Abstract
Lipopolysaccharides (LPS) are potent polyclonal B-lymphocyte activators. Recently, we have shown that LPS inhibits both spontaneous and drug-induced apoptosis in mature B lymphocytes, through cytosolic retention of Bax, a proapoptotic protein of the Bcl-2 family, by preventing its translocation to mitochondria. Research within the last few years has revealed that members of the NF-κB transcription factor regulate cell viability by activating genes involved in mitochondrion-dependent apoptosis. In this report, we examined the effect of sustained LPS stimulation on cytosolic and nuclear proteins of the IκB/NF-κB family to determine which NF-κB pathway, canonical (classical) or noncanonical (alternative), is activated by this agent in mature B cells. Immunoblotting analyses showed that LPS induced a time-dependent degradation of the NF-κB inhibitors IκBβ and IκBɛ (preferentially to isoform IκBα), via IκB kinase β. In addition, we observed that LPS triggered the processing of NF-κB p105 to p50 and that of NF-κB p100 to p52 in parallel with nuclear translocation of active p50 and p52, as NF-κBp50/RelA and NF-κBp52/RelB heterodimers, respectively. These results suggest that sustained stimulation with LPS can activate NF-κB through both classical and alternative pathways.
Bacterial lipopolysaccharide (LPS) or endotoxin, the major component of the outer membrane of gram-negative bacterial cell walls, activates various cell types of the innate and adaptive immune responses (27, 47). LPS has been known for a long time to be a potent activator of B lymphocytes (2, 28). Besides its effect on B-cell activation (33), LPS exhibits a prosurvival activity on both immature (31, 48) and mature (31, 44) B cells. In the immune system, apoptosis plays a fundamental role in the development and homeostasis of lymphocytes and is essential in the negative selection and deletion of autoreactive cells (3, 9, 23). Recently, we reported that LPS protects primary B lymphocytes from both spontaneous and drug-induced apoptosis by preventing mitochondrial translocation of Bax, a proapoptotic protein of the Bcl2 family (45). It has been reported that Bax translocation could be blocked by Bcl-xL, the prosurvival protein of the Bcl2 family (13) whose expression could be enhanced by nuclear factor κB (NF-κB) activation (8).
The pleiotropic NF-κB and Rel transcription factors play an essential role in the regulation of genes involved in various biological processes, such as inflammation, innate and adaptive immune responses, lymphocyte development, and apoptosis (1, 7, 20, 42). NF-κB comprises homo- or heterodimers of five Rel proteins, RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1,) and p52/p100 (NF-κB2). In most resting cell types, NF-κB is kept inactive in the cytoplasm through association with inhibitory members of the IκB family, including IκBα, IκBβ, and IκBɛ, as well as p105 and p100, the precursors of the p50 and p52 subunits, respectively. Phosphorylation of IκB, by a multiunit IκB kinase (IKK) complex consisting of IKKα and IKKβ, results in its proteasomal degradation, thereby permitting nuclear translocation of NF-κB and transcriptional activation of target genes (4, 20, 35).
It is increasingly being recognized that in immune regulation and lymphocyte activation, NF-κB activation can be achieved through two main pathways, termed canonical (classical) and noncanonical (alternative). The canonical pathway is known to be involved in the response of various cell types to pathogen-associated molecular patterns as well as proinflammatory cytokines, such as tumor necrosis factor alpha and interleukin-1. The second NF-κB noncanonical pathway is activated by a specific set of stimuli, including B-cell activating factor (BAFF), lymphotoxin β, and CD40L (6, 49). The canonical NF-κB signaling pathway activates the IKK complex, preferentially IKKβ, that controls the phosphorylation and degradation of most IκB family proteins, including p105, resulting in the processing of p105 to p50 (35, 38). In the noncanonical pathway, it is postulated that the processing of the NF-κB2 precursor p100 to the mature subunit p52 is regulated via an NF-κB-inducing kinase (NIK) pathway which induces generally IKKα-mediated phosphorylation of specific serine residues in the C-terminal domain of p100 and its processing to p52 (35, 38, 41, 51).
So far, LPS has been known to trigger the activation of NF-κB in various B cell lines and immature B cells, thus inducing their differentiation to mature B cells (17, 29, 39). Recently, NF-κB transcription factors have emerged as key regulators of survival for many cell types (1, 34), including B cells (14, 40). However, involvement of the noncanonical NF-κB signaling pathway in the activity of LPS has not yet been demonstrated in mature B lymphocytes. In the present report, we examined the effect of LPS on the IκB and NF-κB families of proteins to investigate the involvement of NF-κB in LPS-induced B-cell stimulation and, subsequently, to determine whether the activation of NF-κB would proceed through the canonical signaling pathway, the noncanonical signaling pathway, or both.
MATERIALS AND METHODS
Animals.
Female BDF1 [(C57BL/6 × DBA/2)F1] mice were purchased from Charles River Laboratories (L'Arbresle, France). Animals were maintained in a sterile microisolator cage system at our central animal care facility and used at 8 to 12 weeks of age.
Culture medium and reagents.
The culture medium used throughout was RPMI 1640 (Gibco, Invitrogen) supplemented with 25 mM HEPES, 2 mM l-glutamine, standard antibiotics, 50 μM 2-mercaptoethanol, and 8% heat-inactivated fetal calf serum (Gibco). Monoclonal anti-Thy-1.2 antibody and Low-Tox rabbit complement were obtained from Cedarlane (Ontario, Canada). LPS from Salmonella enterica serotype Minnesota Re 595 (Re mutant) and 6-diamidino-2-phenylindole (DAPI) were purchased from Sigma Chemicals (St. Louis, MO). A cocktail of protease inhibitors {including 500 μM AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], 150 nM aprotinin, 1 μM E-64, 1 μM leupeptin and 500 μM EDTA} was obtained from Calbiochem (San Diego, CA). Monoclonal anti-B220-PE antibodies were from Pharmingen (San Diego, CA). Antibodies against the following proteins of the IκB/NF-κB family were obtained from Santa Cruz Biotechnology: mouse monoclonal anti-NF-κB p65 RelA (F-6, sc-8008), anti-NF-κB p100/p52 (C-5, sc-7386), anti-c-Rel (B-6, sc-6955), anti-NIK (A-12, sc-8417), anti-Sam 68 (7-1, sc-1238), and rabbit polyclonal IκBα (C-21, sc371), IκBβ (S-20, sc946), IκBɛ (M-364, sc7155), anti-RelB (C-19, sc-226), anti-NF-κB p105/p50 (H-119, sc-7178), and anti-IKKα/β (H-470, sc-7607).
Cell preparation and culture.
Mature B lymphocytes were purified as previously described (25). Briefly, spleen cells were first treated with the anti-Thy-1.2 (CD90) monoclonal antibody at 4°C for 45 min, followed by incubation with Low-Tox rabbit complement at 37°C for 1 h, and then separated on a discontinuous Percoll gradient (GE Healthcare Bio-Sciences, Uppsala, Sweden). More than 98% of the cells recovered at the 55 to 70% interface were found to be B220 positive. B cells were cultured in medium alone or in the presence of LPS (5 μg/ml) for various times, as indicated in the figures below, in 24-well plates (Nunclon) in 5% CO2 at 37°C.
Quantitative apoptosis determination by DAPI staining.
After an overnight incubation, cells cultured at 5 × 105/ml were harvested, fixed in ice-cold 70% ethanol, and kept at −20°C until determination of the DNA content by flow cytometry. For apoptosis analysis, the cellular DNA content was measured after staining fixed cells with DAPI (2.5 μg/ml) at 37°C for 30 min. Cells were analyzed on a PARTEC CA II flow cytometer (Bailly, France) equipped with a 100-W mercury lamp (type HBO). Fluorescence at 455 nm was recorded as a function of the DNA content. Percentages of subdiploid cells, considered apoptotic cells, were determined from the sub-G1 events by using DPAC software on histograms generated from at least 104 cells.
Isolation of cytosolic and nuclear fractions.
Cells (2 × 106/sample) recovered after culture for various times, as indicated in the figures below, were washed twice in Hanks balanced salt solution. To obtain cytosolic and nuclear fractions, the cell pellets were first resuspended in hypotonic buffer A (10 mM HEPES-KOH, pH 7.9, 10 mM NaCl, 400 mM sucrose, 1 mM sodium orthovanadate, and a cocktail of protease inhibitors). After treatment for 20 min at 4°C, 1% Nonidet P-40 was added and vortexed for 10 s. Nuclei were pelleted by centrifugation at 15,000 × g for 1 min, and supernatants containing cytosolic fractions were collected. Pellets containing nuclei were washed once with buffer A and then resuspended in hypertonic buffer B (10 mM HEPES-KOH, pH 7.9, 400 mM NaCl, 25% glycerol, 1% Nonidet P-40, and a protease inhibitor mixture) for 30 min on ice. Supernatants containing nuclear proteins were collected by centrifugation at 15,000 × g for 10 min.
Immunoblots and immunoprecipitations.
Either cytosolic or nuclear fractions were first separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Prestained standards (BMA, Rockland, ME) were used to determine the molecular weights. The proteins were then electroblotted onto Immobilon polyvinylidene fluoride membranes (Pharmacia Biotech) and were detected following incubation for 1 h at room temperature with either monoclonal or polyclonal antibodies as indicated in the figure legends. The blots were incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Jackson Laboratory), or anti-mouse antibodies (Calbiochem, San Diego, CA) and then developed with an enhanced chemiluminescence (ECLplus) procedure as specified by the manufacturer (Bio-Rad). For comparison, Western blots contained equivalent amounts of extracts from 2 × 106 cells/sample. For immunoprecipitation experiments, samples were first incubated with specific antibodies, as indicated in the figure legends, on ice for 4 h, prior to the addition of protein A/G-agarose beads for an additional 4 h. The beads were washed three times and heat denatured in sample buffer to separate the protein from the beads. Immunoprecipitated proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then immunoblot analysis was performed as described above.
RESULTS
Differential effects of LPS on IκB degradation.
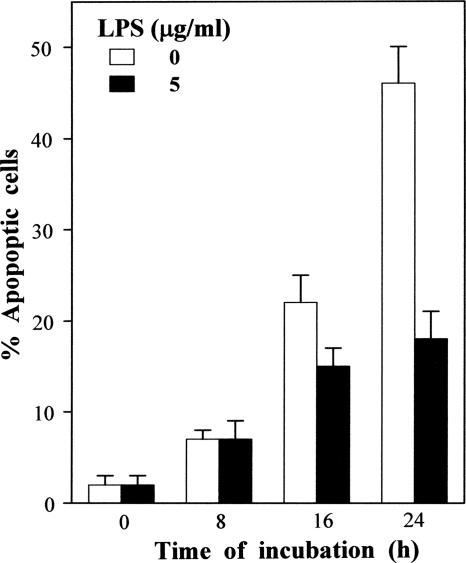
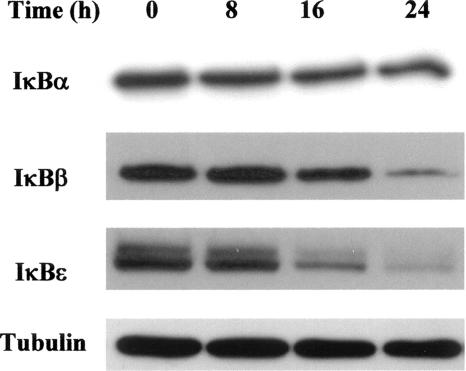
The IκB proteins are well recognized to have distinct roles in the NF-κB family of transcription factors, depending on the nature of the stimuli, the kinetics of the response, and the cellular lineage. Indeed, previous results have clearly shown temporal oscillations in the NF-κB/IκB protein family in early cell responses (up to 6 h) to various stimuli (19). We focused our study on long-term (8-, 16-, or 24-h) LPS stimulation. For the longest time period, as shown in Fig. 1, LPS (5 μg/ml) markedly reduced the spontaneous apoptosis of B cells (from 46% to 18%). To evaluate their respective roles in the regulation of mature B cells, we analyzed the time course of LPS-induced degradation of IκBα, IκBβ, and IκBɛ proteins. Results presented in Fig. 2 show that LPS failed to induce IκBα degradation but that it triggered both IκBβ and IκBɛ protein degradation. In addition, degradation of the IκBβ and IκBɛ proteins occurred earlier and was more pronounced than that of IκBα.
FIG. 1.
Kinetics of the LPS effect on spontaneous apoptosis. B cells were incubated, for the indicated times, in medium alone or with 5 μg/ml of S. enterica LPS. Apoptosis was determined using flow cytometry for 10,000 cells after staining them with the DNA probe DAPI. The percentages of apoptotic cells were determined based on sub-G1 DNA content. These data are representative of at least three individual experiments. Data are expressed as the means ± standard deviations of triplicate experiments.
FIG. 2.
LPS differentially affects levels of cytosolic IκB proteins. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic lysates were prepared and subjected to immunoblot analysis with anti-IκBα, anti-IκBβ, and anti-IκBɛ antibodies. Tubulin levels are shown as loading controls. These blots are representative of at least three individual experiments.
Influence of LPS on cytoplasmic IKKα, IKKβ, and NIK protein levels.
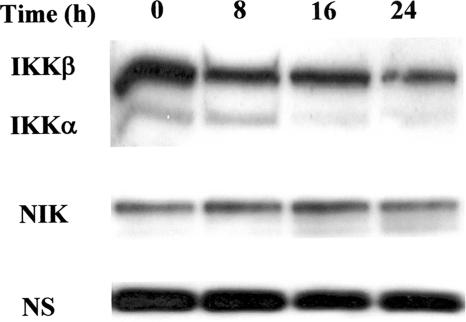
The IKK complex, including the two kinase subunits IKKα and IKKβ, are important components in signaling pathways of upstream NF-κB activation. The responsiveness of IKK to diverse stimuli can regulate the functional pleiotropism of NF-κB, and stimulus-specific temporal control of IKK activity can result in distinct NF-κB signaling profiles (21, 22, 50). We therefore examined the influence of LPS on IKKα/β levels in parallel with its effect on NIK that has been shown to be involved in the alternative NF-κB pathway (51). As shown in Fig. 3, different levels of IKKα, IKKβ, and NIK proteins were observed in freshly prepared B cells. It must be stressed that the constitutive levels of IKKα were very low compared to those of IKKβ. The former vanished almost completely within a 16-h stimulation with LPS. A slight decrease in IKKβ protein levels also became apparent at 24 h. In contrast, the presence of LPS did not significantly change NIK protein levels.
FIG. 3.
Western blot analysis showing IKKα/β and NIK protein levels. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic lysates were prepared and subjected to immunoblot analysis with anti-IΚΚα/β or anti-NIK antibodies. NS, nonspecific. These blots are representative of at least three individual experiments.
LPS activates the canonical NF-κB signaling pathway.
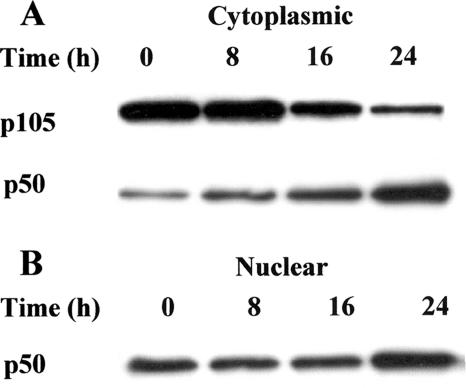
To investigate whether LPS triggers the classical NF-κB signaling pathway, the ability of LPS to stimulate the processing of p105 to p50, and subsequently to trigger p50 nuclear translocation, was determined. By immunoblotting analysis, p105 and p50 protein levels were determined in cytoplasmic and nuclear fractions from B cells cultured with LPS for the times indicated in Fig. 4. Freshly prepared B cells expressed relatively high levels of p105, compared to p50, in the cytoplasmic fraction (Fig. 4A). However, the presence of LPS induced the processing of p105 to active p50, which progressively increased up to 24 h. Active p50 protein was detected in nuclear fractions of mature B cells (Fig. 4B). The presence of LPS did not change p50 levels through 16 h but resulted in a marked increase at 24 h, which is coincidental with p105 processing and with nuclear translocation of active p50 from the cytoplasm.
FIG. 4.
LPS induces the processing of NF-κB p105 to p50 and nuclear p50 translocation. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic (A) and nuclear (B) fractions were prepared and subjected to immunoblot analysis with anti-NF-κB p105/p50 antibodies. Data are representative of at least three individual experiments.
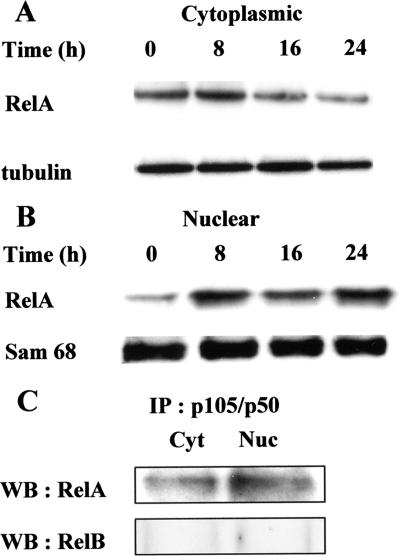
Concerning the current model of canonical NF-κB signaling pathway, active p50 proteins generally translocate to the nucleus as NF-κΒ p50/RelA heterodimers. RelA protein levels were thus analyzed by immunoblot analysis, and results in Fig. 5 show that RelA protein, at the onset of cultures, was found in relatively high levels in cytoplasmic fractions (Fig. 5A), compared to very low levels in nuclear fractions (Fig. 5B). LPS triggered nuclear translocation of RelA, which was detectable at 8 h and was maintained up to 24 h. The levels of nuclear protein Sam 68, used as a loading control for nuclear fractions, were identical at all time periods tested. To determine the ability of RelA to physically interact with p50, the latter protein was immunoprecipitated from cytoplasmic and nuclear fractions recovered after 24 h of stimulation with LPS, and immunoprecipitates were analyzed by Western blotting using anti-RelA antibodies. As shown in Fig. 5C, p50/RelA complexes were observed in both cytoplasmic and nuclear fractions, whereas no p50/RelB complexes were detected by Western blotting performed with anti-RelB antibodies. Taken together, these results suggest that LPS activates the classical NF-κB signaling pathway.
FIG. 5.
LPS induces nuclear translocation of RelA as p50/RelA dimers. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic (A) and nuclear (B) fractions were prepared and subjected to immunoblot analysis with anti-RelA antibodies. Tubulin and Sam 68 levels are shown as loading controls for these fractions, respectively. (C) Cytoplasmic (Cyt) and nuclear (Nuc) fractions, recovered at 24 h, were first immunoprecipitated (IP) with anti-p50 and then subjected to immunoblot (Western blot [WB]) analysis with anti-RelA antibodies. Anti-RelB antibodies were used as negative controls. Data are representative of at least three individual experiments.
LPS activates the noncanonical NF-κB signaling pathway.
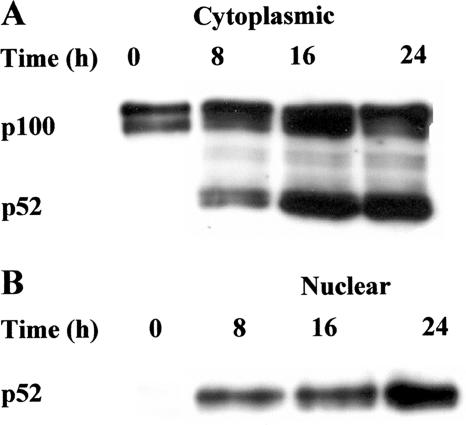
The ability of LPS to stimulate p100 processing, and thus to induce nuclear translocation of p52, was analyzed to determine whether LPS triggers the noncanonical NF-κB signaling pathway. Total p100 and p52 protein levels were determined by Western blotting of cytoplasmic and nuclear fractions from splenic B cells cultured with LPS for the times in Fig. 6. Freshly prepared B cells, in the cytoplasmic fraction, expressed relatively high levels of p100, whereas p52 was almost undetectable (Fig. 6A). However, in the presence of LPS, processing of p100 to p52 was triggered, and very high p52 protein levels were detected by 8 h and 16 h and then slightly declined at 24 h. A marked nuclear translocation of active p52 was observed following stimulation with LPS by 8 h, and significant p52 levels were maintained up to 24 h, whereas nuclear p52 was apparently not detectable in unstimulated B cells (Fig. 6B).
FIG. 6.
LPS induces the processing of NF-κB p100 to p52 and nuclear p52 translocation. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic (A) and nuclear (B) fractions were prepared and subjected to immunoblot analysis with anti-NF-κB p100/p52 antibodies. Data are representative of at least three individual experiments.
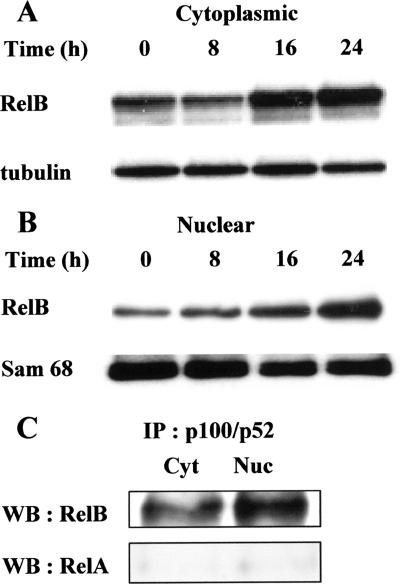
It has been demonstrated that p100 is associated in the cytosol with RelB (43) and that processing of p100 to p52 results in the activation of complexes which, in most circumstances, consist of p52/RelB heterodimers (6). Therefore, RelB protein levels were analyzed in cytoplasmic and nuclear fractions for different time periods as indicated in Fig. 7. Again, tubulin and Sam 68 were used as loading controls for cytoplasmic and nuclear fractions, respectively. Constitutive RelB was observed in the cytosol (Fig. 7A) and the nucleus. Upon stimulation with LPS, both cytoplasmic and nuclear RelB levels were markedly enhanced by 16 h and maintained at 24 h. As revealed by Western blotting analysis using anti-RelA and anti-RelB antibodies, immunoprecipitates of nuclear and cytoplasmic extracts with anti-p52 antibodies showed the presence of p52/RelB dimers in the cytoplasm and the nucleus, whereas p52/RelA complexes were not observed (Fig. 7C). These results thus suggest that LPS activates the alternative NF-κB signaling pathway.
FIG. 7.
LPS induces nuclear translocation of RelB as p52/RelB dimers. B cells (2 × 106) were cultured for various time periods with S. enterica LPS (5 μg/ml). At the end of the incubations, cytoplasmic (A) and nuclear (B) fractions were prepared and subjected to immunoblot analysis with anti-RelB antibodies. Tubulin and Sam 68 levels are shown as loading controls for these fractions, respectively. (C) Cytoplasmic (Cyt) and nuclear (Nuc) fractions, recovered at 24 h, were first immunoprecipitated (IP) with anti-p52 and then subjected to immunoblot (Western blot [WB]) analysis with anti-RelB antibodies. Anti-RelA antibodies were used as negative controls. Data are representative of at least three individual experiments.
DISCUSSION
In this study, we have investigated the effect of LPS stimulation on IκB/NF-κB proteins in murine primary B lymphocytes. The results indicate that LPS could activate both the canonical (classical) and noncanonical (alternative) NF-κB signaling pathways in these cells, concurrently with a decrease in spontaneous apoptosis.
NF-κB activation has been shown to occur in many cell types in response to various stimuli. However, recent reports demonstrate that cell-signaling pathways with NF-κB function are obviously much more complex than was documented in early studies. Particularly, the outcome of NF-κB activation depends on the nature, the kinetics, and the cellular context of its induction (20, 34, 36). Previous studies on the role of NF-κB in mediating LPS activity on B-cell proliferation have been extensively performed with B cell lines and have focused mostly on the canonical p105/p50 NF-κB1 signaling pathway (5, 24, 29, 37).
In the present study, high levels of IκBα, IκBβ, and IκBɛ proteins were found in unstimulated B cells. A sustained B-cell stimulation with LPS induced degradation of both IκBβ and IκBɛ proteins, preferentially to IκBα. IκBɛ levels decreased more rapidly and more significantly than IκBβ. Our data are consistent with previous results showing that IκBɛ levels are reduced much more dramatically than IκBα levels after B-cell stimulation with LPS for 24 h. However, it must be stressed that, in interleukin-4-activated B cells, IκBɛ does not degrade as rapidly as IκBα after LPS stimulation, thus suggesting that the stimulation of degradation of the different IκB proteins not only depends on the stimulus but also exhibits cell activation stage specificity (11). It appears that the canonical and noncanonical NF-κB pathways can be distinguished by their dependence on kinases that regulate the stimulus degradation of IκB proteins IKKβ and IKKα, respectively (20, 21). Here, high basal levels of IKKβ were detected and maintained throughout the experiment following LPS stimulation, whereas IKKα present at lower levels than IKKβ declined up to 24 h. Previous studies have shown that IKKβ-deficient B cells are impaired in mitogenic responses to LPS, anti-CD40, and anti-immunoglobulin M, thus suggesting a key role for IKKβ in B-cell survival and proliferation (26). It has been suggested that IKKα and NIK, the upstream kinase required for activation of the alternative pathway, act upstream of NF-κB2 and RelB. We observed significant levels of NIK that were maintained up to 24 h after stimulation with LPS. In parallel, we observed the processing of NF-κB1 p105 to p50 and that of NF-κB2 p100 to p52 in the cytoplasm, in parallel with nuclear translocation of active p50 and p52 proteins as p50/RelA and p52/RelB heterodimers, respectively. Conversely, p50/RelB and p52/RelA complexes were not detected in our study. These results thus suggest that, in addition to the classical pathway, LPS can trigger NF-κB activation through the alternative pathway. These findings are consistent with previous studies showing for the first time, either in the mouse immature B cell line 70Z/3 (30) or in both the human B cell line IM-9 and human peripheral blood B cells (32), that persistent stimulation with LPS triggers the activation of this noncanonical NF-κB pathway as typically observed during stimulation with lymphotoxin β. Furthermore, Ohmae et al. (32) have demonstrated that alternative-pathway activation by Helicobacter pylori was associated with attenuated apoptosis in B lymphocytes. Interestingly, in a figure representing pathways leading to the activation of NF-κB, Perkins and Gilmore have very recently included LPS in the group of inducers of the noncanonical pathway, which contains CD40 and lymphotoxin β (36). In quiescent mature B cells, following activation with B-lymphocyte stimulator (BlyS or BAFF), the two NF-κB pathways have been shown to function in concert to mediate BlyS survival signals and concomitant activation of Bcl-xL (18). In agreement, we have previously shown the capability of LPS to increase the levels of this antiapoptotic protein (45), thus suggesting that LPS could mediate its survival signals through coordinated activation of both NF-κB pathways and downstream Bcl-xL synthesis. Similarly, other studies have revealed that CD40-mediated responses in mature B cells require dual activation of both NF-κB pathways. However, it was shown in the same study that BAFF and LPS activate the alternative and the classical NF-κB pathways, respectively (52). The unknown source of LPS used by these authors could explain this discrepancy between the results because of its requirement for a signaling receptor different from Toll-like receptor 4 that recognizes LPS from Salmonella used in our study (46). Indeed, in bone marrow granulocytes, it has been shown that Toll-like receptor 2 can interact with many lipid A structures of different LPSs that could lead to either agonistic or antagonistic effects (15). Such dual effects were observed in mature B cells for the TNF receptor superfamily signaling protein TRAF2 as a multifunctional regulator of NF-κB that mediates activation of the canonical pathway but acts as a negative regulator of the noncanonical pathway (16).
The classical and alternative pathways to NF-κB activation have been suggested to have distinct regulatory functions, one mostly involved in innate immunity and the other in adaptive immunity (6). Nevertheless, exacerbated activation of the alternative pathway in B lymphocytes is potentially associated with a wide range of disorders, such as lupus-like disease, ulcerative colitis, and B-cell lymphomas (10, 12, 32). Our findings show the ability of LPS to activate the two NF-κB signaling pathways and provide new data, in the context of primary B cells, concerning the involvement of an alternative p52-dependent NF-κB pathway in long-term LPS stimulation that is associated with decreased apoptosis. It will be important to determine whether the persistent stimulation by LPS could trigger the activation of a distinct p52-dependent target gene repertoire that could have a role in an adaptive immune response or B-cell lymphomas.
Acknowledgments
We thank Nicole Esquirol for her excellent technical assistance.
This work was supported by the Centre National de la Recherche Scientifique (CNRS) and by the Université Paris-Sud (UPS).
Editor: A. Camilli
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Aggarwal, B. B. 2000. Apoptosis and nuclear factor-kappa B: a tale of association and dissociation. Biochem. Pharmacol. 60:1033-1039. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, J., G. Moller, and O. Sjoberg. 1972. Selective induction of DNA synthesis in T and B lymphocytes. Cell. Immunol. 4:381-393. [DOI] [PubMed] [Google Scholar]
- 3.Baixeras, E., L. Bosca, C. Stauber, A. Gonzalez, A. C. Carrera, J. A. Gonzalo, and C. Martinez. 1994. From apoptosis to autoimmunity: insights from the signaling pathways leading to proliferation or to programmed cell death. Immunol. Rev. 142:53-91. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 5.Bendall, H. H., D. C. Scherer, C. R. Edson, D. W. Ballard, and E. M. Oltz. 1997. Transcription factor NF-kappaB regulates inducible Oct-2 gene expression in precursor B lymphocytes. J. Biol. Chem. 272:28826-28828. [DOI] [PubMed] [Google Scholar]
- 6.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 7.Caamaño, J., and C. A. Hunter. 2002. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15:414-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C., L. C. Edelstein, and C. Gelinas. 2000. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol. Cell. Biol. 20:2687-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, J. J. 1999. Apoptosis: mechanisms of life and death in the immune system. J. Allergy Clin. Immunol. 103:548-554. [DOI] [PubMed] [Google Scholar]
- 10.Dejardin, E. 2006. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 72:1161-1179. [DOI] [PubMed] [Google Scholar]
- 11.Doerre, S., K. P. Mesires, K. M. Daley, T. McCarty, S. Knoetig, and R. B. Corley. 2005. Reductions in I kappa B epsilon and changes in NF-kappa B activity during B lymphocyte differentiation. J. Immunol. 174:983-991. [DOI] [PubMed] [Google Scholar]
- 12.Enzler, T., G. Bonizzi, G. J. Silverman, D. C. Otero, G. F. Widhopf, A. Anzelon-Mills, R. C. Rickert, and M. Karin. 2006. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity 25:403-415. [DOI] [PubMed] [Google Scholar]
- 13.Ganju, N., and A. Eastman. 2002. Bcl-X-L and calyculin A prevent translocation of Bax to mitochondria during apoptosis. Biochem. Biophys. Res. Commun. 291:1258-1264. [DOI] [PubMed] [Google Scholar]
- 14.Gerondakis, S., and A. Strasser. 2003. The role of Rel/NF-kappaB transcription factors in B lymphocyte survival. Semin. Immunol. 15:159-166. [DOI] [PubMed] [Google Scholar]
- 15.Girard, R., T. Pedron, S. Uematsu, V. Balloy, M. Chignard, S. Akira, and R. Chaby. 2003. Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J. Cell Sci. 116:293-302. [DOI] [PubMed] [Google Scholar]
- 16.Grech, A. P., M. Amesbury, T. Chan, S. Gardam, A. Basten, and R. Brink. 2004. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity 21:629-642. [DOI] [PubMed] [Google Scholar]
- 17.Gugasyan, R., R. Grumont, M. Grossmann, Y. Nakamura, T. Pohl, D. Nesic, and S. Gerondakis. 2000. Rel/NF-kappaB transcription factors: key mediators of B-cell activation. Immunol. Rev. 176:134-140. [DOI] [PubMed] [Google Scholar]
- 18.Hatada, E. N., R. K. Do, A. Orlofsky, H. C. Liou, M. Prystowsky, I. C. MacLennan, J. Caamano, and S. Chen-Kiang. 2003. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J. Immunol. 171:761-768. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, A., and D. Baltimore. 2006. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 210:171-186. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, A., A. Levchenko, M. L. Scott, and D. Baltimore. 2002. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298:1241-1245. [DOI] [PubMed] [Google Scholar]
- 21.Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 10:129-133. [DOI] [PubMed] [Google Scholar]
- 22.Karin, M. 1999. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18:6867-6874. [DOI] [PubMed] [Google Scholar]
- 23.Krammer, P. H., I. Behrmann, P. Daniel, J. Dhein, and K. M. Debatin. 1994. Regulation of apoptosis in the immune system. Curr. Opin. Immunol. 6:279-289. [DOI] [PubMed] [Google Scholar]
- 24.Krappmann, D., E. Wegener, Y. Sunami, M. Esen, A. Thiel, B. Mordmuller, and C. Scheidereit. 2004. The IκB kinase complex and NF-κB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol. Cell. Biol. 24:6488-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaire, C., K. Andréau, C. Sidoti-deFraisse, A. Adam, and V. Souvannavong. 1999. IL-4 inhibits apoptosis and prevents mitochondrial damage without inducing the switch to necrosis observed with caspase inhibitors. Cell Death Differ. 6:813-820. [DOI] [PubMed] [Google Scholar]
- 26.Li, Z. W., S. A. Omori, T. Labuda, M. Karin, and R. C. Rickert. 2003. IKK beta is required for peripheral B cell survival and proliferation. J. Immunol. 170:4630-4637. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 28.Melchers, F., V. Braun, and C. Galanos. 1975. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J. Exp. Med. 142:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto, S., B. J. Seufzer, and S. D. Shumway. 1998. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol. Cell. Biol. 18:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mordmuller, B., D. Krappmann, M. Esen, E. Wegener, and C. Scheidereit. 2003. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep. 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norvell, A., L. Mandik, and J. G. Monroe. 1995. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J. Immunol. 154:4404-4413. [PubMed] [Google Scholar]
- 32.Ohmae, T., Y. Hirata, S. Maeda, W. Shibata, A. Yanai, K. Ogura, H. Yoshida, T. Kawabe, and M. Omata. 2005. Helicobacter pylori activates NF-kappaB via the alternative pathway in B lymphocytes. J. Immunol. 175:7162-7169. [DOI] [PubMed] [Google Scholar]
- 33.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364-368. [DOI] [PubMed] [Google Scholar]
- 34.Perkins, N. D. 2007. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell. Biol. 8:49-62. [DOI] [PubMed] [Google Scholar]
- 35.Perkins, N. D. 2006. Post-translational modifications regulating the activity and function of the nuclear factor-kappa B pathway. Oncogene 25:6717-6730. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, N. D., and T. D. Gilmore. 2006. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 13:759-772. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, R. J., and S. Ghosh. 1997. Regulation of IκBβ in WEHI 231 mature B cells. Mol. Cell. Biol. 17:4390-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pomerantz, J. L., and D. Baltimore. 2002. Two pathways to NF-kappaB. Mol. Cell 10:693-695. [DOI] [PubMed] [Google Scholar]
- 39.Sen, R. 2006. Control of B lymphocyte apoptosis by the transcription factor NF-kappaB. Immunity 25:871-883. [DOI] [PubMed] [Google Scholar]
- 40.Sen, R., and D. Baltimore. 1986. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell 47:921-928. [DOI] [PubMed] [Google Scholar]
- 41.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 42.Siebenlist, U., K. Brown, and E. Claudio. 2005. Control of lymphocyte development by nuclear factor-kappaB. Nat. Rev. Immunol. 5:435-445. [DOI] [PubMed] [Google Scholar]
- 43.Solan, N. J., H. Miyoshi, E. M. Carmona, G. D. Bren, and C. V. Paya. 2002. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 277:1405-1418. [DOI] [PubMed] [Google Scholar]
- 44.Souvannavong, V., K. Andreau, A. Adam, and R. Chaby. 1999. Effect of synthetic lipids on apoptosis and expression of alkaline phosphatase in B-lymphocytes: influence on lipopolysaccharide action. FEMS Immunol. Med. Microbiol. 26:37-47. [DOI] [PubMed] [Google Scholar]
- 45.Souvannavong, V., C. Lemaire, and R. Chaby. 2004. Lipopolysaccharide protects primary B lymphocytes from apoptosis by preventing mitochondrial dysfunction and Bax translocation to mitochondria. Infect. Immun. 72:3260-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 47.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler-Reya, R. J., and J. G. Monroe. 1996. Lipopolysaccharide prevents apoptosis and induces responsiveness to antigen receptor cross-linking in immature B cells. Immunology 89:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weil, R., and A. Israel. 2004. T-cell-receptor- and B-cell-receptor-mediated activation of NF-kappaB in lymphocytes. Curr. Opin. Immunol. 16:374-381. [DOI] [PubMed] [Google Scholar]
- 50.Werner, S. L., D. Barken, and A. Hoffmann. 2005. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science 309:1857-1861. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 52.Zarnegar, B., J. Q. He, G. Oganesyan, A. Hoffmann, D. Baltimore, and G. H. Cheng. 2004. Unique CD40-mediated biological program in B cell activation requires both type 1 and type 2 NF-kappa B activation pathways. Proc. Natl. Acad. Sci. USA 101:8108-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]