a control. Data combined from four experiments are shown (P = 0.0001; n = 17). The Ig titer of the immune sera was greater than 1:1,200. b. The monoclonal antibody Ec56.5, or an isotype control, was administered to naïve mice 1 day prior and 3 and 7 days post-IOE infection (200 μg/dose). Each group contained four mice. The data shown are representative of two experiments. c. Serum was pooled from E. muris-immunized MHC-II-deficient mice on day 30 postinfection and was administered to naive mice, as described for panel a. The differences were statistically significant (P < 0.0082; n = 5). The Ig titer of the immune serum was greater than 1:200.
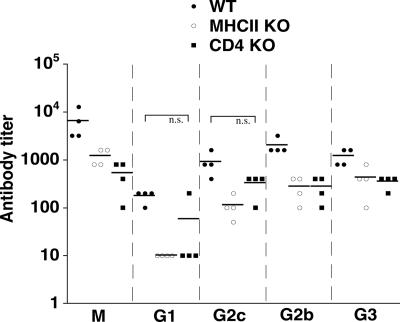
To further address a role for antibodies in immune protection, we utilized the monoclonal anti-OMP-19-specific antibody Ec56.5 (IgG2c) (30). This antibody was highly effective when administered during E. chaffeensis infection in SCID mice (24), and the antibody epitope is conserved in IOE OMP-19 (data not shown). Ec56.5 administration was highly effective during IOE challenge infection (Fig. 5b), further supporting the notion that antibodies can be effective during infection of immunocompetent mice. Since protection was also observed in both CD4- and MHC class II-deficient E. muris-immunized mice, we next addressed whether immune serum from MHC class II-deficient mice could transfer protection to naive C57BL/6 mice. Protection was statistically significant (P = 0.0082), albeit moderate, in the immune serum-treated mice. Although the titers of OMP-19-specific antibodies were lower in the class II MHC- and CD4-deficient mice relative to wild-type mice, perhaps explaining the less efficient protection achieved following serum transfer, they were nevertheless substantial (Fig. 6). Moreover, isotype switching to IgG2c, IgG2b, and IgG3, but not IgG1, was evident in the immunized CD4 T-cell-deficient mice, suggesting that E. muris is capable of mediating B-cell isotype switching in the absence of T-cell help.
FIG. 6.
T-cell-independent antibody responses in E. muris-immunized mice. OMP-19 antibody titers were measured in sera of E. muris-immunized wild-type, MHC class II-, and CD4-deficient mice. Differences in titers between wild-type and gene-targeted strains were statistically significant, except where indicated (n.s.) (Mann-Whitney test with 99% confidence interval; P < 0.05).
DISCUSSION
This study demonstrates that immunity to fatal monocytotropic ehrlichiosis infection can be mediated by B cells and antibodies and can be generated in the absence of CD4 T-cell-mediated help. These findings were unexpected, given that T-cell immunity is often considered a hallmark of immunity to intracellular bacteria. Even in cases where antibodies have been shown to be effective, it has generally been concluded that they play auxiliary roles in protection in normal mice and typically require T-cell-mediated helper functions for their generation. Here we demonstrate that B cells and antibodies can play major roles in immunity to intracellular bacteria. To our knowledge, this is the first study to demonstrate a central role for T-cell-independent immunity in an intracellular bacterial infection. It is possible that T-cell-independent immunity has been overlooked in such infections, or that unusual characteristics of E. muris promote T-cell-independent immunity. We have shown that OMPs are at least one family of antigens that are targeted by the T-cell-independent response. Data from our and others’ studies indicate that OMPs are immunodominant antigens (24, 37, 52), and high-titer OMP antibodies were detected in the CD4- and MHC class II-deficient mice. It is not yet possible to conclude that OMP antibodies were responsible for protection, however, and other antigens may be involved, perhaps ehrlichiae glycoproteins (32). The high-affinity OMP antibody Ec56.5, generated following E. chaffeensis infection, transferred protection to naïve mice, indicating that OMP-19 antibodies have the capacity to provide protection in this infection model. The ehrlichia OMPs are candidate T-cell-independent type 2 antigens, as it is likely that OMPs can initiate T-cell-independent responses by cross-linking antibodies on the surface of the bacterium (42). Thus, other intracellular bacteria that express repetitive outer membrane proteins may also be candidates for T-cell-independent immunity (16, 27, 28, 48).
Although E. muris has been reported to establish persistent infection in immunocompetent hosts (20, 38), bacterial numbers decrease dramatically following peak infection, which occurs on day 9 postinfection. By day 120 postimmunization, E. muris infection was near the limits of detection in spleen, liver, and lungs (38). Moreover, in our studies we were unable to transfer E. muris infection to highly susceptible RAG-deficient mice using spleen cells (1 × 106) obtained from E. muris-infected mice 30 or 60 days postinfection, and treatment of mice with doxycycline at various times post-E. muris immunization did not affect immunity to IOE challenge (C. Bitsaktsis, R. Racine, K. C. MacNamara, and G. Winslow, unpublished data). Thus, although we have not completely resolved whether low-level persistent infection and/or inflammation contributes to long-term immunity in our model, current data suggest that this will unlikely be the case. Moreover, induction of inflammation in the peritoneum using L. monocytogenes prior to IOE challenge failed to generate protection, providing further support that immunity was a not a consequence of nonspecific innate inflammatory processes.
Our findings differ somewhat from those reported by Ismail et al., who demonstrated that polyclonal antibodies obtained from E. muris-immunized mice were insufficient for protection following fatal IOE challenge (17). One possible explanation for the discordance is that the IOE challenge doses, as well as the regimens of serum administration, differed between the two studies. Serum administration did not protect all challenged mice in our studies, perhaps because it is difficult to obtain high concentrations of the administered antibodies in the recipient mice following passive serum transfer. Another explanation is that both B cells and antibodies play important roles in immunity during intracellular ehrlichia infection.
Although we reported previously that B cells and/or antibodies are essential during low-dose IOE infection, we have also demonstrated that IFN-γ production by CD4 T cells is required for protection during primary low-dose IOE infection (4). Why, then, is immunity to low-dose IOE infection B cell and CD4 T cell dependent, whereas heterologous immunity to high-dose IOE challenge is CD4 T cell independent? The observation that CD8-deficient mice were protected by E. muris immunization suggests that CD8 T cells do not substitute for CD4 T cells in the latter's absence. Although B cells were necessary for low-dose IOE immunity, the antibody responses in low-dose IOE-infected mice were relatively poor (51). Therefore, we propose that in the presence of a high-titer antibody response, as had been detected following E. muris immunization (17, 51), CD4 T cells are not required for immunity to IOE challenge infection. However, in the absence of a strong humoral response, CD4 T cells are required for immunity after low-dose IOE infection. The requirement for B cells in the low-dose IOE-infected mice could have been due instead to a role for B cells in generating protective CD4 T-cell responses (22). Why low-dose IOE infection does not generate high-titer antibody responses is not yet known, but this observation may explain why low-dose IOE infection does not generate immunity to high-dose IOE challenge infection (4).
Ehrlichia infections can persist in low numbers in both naturally and experimentally infected animals in the presence of high-titer antibodies (12, 38, 52), indicating that antibodies are not always sufficient for sterile immunity. We have proposed that antibodies are effective when the ehrlichiae are exposed extracellularly (25), and so intracellular ehrlichiae are likely controlled by T cells in most ehrlichiae infections. IOE, perhaps due to differences in its intracellular life cycle, may represent a special case, where antibodies can be effective even in the absence of T cells. Nevertheless, even during IOE infection, CD4 T-cell-derived IFN-γ, as well as other cytokines, likely contributes to immunity. This has been difficult to assess experimentally in our model, however, as susceptibility to fatal IOE infection occurs within a narrow range of infectious doses, between 500 and 1,000 bacteria. OMP-19 antibody titers were significantly higher in wild-type relative to CD4- and MHC class II-deficient mice, and so CD4 T cells likely also provide a component of B-cell help to facilitate antibody production. Studies of immunity in animals infected via the natural route of transmission have not yet been performed, and so it is also possible that tick feeding may modify mechanisms of immunity, as has been observed during other tick-borne infection models (41, 43).
The observation that immunity to IOE challenge infection can occur in a CD4 T-cell-independent fashion was also unexpected. Although it is possible that the apparent T-cell-independent immunity was due to remaining small populations of CD4-like T cells in the CD4- and/or MHC class II-deficient mice, we consider this unlikely, given that both CD4 and IFN-γ depletion during IOE challenge infection failed to impair protective immunity. Moreover, CD4 T-cell-mediated B-cell helper functions were not observed in a study of influenza virus infection of CD4- and MHC class II-deficient mice (23). To our knowledge, ours is the first example where immunity to an intracellular bacterium could be generated in the absence of type I cytokines such as IFN-γ and TNF-α.
The class of antibody responsible for E. muris-induced immunity has also not yet been determined. T-cell-independent antibody responses are typically associated with IgM and IgG3 utilization (29), although isotype-switched antibodies are often associated with T-cell-independent immunity (34). However, since isotype-switched OMP-specific antibodies were observed in the CD4- and MHC class II-deficient mice, it is possible that protection was mediated by antibodies of several classes, or by particular isotypes, as was observed following transfer of monoclonal antibodies to SCID mice (24). Although B-cell isotype switching can occur in the absence of T-cell help (45, 46), this too was an unexpected finding after E. muris infection, especially considering that the class-switched antibody responses in the CD4 T-cell- and class II MHC-deficient mice, although generally lower than those observed in wild-type mice, were nonetheless substantial. We envision several possible explanations for the apparent T-cell-independent class switching. One possibility is that B-cell help for isotype switching is provided by NKT cells. It has been demonstrated that the ehrlichiae, including E. muris, express exogenous glycolipid CD1d antigens that are capable of priming NKT cells (3, 18, 31). NKT cell-mediated B-cell helper functions have been observed in other studies (11, 13), and CD1d expression by marginal zone B cells was shown to be essential during borrelia infection (2). OMP-19 antibody titers in E. muris-infected CD1d-deficient mice were similar to titers in wild-type mice (G. Winslow, unpublished data), although it is possible that CD4 T-cell-dependent B-cell help available in these mice masked CD1d-dependent helper activity.
A second explanation for the observation that class switching occurred in the absence of CD4 T cells is that dendritic cells or γδ T cells provided B-cell helper function (7, 49) or, alternatively, that other forms of help can be mediated in the absence of CD4 T cells. For example, recent findings indicate that triggering via Toll-like receptors (TLRs) can mediate B-cell isotype switching (15, 21). Although the ehrlichiae do not express lipopolysaccharide or peptidoglycan, we have observed a requirement for TLR2 during low-dose IOE infection (C. Bitsaktsis, K. C. MacNamara, and G. Winslow, unpublished data), suggesting a possible role for TLRs in B-cell isotype switching during ehrlichia infection.
Our studies also suggest that highly effective, long-lived B-cell responses can be generated in both wild-type and CD4 T-cell-deficient mice. This was evident from our findings that protection against IOE challenge was observed as long as 110 days after E. muris immunization. It is not yet clear if protection is associated with a particular memory B-cell population or if effector B cells are maintained as a consequence of a persistent low-level infection and/or inflammation following E. muris infection. Given that at least a component of the protection observed in our model was due to T-cell-independent antibodies, our data may support other observations that T-cell-independent antigens can generate immunological memory (1, 36).
E. muris infection generates highly effective heterologous immunity, but E. chaffeensis infection does not (4). This is despite the fact that both infections generate high-titer OMP antibody responses. Although we have proposed OMPs as candidates for protective T-cell-independent responses, it is possible that other antigens selectively expressed by E. muris and E. chaffeensis are essential for antibody-mediated protection. Alternatively, the quality (i.e., affinity, isotype utilization) of the humoral responses to the two pathogens may differ in important ways. The fact that CD4-deficient AIDS patients are in some cases susceptible to E. chaffeensis infections (39) suggests either that E. chaffeensis does not generate protective T-cell-independent immunity in humans or that other factors contribute to susceptibility in these individuals. Nevertheless, our study demonstrates that highly effective T-cell-independent humoral immunity can be generated following heterologous ehrlichia infection in mice, and this supports the findings from our previous studies that antibodies provide a major component of host defense during these intracellular bacterial infections.
Acknowledgments
We thank the Wadsworth Center Immunology Core Facility and Kathryn Hogle and Frances Hetherington for excellent technical assistance. We also thank David Woodland of the Trudeau Institute for helpful discussion and David Lawrence of the Wadsworth Center for assistance with the Listeria experiment.
This work was supported by U.S. Public Health Service grant R01 AI064678 to G.W.
We have no conflicting financial interests.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 2.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac, A., O. Lantz, M. E. Quimby, J. W. Yewdell, J. R. Bennick, and R. R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863-865. [DOI] [PubMed] [Google Scholar]
- 4.Bitsaktsis, C., J. Huntington, and G. M. Winslow. 2004. Production of interferon-γ by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 5.Bitsaktsis, C., and G. Winslow. 2006. Fatal recall responses mediated by CD8 T cells during intracellular bacteria infection. J. Immunol. 177:4644-4651. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends. Microbiol. 6:102-107. [DOI] [PubMed] [Google Scholar]
- 7.Colino, J., Y. Shen, and C. M. Snapper. 2002. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 195:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 9.Feng, H. M., and D. H. Walker. 2004. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect. Immun. 72:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, H. M., T. Whitworth, J. P. Olano, V. L. Popov, and D. H. Walker. 2004. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine 21(Suppl. 2):S48-S54. [DOI] [PubMed] [Google Scholar]
- 12.Ganta, R. R., M. J. Wilkerson, C. Cheng, A. M. Rokey, and S. K. Chapes. 2002. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 70:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen, D. S., M. A. Siomos, T. De Koning-Ward, L. Buckingham, B. S. Crabb, and L. Schofield. 2003. CD1d-restricted NKT cells contribute to malarial splenomegaly and enhance parasite-specific antibody responses. Eur. J. Immunol. 33:2588-2598. [DOI] [PubMed] [Google Scholar]
- 14.Harris, D. P., L. Haynes, P. C. Sayles, D. K. Duso, S. M. Eaton, N. M. Lepak, L. L. Johnson, S. L. Swain, and F. E. Lund. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1:475-482. [DOI] [PubMed] [Google Scholar]
- 15.He, B., X. Qiao, and A. Cerutti. 2004. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J. Immunol. 173:4479-4491. [DOI] [PubMed] [Google Scholar]
- 16.Huntley, J. F., P. G. Conley, K. E. Hagman, and M. V. Norgard. 2007. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol. 189:561-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786-1800. [DOI] [PubMed] [Google Scholar]
- 18.Jayawardena-Wolf, J., and A. Bendelac. 2001. CD1 and lipid antigens: intracellular pathways for antigen presentation. Curr. Opin. Immunol. 13:109-113. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara, M., C. Suto, Y. Rikihisa, S. Yamamoto, and Y. Tsuboi. 1993. Characterization of ehrlichial organisms isolated from a wild mouse. J. Clin. Microbiol. 31:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara, M., C. Suto, S. Shibata, M. Futohashi, and Y. Rikihisa. 1996. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol. Immunol. 40:575-581. [DOI] [PubMed] [Google Scholar]
- 21.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- 22.Lee, B. O., J. Moyron-Quiroz, J. Rangel-Moreno, K. L. Kusser, L. Hartson, F. Sprague, F. E. Lund, and T. D. Randall. 2003. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J. Immunol. 171:5707-5717. [DOI] [PubMed] [Google Scholar]
- 23.Lee, B. O., J. Rangel-Moreno, J. E. Moyron-Quiroz, L. Hartson, M. Makris, F. Sprague, F. E. Lund, and T. D. Randall. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175:5827-5838. [DOI] [PubMed] [Google Scholar]
- 24.Li, J. S., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 25.Li, J. S., and G. Winslow. 2003. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Inf. Immun. 71:4229-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, F. K. Chu, and G. Winslow. 2001. Outer membrane protein specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 27.Lin, Q., Y. Rikihisa, N. Ohashi, and N. Zhi. 2003. Mechanisms of variable p44 expression by Anaplasma phagocytophilum. Infect. Immun. 71:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohr, C. V., K. A. Brayton, V. Shkap, T. Molad, A. F. Barbet, W. C. Brown, and G. H. Palmer. 2002. Expression of Anaplasma marginale major surface protein 2 operon-associated proteins during mammalian and arthropod infection. Infect. Immun. 70:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, F., and J. F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195-201. [DOI] [PubMed] [Google Scholar]
- 30.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 31.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu, 3rd, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 32.McBride, J. W., X. J. Yu, and D. H. Walker. 2000. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and Ehrlichia canis. Infect. Immun. 68:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McSorley, S. J., and M. K. Jenkins. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect. Immun. 68:3344-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee, S., S. C. Lee, and A. Casadevall. 1995. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect. Immun. 63:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obukhanych, T. V., and M. C. Nussenzweig. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Erlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olano, J. P., G. Wen, H. M. Feng, J. W. McBride, and D. H. Walker. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165:997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paddock, C. D., S. M. Folk, G. M. Shore, L. J. Machado, M. M. Huycke, L. N. Slater, A. M. Liddell, R. S. Buller, G. A. Storch, T. P. Monson, D. Rimland, J. W. Sumner, J. Singleton, K. C. Bloch, Y. W. Tang, S. M. Standaert, and J. E. Childs. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586-1594. [DOI] [PubMed] [Google Scholar]
- 40.Pearce, E. L., D. J. Shedlock, and H. Shen. 2004. Functional characterization of MHC class II-restricted CD8+CD4− and CD8−CD4− T cell responses to infection in CD4−/− mice. J. Immunol. 173:2494-2499. [DOI] [PubMed] [Google Scholar]
- 41.Ramamoorthi, N., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, D. Fish, J. Anguita, M. V. Norgard, F. S. Kantor, J. F. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snapper, C. M., and J. Mond. 1996. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J. Immunol. 157:2229-2233. [PubMed] [Google Scholar]
- 43.Sukumaran, B., S. Narasimhan, J. F. Anderson, K. DePonte, N. Marcantonio, M. N. Krishnan, D. Fish, S. R. Telford, F. S. Kantor, and E. Fikrig. 2006. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J. Exp. Med. 203:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun, W., J. W. Ijdo, S. R. Telford III, E. Hodzic, Y. Zhang, S. W. Barthold, and E. Fikrig. 1997. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J. Clin. Investig. 100:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szomolanyi-Tsuda, E., and R. M. Welsh. 1996. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J. Exp. Med. 183:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szomolanyi-Tsuda, E., and R. W. Welsh. 1998. T-cell independent antiviral antibody responses. Curr. Opin. Immunol. 10:431-435. [DOI] [PubMed] [Google Scholar]
- 47.Tyznik, A. J., J. C. Sun, and M. J. Bevan. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J. Exp. Med. 199:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Vliet, A. H., F. Jongejan, M. van Kleef, and B. A. van der Zeijst. 1994. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect. Immun. 62:1451-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe, N., K. Ikuta, S. Fagarasan, S. Yazumi, T. Chiba, and T. Honjo. 2000. Migration and differentiation of autoreactive B-1 cells induced by activated gamma/delta T cells in antierythrocyte immunoglobulin transgenic mice. J. Exp. Med. 192:1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winslow, G. M., E. Yager, and J. Li. 2003. Mechanisms of humoral immunity during Ehrlichia chaffeensis infection. Ann. N. Y. Acad. Sci. 990:435-443. [DOI] [PubMed] [Google Scholar]
- 51.Yager, E., C. Bitsaktsis, B. Nandi, J. W. McBride, and G. Winslow. 2005. An essential role for humoral immunity during ehrlichia infection in immunocompetent mice. Infect. Immun. 73:8009-8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, J., H. Guo, G. Winslow, and X. Yu. 2004. Expression of members of the 28-kDa major outer membrane protein family of Ehrlichia chaffeensis during persistent infection. Infect. Immun. 72:4336-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]