Abstract
The proteins that comprise anthrax toxin self-assemble at the mammalian cell surface into a series of toxic complexes, each containing a heptameric form of protective antigen (PA) plus up to a total of three molecules of the enzymatic moieties of the toxin (lethal factor [LF] and edema factor [EF]). These complexes are trafficked to the endosome, where the PA heptamer forms a pore in the membrane under the influence of low pH, and bound LF and EF unfold and translocate through the pore to the cytosol. To explore the hypothesis that the PA pore can translocate multiple, cross-linked polypeptides simultaneously, we cross-linked LFN, the N-terminal domain of LF, via an introduced cysteine at its N or C terminus and characterized the products. Both dimers and trimers of LFN retained the ability to bind to PA pores and block ion conductance, but they were unable to translocate across the membrane, even at high voltages or with a transmembrane pH gradient. The multimers were remarkably potent inhibitors of toxin action in mammalian cells (20- to 50-fold more potent than monomeric LFN) and in a zebrafish model system. These findings show that the PA pore cannot translocate multimeric, cross-linked polypeptides and demonstrate a new approach to generating potent inhibitors of anthrax toxin.
Bacillus anthracis causes pathology in infected human or animal hosts in part through the concerted action of three proteins, collectively termed anthrax toxin. The toxin consists of two enzymatic moieties, termed lethal factor (LF) and edema factor (EF), and a transport protein, termed protective antigen (PA), that delivers both LF and EF to the cytosol. LF is a 90-kDa zinc-dependent metalloprotease that cleaves mitogen-activated protein kinase kinases (6, 20, 24), and EF is an 89-kDa calmodulin-dependent adenylate cyclase (12). The intracellular actions of these enzymes impair the functions of various cells and can lead to the death of infected hosts.
Delivery of LF and EF to the cytosol begins with binding of PA (83 kDa) to a receptor. Two receptors have been identified: ANTXR1 (for anthrax toxin receptor 1; also known as ATR/TEM8) and ANTXR2 (for anthrax toxin receptor 2; also known as CMG2) (3, 23). Receptor-bound PA is proteolytically processed by furin or a furin-like protease (19), resulting in the removal of a 20-kDa fragment (PA20) from the N terminus. The remaining, receptor-bound fragment (PA63, 63 kDa) spontaneously oligomerizes, forming a ring-shaped heptamer, called the prepore, which is capable of binding up to three molecules of LF and/or EF with high affinity (17, 18). The resulting toxic complexes are internalized, and the low pH within the endosome promotes a conformational change in the prepore moiety that allows it to insert into endosomal membranes and form a pore. The conformational transition of the prepore to the pore depends on the association of the 2β2-2β3 loops of the seven PA63 subunits to form a membrane-spanning, 14-stranded β barrel (1, 21, 22).
Recent evidence shows that the pore plays an active, chaperone-like role in the translocation of LF and EF across membranes (8, 15). Translocation requires unfolding of the enzymatic factors (26), and there is evidence that the pH gradient across the endosomal membrane drives the translocation process (7). The seven Phe-427 residues of PA63 form what we have termed the Phe clamp, a structure in the pore lumen that is believed to interact directly with the translocating polypeptide chain to promote its passage across the membrane (8).
The PA binding domain of LF, termed LFN, corresponds to the N-terminal 263 amino acids of LF. LFN binds to the prepore with high affinity (KD of ∼1 nM), and, when isolated as a discrete protein, this domain alone can be shown to translocate through the pore (28). Also, some fusion proteins containing LFN fused to heterologous proteins are able to undergo PA-dependent translocation into cells (16, 26) or across planar lipid bilayers (9, 10, 14, 28). The crystallographic structure of LF shows LFN to be a discrete helix-rich domain with a disordered N-terminal region that is essential for translocation. The disordered region, corresponding to the first ∼30 amino acids and densely populated with acidic and basic residues, is believed to enter the pore and initiate N-terminal-to-C-terminal translocation of LF and EF across the membrane.
Although much has been learned in recent years about the translocation of anthrax toxin, many questions remain unanswered. Here we have addressed the question of whether multimeric, cross-linked polypeptides can be translocated through the heptameric PA63 pore simultaneously. The finding that cross-linked forms of the N-terminal domain of LF do not translocate led to the discovery that such multimers are potent inhibitors of anthrax toxin, both in cultured mammalian cells and in a vertebrate animal model.
MATERIALS AND METHODS
Plasmid construction.
The expression construct pET15b-LFN has been described previously (9). The mutations A1C and R263C were introduced into LFN using QuikChange site-directed mutagenesis (Stratagene). The expression construct pET15b-LFN-(Gly4Ser)3-Cys was made by amplifying LFN by PCR using a primer that contained an in-frame addition of (Gly4Ser)3 repeats followed by a cysteine residue and stop codon. The DNA sequence encoding a fusion protein between LFN and the catalytic domain of diphtheria toxin (DTA) was introduced into pET15b (Novagen) using 5′ NcoI and 3′ BamHI sites. A small polylinker containing the restriction sites XhoI, SacI, and NotI and an additional base to maintain the reading frame was introduced between the two protein domains. All constructs were verified by DNA sequencing.
Preparation of proteins.
Recombinant LFN and PA were purified as previously described (9, 27). PA was activated and heptamerized as previously described (5). Recombinant LFN-DTA was expressed in BL21(DE3) Escherichia coli cells (Novagen). Growth and expression of LFN-DTA were carried out in a 5-liter Bioflo 110 fermentor (New Brunswick Scientific). Cells were grown at 37°C in ECPM1 medium (2) to and optical density at 600 nm of ∼5, sparged with a 60% air-O2 mixture, and induced at 30°C with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Bacteria were harvested and lysed with a French press and sonication in 20 mM Tris (pH 8.5)-1 M NaCl. The lysate was centrifuged at 30,000 × g, and the supernatant was applied to an Ni2+-chelated Sepharose column (Amersham Pharmacia). LFN-DTA was eluted from the column using a 35 mM to 385 mM imidazole gradient. The His6 tag was removed from LFN-DTA using bovine α-thrombin (Enzyme Research Laboratories) overnight at room temperature. Thrombin was removed from cleaved LFN-DTA using a Q-Sepharose anion-exchange column (Amersham Pharmacia).
Cross-linking.
N-terminal LFN dimers and trimers (using A1C LFN) and C-terminal LFN dimers (using R263C LFN) and trimers [using LFN-(Gly4Ser)3-Cys] were formed as follows. Protein was kept at 450 μM in 20 mM Tris (pH 8.5), 150 mM NaCl, and 500 μM tris-(2-carboxyethyl)phosphine. The pH of the solution was lowered to 7.5, and cross-linker (bismaleimidoethane for dimer formation and tris-[2-maleimidoethyl]amine for trimer formation [both from Pierce]) was added to protein at a 1:10 molar ratio. The reaction mixture was incubated at 4°C for 30 min. The addition of cross-linker to protein at a 1:10 molar ratio and incubation at 4°C for 30 min were repeated five times. Finally, the molar ratio of cross-linker to protein was raised to 1:1, and the reaction mixture was incubated overnight at 4°C. Dimeric and trimeric proteins were separated from monomeric protein using size exclusion chromatography over an S-200 column (Amersham Pharmacia).
Planar lipid bilayer translocation assay.
Translocation of LFN variants across black lipid membranes was measured as previously described (28). Briefly, a lipid bilayer of diphytanoylphosphatidylcholine was formed across a 0.2-mm-diameter hole of a Delrin cup. The membrane separated two compartments containing 10 mM MES (morpholineethanesulfonic acid), 10 mM KH2PO4, 10 mM sodium oxalate, 100 mM KCl, and 1 mM EDTA (pH 5.5). Once membranes were formed, heptameric PA was added to the cis compartment, and a steady-state current level was reached (at a transmembrane voltage of +20 mV). LFN was added to the cis compartment to a final concentration of 10 nM, and blockage of PA channels was measured by monitoring the decrease in current. The cis compartment was slowly perfused with a two-syringe system to remove unbound LFN (Hamilton). The fraction of channels blocked by LFN at any given time was calculated using the equation Fb = It/Io, where Fb is the fraction of channels blocked, It is the current at any given time, and Io is the steady-state current of open channels. Data were plotted either as the fraction of channels unblocked as a function of time or as the maximum percentage of channels blocked.
Translocation was initiated by increasing the voltage to +50 mV and was monitored by current increase across the membrane. The fraction of channels unblocked at any given time was calculated using the equation Fu = (It + Ic)/ (Io + Ic), where Fu is the fraction of unblocked channels, It is the current at any given time, Ic is the current of blocked channels after addition of LFN, and Io is the steady-state current of open channels. Data were plotted either as the fraction of channels unblocked as a function of time or as the maximum percentage of channels unblocked.
Cell culture.
CHO-K1 cells (ATCC CCL-61) were grown in Ham's F-12 medium supplemented with 10% fetal calf serum, 500 units/ml penicillin G, and 500 units/ml streptomycin sulfate. Cells were maintained as monolayers and grown in a humidified atmosphere at 5% CO2.
Inhibition of protein synthesis.
A protein synthesis assay was used to measure the ability of LFN variants to inhibit the translocation of LFN-DTA into cells (16). CHO-K1 cells (5 × 104 cells) were incubated with 100 pM PA83, 100 pM LFN-DTA, and various concentrations of either monomeric or multimeric LFN for 4 h at 37°C. Cells were then incubated with leucine-deficient medium supplemented with 1 μCi of [3H]leucine/ml for 1 h at 37°C and washed with phosphate-buffered saline. Cells were incubated twice for 10 min each with cold 5% trichloroacetic acid. The protein precipitate was solubilized in 100 μl 0.2 M KOH and neutralized with an equal volume of 0.1 M HCl. Tritiated protein was determined by scintillation counting. Fifty percent inhibitory concentrations (IC50s) were calculated by fitting data to the equation y = a + (b − a)/[1 + (x/c)d], where a is the y minimum, b is the y maximum, c is the IC50, and d is the slope.
Zebrafish studies.
All zebrafish protocols were approved by the Institutional Animal Care and Use Committee of Children's Hospital, Boston. Breeding fish were maintained at 28.5°C on a 14-h light/10-h dark cycle. Embryos were collected by natural spawning, and raised in 10% Hanks saline at 32°C. Wild-type fish of the AB strain or a transgenic zebrafish line in which endothelial cells were labeled with enhanced green fluorescent protein (EGFP) [Tg(fli1:EGFP)y1] (11) were used in these experiments. Microinjections were carried out as described by Weinstein et al. (25), with modifications described by Chan and Serluca (4). Combinations of PA, LFN-DTA, LFN, N-terminal LFN dimer, and N-terminal LFN trimer were prepared before injection. Phenol red (0.05%) was added under each condition for visibility during injection, and samples were loaded into microinjection needles. Volumes of 30 nl or less were delivered into the anterior cardinal veins of embryos anesthetized with tricaine (Sigma) at 48 h postfertilization, resulting in injection of ∼50 fmol PA, 11.5 fmol LFN-DTA, and various amounts of inhibitor (n = 20 per treatment condition). After injection, embryos were transferred into fresh medium for recovery. Subjects were scored for attenuation of toxin effects under a dissecting scope at 20 h postinjection (hpi) to allow visualization of EGFP-labeled vasculature. Statistical analysis was conducted with Student's t test using SigmaStat 3.0 software.
RESULTS
Synthesis and characterization of LFN multimers.
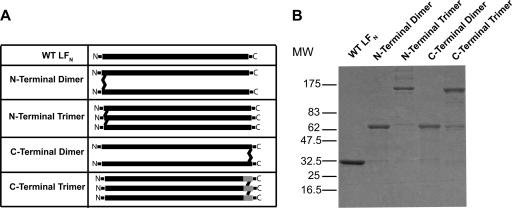
Multimeric forms of LFN were constructed by introducing Cys residues at either the N terminus (A1C) or the C terminus [R263C, LFN-(Gly4Ser)3-Cys] of LFN (Fig. 1A). Dimers cross-linked at the N or C terminus were prepared by incubating the A1C and R263C preparation, respectively, with the cysteine-reactive bifunctional cross-linker bismaleimidoethane. The corresponding trimers were generated by incubating the A1C and LFN-(Gly4Ser)3-Cys preparations with the trifunctional cross-linker tris-[2-maleimidoethyl]amine. Both cross-linking reagents react with thiols to form nonreducible thioether linkages. We were successful in obtaining C-terminal trimers with the LFN-(Gly4Ser)3-Cys preparation (Fig. 1B) but, for unknown reasons, not with R263C.
FIG. 1.
Cross-linking of LFN. (A) Schematic representation of constructs used in this study. The LFN used contained an extra three amino acids at the N terminus due to the thrombin recognition site. (B) Cross-linked proteins were purified as described in Materials and Methods, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and stained with Coomassie brilliant blue. WT, wild type; MW, molecular weight.
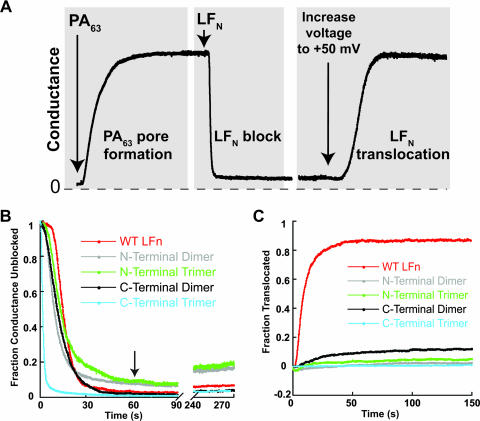
The multimers were purified by size exclusion chromatography and tested for the ability to undergo PA-dependent translocation across planar phospholipid bilayers, according to the protocol illustrated in Fig. 2A (28). PA63 prepore was added to one compartment (defined as the cis compartment) of a planar lipid bilayer apparatus containing 100 mM KCl buffered at pH 5.5, and pore formation was monitored at a transmembrane voltage of +20 mV. After the conductance plateaued, the cis compartment was perfused to remove residual PA, and LFN was added, causing rapid blockage of current as the protein bound to the pore. Following a second perfusion step to remove residual free LFN, we increased the voltage to +50 mV to induce translocation of bound LFN to the trans compartment. Translocation was manifested by the increase in conductance as LFN exited the pore into the trans compartment.
FIG. 2.
Cross-linked LFN cannot translocate across artificial membranes. (A) Schematic representation of translocation as assessed in planar lipid bilayers. Binding and translocation of LFN were monitored by conductance. Pores initially form in membranes after addition of heptameric PA63. Addition of LFN rapidly lowers conductance, mimicking closed channels. Translocation, which is initiated by increasing the voltage to +50 mV, promotes passage of LFN to the trans side of the membrane. (Modified from reference 13 with permission of the publisher.) (B) PA pores were formed in membranes at a transmembrane voltage of +20 mV and incubated in the presence of 10 nM monomeric or multimeric LFN constructs. Ion conductance was measured during LFN incubation and perfusion of the cis chamber. (C) PA channels blocked with monomeric or multimeric LFN were subjected to an increase in transmembrane voltage to +50 mV, and ion conductance was monitored until a steady state was reached. WT, wild type.
As shown in Fig. 2B, both dimeric and trimeric forms of LFN were effective conductance blockers, demonstrating that they retained the ability to bind to the LF/EF sites of the pore. Blockage by the monomer or the C-terminally cross-linked (C-linked) forms was close to 100%, whereas blockage by N-terminally cross-linked (N-linked) multimers was only ∼90%. Also, blockage by the multimers was more rapid than that by the monomer, with the C-linked trimer showing remarkably fast kinetics. Two other C-linked trimers, prepared from LFN-(Gly4Ser)2-Cys or LFN-(Gly4Ser)4-Cys, were found to show the same fast kinetics of blockage as trimer prepared from LFN-(Gly4Ser)3-Cys (data not shown). Finally, blockage by the N-linked forms was more readily lost during perfusion than that of the monomer or the C-linked forms (Fig. 2B, see 240- to 270-s time points).
Consistent with earlier findings, raising the voltage to +50 mV induced bound, monomeric LFN to translocate through the pore (28). Translocation was rapid, with a half-time of several seconds, and conductance was restored to ∼80% of the original value within a minute. However, none of the LFN multimers was able to translocate to a significant degree, regardless of the site of cross-linking (Fig. 2C). Furthermore, translocation did not occur even at a high transmembrane voltage (+100 mV) or when we applied a transmembrane proton gradient by raising the pH of the trans compartment to neutrality (data not shown).
Multimeric forms of LFN are potent inhibitors of toxin action on cells.
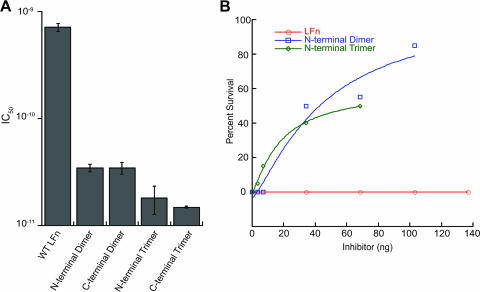
The fact that multimeric forms of LFN were able to bind to the PA pore but did not translocate suggested that they might function as potent inhibitors of toxin action. Such forms would be expected to bind to LF/EF binding sites on the PA heptamer more strongly than monomeric LFN and could potentially form dead-end translocation complexes with the pore, effectively inactivating it. We therefore measured the ability of the multimers to inhibit the PA-dependent action of LFN-DTA on CHO-K1 cells. LFN-DTA is translocated in an manner analogous to that of LF and EF (16), and, once in the cytosol, it catalyzes the ADP-ribosylation of EF-2, causing inhibition of protein synthesis. The inhibition of incorporation of radiolabeled leucine into newly synthesized proteins may therefore be used as an index of toxin action. As illustrated in Fig. 3A, the LFN multimers gave IC50s of between 15 and 35 pM, representing a 20- to 50-fold increase in inhibitory activity relative to monomeric LFN (IC50, ∼700 pM). The two trimers were more potent than the dimers.
FIG. 3.
Multimeric LFN is a potent inhibitor of toxin action in cells and in zebrafish embryos. (A) CHO-K1 cells were incubated in the presence of PA, LFN-DTA, and various concentrations of inhibitor. Toxin translocation was monitored through the incorporation of [3H]leucine into newly synthesized proteins, and IC50s were calculated as described in Materials and Methods. (B) Zebrafish embryos were microinjected with PA, LFN-DTA, and various concentrations of inhibitor (n = 20 for each data point). Protection as described in the text was scored as percent embryo survival. Inhibition experiments using trimer and dimer doses of 68.5 ng were repeated two times, in which significant increases in the survival of embryos treated with each multimer were observed (trimer, P < 0.001; dimer, P < 0.001). Statistics were completed using Student's t test. WT, wild type. Error bars indicate standard deviations.
Multimers inhibit toxin action in zebrafish.
To corroborate and extend the results from cell culture, we tested the ability of multimeric LFN to inhibit LFN-DTA-induced toxicity in a zebrafish embryonic vascular model. Coinjection of LFN-DTA and PA induced rapid cell death in zebrafish embryos as early as 2 hpi, with tissues becoming necrotic by 8 hpi. When injected singly, neither LFN-DTA nor PA produced observable effects at the latest time point, 20 hpi. Death (0% survival) was scored as severe necrosis and a complete lack of circulation at 20 hpi. Survival was defined as minimal to no tissue necrosis and retention of aortic blood flow. By these criteria, multimeric LFN protected against LFN-DTA effects, whereas monomeric LFN did not (Fig. 3B).
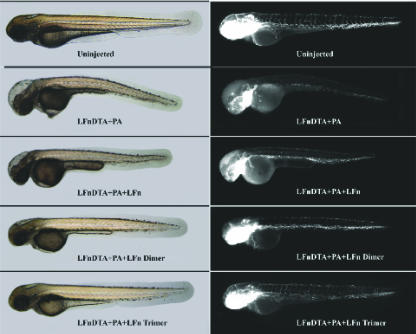
Since the vasculature is directly exposed to injected toxin, we examined toxin effects on the endothelium using a transgenic zebrafish line in which endothelial cells expressed EGFP under the control of a fli-1 promoter [Tg(fli1:EGFP)y1]. Various concentrations of monomeric or multimeric LFN were combined with LFN-DTA and PA and tested in comparison with controls. At 20 hpi, phenotypic changes were scored by overall morphology using phase-contrast microscopy, and changes in endothelial cells were scored under fluorescence (Fig. 4). A titratable increase in survival was observed in the presence of multimeric LFN but not monomeric LFN (Fig. 3B). Indeed, in the presence of multimeric LFN, the vasculature of toxin-injected embryos closely resembled that of uninjected control fish, especially in the presence of the LFN trimer (Fig. 4). Thus, multimerization of LFN increased its potency as a toxin inhibitor, not only in cell culture but also in a vertebrate animal model.
FIG. 4.
Multimeric LFN protects against LFN-DTA effects on zebrafish vasculature. Zebrafish embryos expressing EGFP under the control of a fli-1 promoter [Tg(fli1:EGFP)y1] (11) were injected with PA, LFN-DTA, and/or 68.5 ng of inhibitor as indicated (representative embryos are depicted; n = 20). Photographs of the same embryos were taken for each pair of phase-contrast (left panels) and fluorescence (right panels) micrographs. Loss of fluorescence in LFN-DTA- and PA-injected panels indicates endothelial cell death.
DISCUSSION
Our findings show that dimers and trimers of LFN formed by chemically cross-linking the N or C terminus do not translocate and, as a result, are potent blockers of toxin action. All of the multimers retained the ability to bind to the PA63 pore in planar phospholipid bilayers and to block ion conductance by the pore. Multimers cross-linked at the N terminus were less effective in blocking conductance (∼90% inhibition) than those cross-linked at the C terminus (∼98% inhibition). The C-linked multimers had free, unconstrained N termini that were apparently able to enter the pore and interact with the Phe clamp to form a tight seal against ion passage, as shown earlier with native LFN (8). Cross-linking of the N termini must prevent formation of this seal or make the seal less tight.
The effect of cross-linking of LFN on its affinity for the pore could not be easily predicted, because of potentially opposing effects of increased valence, which should strengthen the interaction, and structural perturbation, which would most likely weaken it. We did not measure affinity directly, but the demonstration that conductance blockage by the multimers was complete within a minute and was not rapidly relieved upon perfusion indicates that cross-linking did not cause a major decrease in affinity. Conductance blockage was relieved during perfusion to a greater degree by the N-linked multimers than by the C-linked forms, suggesting that altering the interaction of the N termini with the Phe clamp diminishes affinity. These data support the notion that interaction of the N terminus of LFN with the Phe clamp increases affinity of the protein for the pore.
Despite the fact that all of the multimers were able to bind to the pore and block its conductance, none of them translocated across the membrane under conditions known to promote translocation of monomers. There are at least two possible explanations for the inability to translocate. (i) Translocation might be restricted by the pore's physical dimensions, most likely its diameter at the narrowest point. There is clear evidence that the tertiary structure of a protein must be disrupted for translocation to occur (26). Consistent with this, the lumen of the transmembrane 14-strand β barrel of the pore has been estimated to be on the order of 15 Å, enabling it to accommodate a single α helix or an extended polypeptide but not a folded domain (7). Even narrower points in the translocation pathway may exist elsewhere in the pore, perhaps at the Phe clamp. Whether two or more extended polypeptides could be accommodated in the pore's β barrel or by the Phe clamp is difficult to predict. (ii) Translocation might be restricted by the pore's inability to pass certain chemical configurations generated by cross-linking. Little is known about the ability of the pore to pass nonpeptidic chemical structures, and it is possible that it might not accommodate chemical configurations generated by reaction of the cross-linking agents with the Cys residues introduced into LFN.
The mechanism by which translocation is blocked for the N-linked multimers may differ from that for the C-linked multimers, and indeed, more than one mechanism may contribute to the inactivity of either class of multimers. Our finding that N-linked forms of LFN apparently did not interact with the Phe clamp to form a tight seal against ion flow suggests that the force-generating proton gradient across the membrane may be dissipated locally. Because the N termini of the C-linked multimers are unencumbered, one can envision that the N terminus of an LFN domain would enter the pore and interact with the Phe clamp to form a tight seal to initiate threading of that domain through the pore. Complete translocation could be limited by inability of the Phe clamp or the transmembrane β barrel to accommodate the cross-linked intersection with the partner chain(s) or to translocate the partner chain in a C- to N-terminal direction, leading ultimately to a dead-end complex. Thus, our findings are consistent with the hypothesis that the PA63 pore can translocate no more than a single polypeptide at once, but why cross-linked forms of LFN do not translocate remains uncertain.
The ability of LFN multimers to bind to the pore strongly but not translocate through it makes them potent inhibitors of toxin action. The dimers were ∼20-fold more potent inhibitors than monomeric LFN in a mammalian cell culture-based assay, and the trimers were even more potent, with the C-linked trimer showing the highest potency (∼50-fold greater than that of the monomer). The higher activity of the trimers may be related to greater avidity for the pore, based on valence. Two of the multimers were tested in a zebrafish model and shown to be potent inhibitors of the chimeric enzymatic effector LFN-DTA when coinjected together with PA. Less than 100 ng of either a dimer or trimer raised zebrafish survival to >50% under conditions where 140 ng of monomeric LFN caused no increase in survival. Also, the multimers blocked morphological changes induced in the zebrafish by the chimeric toxin.
Our data demonstrate successful protection against toxin action in vivo by multimeric LFN. The need for an effective means to inhibit anthrax toxin action in unimmunized individuals infected with B. anthracis is well recognized, and our results offer a new way in which a potent anthrax toxin inhibitor can be generated by chemically modifying a component of the toxin. In addition, cross-linked forms of LFN might also elicit toxin-neutralizing antibodies against LF, giving these forms dual protective functions.
Acknowledgments
We thank Ruth-Anne Pimental for help in preparing the proteins used in this study.
R.J.C. holds equity in PharmAthene, Inc., and is a consultant for CombinatoRx, Inc.
This work was supported by funds from NIH grants AI-022021 (to R.J.C.) and AI056234 (to J.C.) and from the New England Regional Center of Excellence in Biodefense and Emerging Infectious Diseases (to R.J.C.). R.A.M. was supported by an NRSA Fellowship.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Benson, E. L., P. D. Huynh, A. Finkelstein, and R. J. Collier. 1998. Identification of residues lining the anthrax protective antigen channel. Biochemistry 37:3941-3948. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, A., and M. Payton. 1995. Production of recombinant proteins, p. 5.3.1-5.3.18. In J. E. Coligan, B. M. Dunn, H. L. Plough, D. W. Speicher, and P. T. Wingfield (ed.), Current protocols in protein science. John Wiley and Sons, Inc., New York, NY.
- 3.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 4.Chan, J., and F. C. Serluca. 2004. Chemical approaches to angiogenesis. Methods Cell Biol. 76:475-487. [PubMed] [Google Scholar]
- 5.Cunningham, K., D. B. Lacy, J. Mogridge, and R. J. Collier. 2002. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc. Natl. Acad. Sci. USA 99:7049-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, et al. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 7.Krantz, B. A., A. D. Trivedi, K. Cunningham, K. A. Christensen, and R. J. Collier. 2004. Acid-induced unfolding of the amino-terminal domains of the lethal and edema factors of anthrax toxin. J. Mol. Biol. 344:739-756. [DOI] [PubMed] [Google Scholar]
- 8.Krantz, B. A., R. A. Melnyk, S. Zhang, S. J. Juris, D. B. Lacy, et al. 2005. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 309:777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy, D. B., M. Mourez, A. Fouassier, and R. J. Collier. 2002. Mapping the anthrax protective antigen binding site on the lethal and edema factors. J. Biol. Chem. 277:3006-3010. [DOI] [PubMed] [Google Scholar]
- 10.Lacy, D. B., H. C. Lin, R. A. Melnyk, O. Schueler-Furman, L. Reither, et al. 2005. A model of anthrax toxin lethal factor bound to protective antigen. Proc. Natl. Acad. Sci. USA 102:16409-16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson, N. D., and B. M. Weinstein. 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248:307-318. [DOI] [PubMed] [Google Scholar]
- 12.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melnyk, R. A., and R. J. Collier. 2006. A loop network within the anthrax toxin pore positions the phenylalanine clamp in an active conformation. Proc. Natl. Acad. Sci. USA 103:9802-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melnyk, R. A., K. M. Hewitt, D. B. Lacy, H. C. Lin, C. R. Gessner, et al. 2006. Structural determinants for the binding of anthrax lethal factor to oligomeric protective antigen. J. Biol. Chem. 281:1630-1635. [DOI] [PubMed] [Google Scholar]
- 15.Miller, C. J., J. L. Elliott, and R. J. Collier. 1999. Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38:10432-10441. [DOI] [PubMed] [Google Scholar]
- 16.Milne, J. C., S. R. Blanke, P. C. Hanna, and R. J. Collier. 1995. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 15:661-666. [DOI] [PubMed] [Google Scholar]
- 17.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 269:20607-20612. [PubMed] [Google Scholar]
- 18.Mogridge, J., K. Cunningham, D. B. Lacy, M. Mourez, and R. J. Collier. 2002. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc. Natl. Acad. Sci. USA 99:7045-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molloy, S. S., P. A. Bresnahan, S. H. Leppla, K. R. Klimpel, and G. Thomas. 1992. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 267:16396-16402. [PubMed] [Google Scholar]
- 20.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 1999. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 462:199-204. [DOI] [PubMed] [Google Scholar]
- 21.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 22.Qa'dan, M., K. A. Christensen, L. Zhang, T. M. Roberts, and R. J. Collier. 2005. Membrane insertion by anthrax protective antigen in cultured cells. Mol. Cell Biol. 25:5492-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, et al. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706-711. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein, B. M., D. L. Stemple, W. Driever, and M. C. Fishman. 1995. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1:1143-1147. [DOI] [PubMed] [Google Scholar]
- 26.Wesche, J., J. L. Elliott, P. O. Falnes, S. Olsnes, and R. J. Collier. 1998. Characterization of membrane translocation by anthrax protective antigen. Biochemistry 37:15737-15746. [DOI] [PubMed] [Google Scholar]
- 27.Wigelsworth, D. J., B. A. Krantz, K. A. Christensen, D. B. Lacy, S. J. Juris, et al. 2004. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J. Biol. Chem. 279:23349-23356. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, S., E. Udho, Z. Wu, R. J. Collier, and A. Finkelstein. 2004. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys. J. 87:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]