Abstract
Nontypeable Haemophilus influenzae (NTHi) is a common gram-negative respiratory pathogen. To determine the role of the proinflammatory cytokine interleukin 18 (IL-18) during NTHi pneumonia, normal wild-type (WT) and IL-18 knockout (KO) mice were intranasally infected with NTHi. IL-18 KO mice displayed a delayed clearance of NTHi from the respiratory tract, resulting in >20-fold higher bacterial loads in their lungs at 24 h after infection, preceded by a strongly attenuated pulmonary innate immune response as determined by cytokine and chemokine induction and histopathology. These data identify IL-18 as part of an adequate innate immune response during NTHi pneumonia.
Nontypeable Haemophilus influenzae (NTHi) is a gram-negative bacterium that is a commensal organism in the human respiratory tract and an important cause of localized infections, such as middle ear infection, sinusitis, and conjunctivitis. Furthermore, NTHi is a common cause of exacerbations in asthma and chronic obstructive pulmonary disease and has been implicated as a frequent cause of community-acquired pneumonia (16).
The innate immune system is crucial for the initiation of an efficient immune response against invading pathogens (2). Toll-like receptors (TLRs), expressed by immune cells, can detect conserved motifs expressed by bacteria and other microorganisms and thereby activate the immune response (2). NTHi abundantly expresses lipooligosaccharides on its cell wall. Lipooligosaccharides and lipopolysaccharides contain a lipid A portion that is recognized by TLR4. Interaction of lipid A with TLR4 triggers the activation of two distinct signaling pathways, one that relies on the common TLR adaptor molecule MyD88 (myeloid differentiation primary-response protein 88) and one that proceeds via TRIF (TIR-domain-containing-adaptor-protein-inducing beta interferon) (2). Recently, we investigated the roles of TLR4, MyD88, and TRIF in the initiation of inflammation after infection with NTHi (17). We found that the MyD88-dependent but not the TRIF-dependent pathway of TLR4 signaling is important for the clearance of NTHi from the mouse respiratory tract (17). However, MyD88 not only is essential for TLR signaling but also mediates cell activation induced by interleukin 1 (IL-1) and IL-18 (1). Therefore, in the present study we sought to identify the role of the proinflammatory cytokine IL-18 during lung infection with NTHi.
(These data were presented as a poster on the 6th Joint Meeting of the International Cytokine Society, the International Society for Interferon and Cytokine Research, and the European Cytokine Society, Vienna, Austria, 2006 [abstract 02-12/P].)
MATERIALS AND METHODS
Animals.
Pathogen-free 8- to 10-week-old wild-type (WT) C57BL/6 mice were purchased from Harlan Sprague Dawley Inc. (Horst, The Netherlands). IL-18 knockout (KO) mice, backcrossed six times to a C57BL/6 background, were generated as described previously (15). Age- and sex-matched animals were used in all experiments. The Animal Care and Use of Committee of the University of Amsterdam approved all experiments.
Experimental infection.
H. influenzae strain 12 (kindly donated by S. J. Barenkamp, St. Louis, MO) is a clinical isolate that has been used by our and other laboratories in investigations on murine pneumonia (9, 17). For preparation of the inoculum, bacteria from frozen aliquots were streaked onto a chocolate agar plate and incubated overnight at 37°C in a 5% CO2 incubator. Next, bacteria obtained from the chocolate agar plate were grown for ±3 h to mid-logarithmic phase in brain heart infusion broth supplemented with 10 μg/ml hemin and 3.5 μg/ml NAD at 37°C (all reagents were from Difco, Detroit, MI). Bacteria were harvested by centrifugation at 1,500 × g for 15 min, washed, and resuspended in sterile isotonic saline at a concentration of 1 × 107 CFU/50 μl, as determined by plating serial 10-fold dilutions on chocolate agar plates. Pneumonia was induced by intranasal (i.n.) inoculation of 50 μl (107 CFU) bacterial suspension as described before (9, 17). For this procedure, mice were lightly anesthetized by inhalation of isoflurane (Upjohn, Ede, The Netherlands).
Determination of bacterial outgrowth.
At 6, 24, and 44 h and 10 days after infection, mice were anesthetized with Hypnorm (Janssen Pharmaceutica, Beerse, Belgium; active ingredients, fentanyl citrate and fluanisone) and midazolam (Roche, Mijdrecht, The Netherlands) and sacrificed by bleeding from the vena cava inferior. The lungs were harvested and homogenized at 4°C in 4 volumes of sterile saline using a tissue homogenizer (Biospec Products, Bartlesville, OK). CFU were enumerated from serial dilutions of lung homogenates and blood, which were plated on chocolate agar plates and incubated at 37°C at 5% CO2 for 20 h before colonies were counted.
Preparation of lung tissue for cytokine measurements.
For cytokine measurements, lung homogenates were diluted 1:2 in lysis buffer containing 300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 2% Triton X-100, AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride], EDTA-Na2, pepstatin, and leupeptin (each at 8 μg/ml; pH 7.4) and incubated on ice for 30 min. Homogenates were centrifuged at 1,500 × g at 4°C for 15 min, and supernatants were stored at −20°C until assays were performed.
Cytokines.
For detection of IL-1α, IL-1β, IL-6, keratinocyte-derived chemokine (KC), and MIP-1α, a Bio-Plex cytokine array was used (Bio-Rad Laboratories, Inc., Hercules, CA; detection limits, 7.8 pg/ml). tumor necrosis factor (TNF) was measured using a specific enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN; detection limit, 31.25 pg/ml).
Histology.
Lungs for histology were harvested after infection, fixed in 10% formalin, and embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin and eosin and analyzed by a pathologist who was blinded to groups. To score lung inflammation and damage, the entire lung surface was analyzed with respect to the following parameters (9, 17): interstitial inflammation, edema, endothelialitis, bronchitis, and pleuritis. Each parameter was graded on a scale of 0 to 4, as follows: 0, absent; 1, mild; 2, moderate; 3, severe; 4, very severe. The total lung inflammation score was the sum of the scores for each parameter, with the maximum being 20. Granulocyte staining was done as described previously (7).
Statistical analysis.
All data are expressed as means ± standard errors of the means unless otherwise indicated. When two groups were compared at multiple time points, a two-way analysis of variance (ANOVA) was used (GraphPad Prism version 4.00; GraphPad Software; San Diego, CA). If appropriate, ANOVAs were followed by a Bonferroni posttest. Cytokine data were analyzed by a Mann-Whitney U test. Statistical analyses of bacterial counts were performed after log transformation. P values of <0.05 were considered statistically significant.
RESULTS
IL-18 contributes to pulmonary clearance of NTHi in vivo.
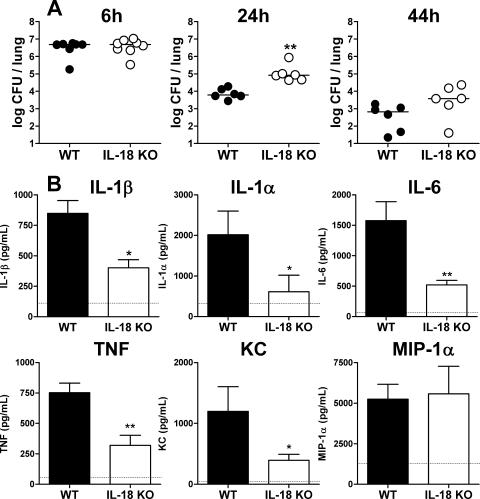
In order to gain insight into the antibacterial host defense in mice lacking IL-18, we infected WT and IL-18 KO mice with NTHi and sacrificed them after 6, 24, and 44 h as well as after 10 days. Bacterial counts were similar in the two mouse strains after 6 h of infection (Fig. 1A). Although both mouse strains were able to clear the infection during the following days, IL-18 KO mice had substantially higher bacterial loads at 24 h after infection. At this time point, bacterial counts were >20-fold higher in IL-18 KO mice compared to WT mice (P < 0.01). At 44 h after infection, IL-18 KO mice still tended to have increased bacterial loads in their lungs, but the difference from WT mice was not statistically significant. Ten days after infection, both WT and IL-18 KO mice had cleared NTHi from their lungs. Hence, these data suggest that IL-18 contributes to an early effective clearance of NTHi from the respiratory tract.
FIG. 1.
(A) Bacterial clearance. WT and IL-18 KO mice were infected i.n. with 1 × 107 CFU of NTHi. Mice were sacrificed at 6, 24, and 44 h postinfection, and bacterial loads were determined in lung homogenates. (B) Levels of IL-1β, IL-1α, IL-6, TNF, KC and MIP-1α measured in lung homogenates of WT and IL-18 KO mice obtained after 6 h of infection, when bacterial loads were equal. Dotted lines represent baseline lung levels in uninfected mice. Data are means ± standard errors of the means for seven or eight mice per group. CFU data were analyzed after log transformation using two-way ANOVA followed by a Bonferroni posttest, and cytokine/chemokine data were compared using a Mann-Whitney U test. * and **, P < 0.05 and P < 0.01 (KO versus WT) at the indicated time points.
IL-18 is important for early cytokine and chemokine production in the lung after NTHi infection in vivo.
The success of combating pulmonary infections strongly depends on the efficacy of the local inflammatory response elicited (6). In order to study the extent and the kinetics of the inflammatory response, we sacrificed WT and IL-18 KO mice at multiple time points after infection and measured the concentrations of proinflammatory cytokines (IL-1β, IL-1α, TNF, and IL-6) and chemokines (KC and MIP-1α) in lung homogenates (Fig. 1B). After 6 h of infection, when the bacterial loads were still similar in the two mouse strains, the pulmonary levels of all inflammatory mediators except for MIP-1α were strongly reduced in the IL-18 KO mice. Interestingly, from 24 h onward, there were no differences in pulmonary cytokine/chemokine levels between IL-18 KO and WT mice (data not shown).
IL-18 KO mice demonstrate delayed lung inflammation after NTHi infection in vivo.
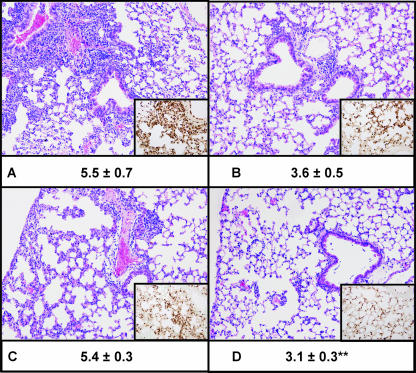
To obtain further insight into the involvement of IL-18 in the inflammatory response in the lung upon infection of the airways with NTHi, we semiquantitatively scored lung histology slides generated from WT and IL-18 KO mice at various time points after infection (Fig. 2). In line with their attenuated initial cytokine/chemokine response, IL-18 KO mice displayed reduced lung inflammation at 6 (P = 0.065) and 24 h postinfection compared with WT mice (Fig. 2). At later time points, there were no differences in lung inflammation between the two strains (data not shown). In agreement with this observation, staining of granulocytes in the lungs by using an antibody against the neutrophil marker Ly6-G revealed fewer neutrophils in the lungs of IL-18 KO mice than in WT mice at both 6 and 24 h postinfection. Of note, hardly any neutrophils can be found in the lungs of naïve mice (10, 18).
FIG. 2.
Representative lung histology of WT (A and C) and IL-18 KO (B and D) mice 6 (A and B) and 24 (C and D) h after i.n. infection with 1 × 107 CFU of NTHi. The lung sections (hematoxylin and eosin stained) are representative of seven or eight animals per group per time point. Histopathological scores (given as mean ± standard error under the corresponding image) were analyzed by Mann-Whitney U tests. Original magnification, ×10. (Insets) Ly6 staining for neutrophils (original magnification, ×20). **, P < 0.01 (KO versus WT).
DISCUSSION
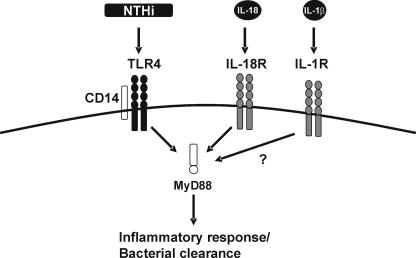
NTHi is a commensal gram-negative bacterium of the human nasopharynx which is able to infect the epithelium of the upper and lower respiratory tracts in patients with underlying lung diseases like chronic obstructive pulmonary disease, chronic bronchitis, and cystic fibrosis (16). NTHi is recognized as an important cause of infection of the lower airways and one of the most common causes of community-acquired bacterial pneumonia in adult populations (4). Therefore, it is important to obtain insight into the innate immune response to NTHi respiratory tract infection. We reported previously that the MyD88-dependent pathway of TLR4 signaling is important in the host defense against NTHi (17). In that study, we subjected MyD88 KO animals to the infection model used in the present study and found a reduced early inflammatory response and attenuated bacterial clearance (17). We argued that the observed effects could be due not only to reduced TLR4 signaling but also to reduced function of other MyD88-dependent receptors, such as the IL-18 receptor. Here, we demonstrate that although the observed effects were not as strong as those observed in TLR4 or MyD88 KO animals, lack of IL-18 signaling indeed results in reduced pulmonary inflammation and delayed clearance of NTHi (Fig. 3).
FIG. 3.
Schematic overview. We previously demonstrated that recognition of NTHi is mediated by CD14 and TLR4 (17). Subsequently, it was reported that the MyD88-dependent route of TLR4 signaling enhances the inflammatory response and bacterial clearance (17). Moreover, we demonstrated that host defense against NTHi was not dependent on TNF or the platelet-activating factor (3, 9). Here, we found that the MyD88-dependent IL-18 is important for the inflammatory response and antibacterial host defense. It will be of interest to determine the role of IL-1, whose signaling is also dependent on MyD88, in this experimental model.
Previous studies in our laboratory implicated IL-18 in the host response to bacterial pneumonia, with IL-18 KO mice displaying impaired innate immune responses during pneumococcal pneumonia (8) and murine melioidosis induced by Burkholderia pseudomallei (19) but an improved host defense against pulmonary infection with Pseudomonas aeruginosa (13). Together with our current findings of a delayed clearance of NTHi from the airways, these data suggest that the role of endogenous IL-18 depends on the respiratory pathogen used. In this respect, it is interesting that the prototypic proinflammatory cytokine TNF also plays variable roles in different models of bacterial pneumonia: whereas elimination of TNF strongly impairs host defense against Streptococcus pneumoniae pneumonia (12), this intervention improves host defense against Pseudomonas pneumonia (14) and does not affect the course of NTHi pneumonia (9). At present, the cause of these differential functions of proinflammatory cytokines in pneumonia caused by distinct microorganisms is not clear. Investigations to identify the specific roles of individual members of the cytokine network in experimental pneumonia caused by different clinically relevant respiratory pathogens are warranted. The current results strongly suggest that IL-18 is essential for a swift stimulation of the inflammatory response to NTHi airway infection and that this early response contributes to a rapid clearance of this bacterium from the lungs. Infection with NTHi in WT mice results in a relatively short but strong inflammatory response. However, the extent of this response is limited, and it is already diminished after 44 h. During more detrimental pneumonia that causes death of the animal, such as Streptococcus pneumoniae-induced pneumonia, excessive inflammation can lead to irreversible lung damage. In this model, in which the infection resolves naturally and no dissemination of bacteria occurs, a swift and strong inflammatory response that sterilizes the pulmonary compartment is preferable. Ultimately, we believe that IL-18 is an important mediator of local host responses during mild infections. From our point of view, the severity of the infection, the balance between good and bad inflammation, the underlying disease state of the host and the character of the infecting agent determine the role of an individual cytokine, such as IL-18.
Here, we found that the MyD88-dependent IL-18 is important for the inflammatory response and antibacterial host defense (Fig. 3). Although IL-18 was originally identified as a gamma interferon-inducing factor, it is now widely acknowledged as a mediator with broad proinflammatory properties. Pro-IL-18 is cleaved by caspase 1, an active enzyme of the NALP3 inflammasome, a protein complex that is considered essential for adequate induction of innate immune responses to invading pathogens (5, 11). Based on our results, the use of caspase 1 inhibitors would not benefit the host in this experimental model unless bioactive IL-1β plays an important role in host defense against NTHi (which is not known). Experiments with caspase 1 inhibitors would, however, dissect the roles of TLR signaling and the inflammasome. The inflammasome consists of cytosolic pattern recognition receptors that are important for intracellular immune surveillance. For example, members of the NALP subfamily of nucleotide-binding oligomerization domain-like receptors can recognize bacterial RNA, toxins, peptidoglycans, or endogenous danger signals (5, 11), and it is likely that in addition to TLRs, the inflammasome is an important part of the host defense against bacterial pathogens such as NTHi. Our findings with IL-18-deficient animals contribute to this notion. It will be of interest to study the roles of nucleotide-binding oligomerization domain-like receptors, caspase 1, and IL-1 in this model of pulmonary infection.
Acknowledgments
We thank Regina de Beer, Marieke ten Brink, and Joost Daalhuisen for expert technical assistance.
We received no external financial support.
We have no commercial or other association that might pose a conflict of interest.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Branger, J., C. W. Wieland, S. Florquin, N. A. Maris, J. M. Pater, P. Speelman, T. Shimizu, S. Ishii, and T. van der Poll. 2004. Platelet-activating factor receptor-deficient mice show an unaltered clearance of nontypeable Haemophilus influenzae from their respiratory tract. Shock 22:543-547. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, G. D., Jr. 1999. Commentary on the 1993 American Thoracic Society guidelines for the treatment of community-acquired pneumonia. Chest 115:14S-18S. [DOI] [PubMed] [Google Scholar]
- 5.Drenth, J. P., and J. W. van der Meer. 2006. The inflammasome—a linebacker of innate defense. N. Engl. J. Med. 355:730-732. [DOI] [PubMed] [Google Scholar]
- 6.Knapp, S., M. J. Schultz, and T. Poll. 2005. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock 24(Suppl. 1):12-18. [DOI] [PubMed] [Google Scholar]
- 7.Knapp, S., C. W. Wieland, C. van 't Veer, O. Takeuchi, S. Akira, S. Florquin, and T. van der Poll. 2004. Toll-like receptor 2 plays a role in the early immune response to murine pneumococcal pneumonia but does not contribute to antibacterial host defense. J. Immunol. 172:3132-3138. [DOI] [PubMed] [Google Scholar]
- 8.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 9.Maris, N. A., S. Florquin, C. van't Veer, A. F. de Vos, W. Buurman, H. M. Jansen, and T. van der Poll. 2006. Inhalation of beta 2 agonists impairs the clearance of nontypable Haemophilus influenzae from the murine respiratory tract. Respir. Res. 7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maris, N. A., K. F. van der Sluijs, S. Florquin, A. F. de Vos, J. M. Pater, H. M. Jansen, and T. van der Poll. 2004. Salmeterol, a β2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 286:L1122-L1128. [DOI] [PubMed] [Google Scholar]
- 11.Ogura, Y., F. S. Sutterwala, and R. A. Flavell. 2006. The inflammasome: first line of the immune response to cell stress. Cell 126:659-662. [DOI] [PubMed] [Google Scholar]
- 12.Rijneveld, A. W., S. Florquin, J. Branger, P. Speelman, S. J. Van Deventer, and T. van der Poll. 2001. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J. Immunol. 167:5240-5246. [DOI] [PubMed] [Google Scholar]
- 13.Schultz, M. J., S. Knapp, S. Florquin, J. Pater, K. Takeda, S. Akira, and T. van der Poll. 2003. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect. Immun. 71:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skerrett, S. J., T. R. Martin, E. Y. Chi, J. J. Peschon, K. M. Mohler, and C. B. Wilson. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276:L715-L727. [DOI] [PubMed] [Google Scholar]
- 15.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 16.Turk, D. C. 1984. The pathogenicity of Haemophilus influenzae. J. Med. Microbiol. 18:1-16. [DOI] [PubMed] [Google Scholar]
- 17.Wieland, C. W., S. Florquin, N. A. Maris, K. Hoebe, B. Beutler, K. Takeda, S. Akira, and T. van der Poll. 2005. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J. Immunol. 175:6042-6049. [DOI] [PubMed] [Google Scholar]
- 18.Wieland, C. W., S. Knapp, S. Florquin, A. F. de Vos, K. Takeda, S. Akira, D. T. Golenbock, A. Verbon, and T. van der Poll. 2004. Non-mannose-capped lipoarabinomannan induces lung inflammation via toll-like receptor 2. Am. J. Respir. Crit. Care Med. 170:1367-1374. [DOI] [PubMed] [Google Scholar]
- 19.Wiersinga, W. J., C. W. Wieland, G. J. van der Windt, A. de Boer, S. Florquin, A. Dondorp, N. P. Day, S. J. Peacock, and T. van der Poll. 2007. Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect. Immun. 75:3739-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]