Abstract
MARK, a kinase family related to PAR-1 involved in establishing cell polarity, phosphorylates microtubule-associated proteins (tau/MAP2/MAP4) at KXGS motifs, causes detachment from microtubules, and their disassembly. The sites are prominent in tau from Alzheimer’s disease brains. We studied the activation of MARK and identified the upstream kinase, MARKK, a member of the Ste20 kinase family. It phosphorylates MARK within the activation loop (T208 in MARK2). A fraction of MARK in brain tissue is doubly phosphorylated (at T208/S212), reminiscent of the activation of MAP kinase; however, the phosphorylation of the second site in MARK (S212) is inhibitory. In cells the activity of MARKK enhances microtubule dynamics through the activation of MARK and leads to phosphorylation and detachment of tau or equivalent MAPs from microtubules. Overexpression of MARK eventually leads to microtubule breakdown and cell death, but in neuronal cells the primary effect is to allow the development of neurites during differentiation.
Keywords: Alzheimer's disease/cell polarity/kinase regulation/microtubules/tau
Introduction
The kinase MARK (microtubule affinity regulating kinase) was initially detected because of its ability to phosphorylate tau protein, a neuronal microtubule-associated protein. This causes detachment of tau and destabilization of microtubules, the tracks of axonal transport (Drewes et al., 1997). Its abnormal phosphorylation and aggregation are hallmarks of Alzheimer’s disease. MARK phosphorylates tau and related microtubule-associated proteins (MAPs: MAP2, MAP4) at serines within the 3–4 KXGS motifs in the microtubule-binding domain. There are a family of MARKs whose expression in cells can disrupt microtubules and cause degeneration. They are homologous to the KIN1/PAR-1/Snf1 kinase family of the CaMK group II (Hanks and Hunter, 1995). Their common feature is the involvement in generating cell polarity, e.g. in yeast budding, partitioning of the Caenorhabditis elegans zygote, or embryonic axis formation in Drosophila (Guo and Kemphues, 1995; Shulman et al., 2000; Tomancak et al., 2000). The mechanisms of action have only been partially elucidated; they may involve signalling pathways such as the Wnt pathway which leads to the phosphorylation of dishevelled through the activation of PAR-1 in the fruit fly (Sun et al., 2001). In mammalian polarized cells such as epithelia or neurons, MARK/PAR-1 plays a role in asymmetric organization (Böhm et al., 1997; Biernat et al., 2002). One open issue is the activation by upstream factors. MARKs from brain contain a doubly phosphorylated peptide in the activation loop of the catalytic domain (Johnson et al., 1996); T215/S219 in MARK1 or T208/S212 in MARK2. Many kinases are activated by phosphorylation of a threonine in the activation loop (T208 in MARK2, by homology with PKA) which forms an ion pair with an arginine and a lysine (R174 and K96 in MARK2) that are important for fixing the loop. Some kinases require dual phosphorylation at the threonine and a downstream tyrosine, e.g. MAP kinases which are activated by a MAPKK (or MEK), which in turn becomes activated by upstream extracellular signals (Chen et al., 2001). Thus, given the similarity of phosphorylation sites in the activation loop, we suspected that there must be activating kinase(s) of MARK. Indeed, when MARK was dephosphorylated by PP-2a, the activity was abolished. Here we show that MARK is activated by a kinase purified from brain, termed MARKK, which is highly homologous to TAO-1 (Hutchison et al., 1998) and belongs to the Ste20 family of kinases. Activation of MARK is achieved by phosphorylation of a single residue, T208, by MARKK. It requires S212 to be present, but not phosphorylated, because this phosphorylation is inhibitory. In CHO cells, overexpression of MARKK triggers a cascade of activation of MARK and phosphorylation of MAPs at KXGS motifs, leading to increased microtubule dynamics, subsequent breakdown of microtubules and cell death. In neuronal cells (PC12), MARKK and MARK increase their activity upon differentiation by NGF. This leads to phosphorylation of tau at KXGS motifs, increased microtubule dynamics, and enables initiation of neurites. The three components MARKK, MARK, and phospho-tau, colocalize and are concentrated at the plasma membrane and growth cones.
Results
Activity of MARK and activating factors
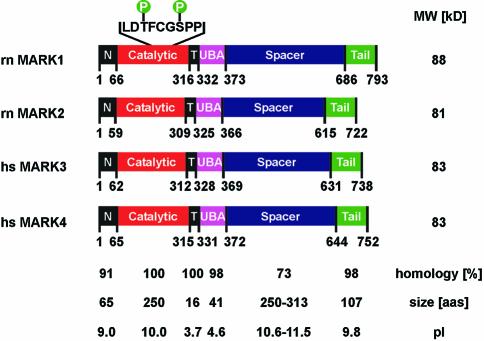
The search for a kinase that phosphorylates the KXGS motifs of tau from a fetal rat brain cDNA library yielded 4 isoforms, MARK1–4 (Figure 1; Drewes et al., 1997). A common feature of all catalytic domains of the MARK family is the presence of two Ser/Thr residues in the activation loop, spaced by three residues, which are important for activity. Both, T208 and S212 in MARK2, were phosphorylated in the native kinase prepared from brain (Drewes et al., 1997). The first (T208) aligns with a phosphorylatable Thr in PKA, Cdk2, or MAP kinase. The second (S212) aligns with a Ser/Thr residue highly conserved among kinases, close to the second phosphorylatable residue in MAP kinase (Y185). This suggested the possibility of a two-step activation mechanism reminiscent of MAP kinase (Chen et al., 2001). To investigate the activation we expressed MARK in Escherichia coli and probed activities against the tau peptide TR1 (255NVKSKIGSTENLK267) containing S262, the major target of native MARK and the major site controlling tau–microtubule interactions. Recombinant MARKs had a low basal activity since they were not phosphorylated in E.coli. This allows one to determine the influence of certain residues and the activation by upstream kinases. Most experiments were performed with the MARK2 isoform, as we did not observe major differences between the MARKs with regard to tau. Extracts from pig brain were fractionated (Table I) and tested for their activation of MARK2. The highest gain (∼80-fold) was achieved by affinity chromatography using the catalytic domain of MARK2 (residues 1–368), inactivated by mutations T208A and S212A. Figure 2A compares the elution profile from the G200 gel filtration column (bottom curve) with MARK activation (top curve). The peak (fractions 13–14) corresponds to an ∼330 kDa mass, several times larger than the activating kinase (see below), suggesting that the MARK activating factor (MAF) is a complex of proteins. Next we tested the activation of MARK2 by fraction 14. MARK2 alone had a low basal activity (Figure 2B, bottom curve), but with MAF the activity increased ∼10-fold (upper curve). This was accompanied by the phosphorylation of MARK2, as expected for the activation mechanism. The SDS gel of Figure 2C (top panel) shows that the Mr of MARK2 shifts upward from ∼88 to ∼97 kDa in the presence of the activating factor (left), but not in its absence (right); this shift is also found with natively active MARK2 (Drewes et al., 1997). Likewise, the incorporation of phosphate was also strongly increased by the activating factor (Figure 2C, bottom).
Fig. 1. Diagram of MARK1-4 (DDBJ/EMBL/GenBank accession Nos Z83868, Z83869, AF240782 and AY057448). Domains: N = header, C = catalytic, T = membrane-targeting, UBA = ubiquitin-associated, S = spacer, T = tail.
Table I. Enrichment of MARK activating factor from brain extract.
| Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Recovery (%) | Purification (fold) | |
|---|---|---|---|---|---|
| Brain extract | 25000 | n.d. | – | – | – |
| Phosphocellulose | 2330 | 450 | 0.19 | 100 | 1 |
| Q-Sepharose | 376 | 90 | 0.24 | 20 | 1.3 |
| MonoS | 31 | 24 | 0.77 | 5.3 | 4 |
| rMARK-Sepharose | 0.35 | 20 | 57 | 4.4 | 300 |
| G200 | 0.043 | 18 | 418 | 0.4 | 2200 |
Purification steps include chromatography by phosphocellulose, Q-sepharose, MonoS, rMARK-Sepharose (where the inactive MARK2 catalytic domain with the double mutation T208A/S212A and N-terminal His10-tag was coupled to Ni-NTA beads, allowing upstream kinases to bind), and G200 gel filtration.
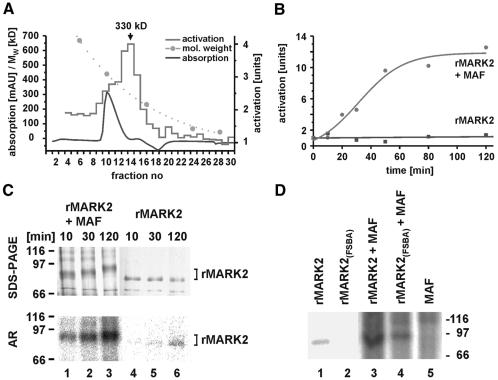
Fig. 2. Activation of MARK2. (A) Protein and activity profile of G200 gel filtration column. Fractions of brain extract were tested for the activation of MARK to phosphorylate the TR1 peptide. Most of the protein elutes around fraction 10 (bottom), but the activity peaks in fractions 13–14 (top, arrow). Calibration of column is shown as dotted line (catalase, ferritin, aldolase, serum albumin, ovalbumin). (B) Activation of MARK2 by MARK-activating factor. Bottom: recombinant MARK2 alone phosphorylates the TR1 peptide at a constant low rate (55 nmol/min/mg). With MAF (top) the activity of MARK2 increases ∼10-fold. (C) Phosphorylation of MARK2 increases with activation by MAF. Top left: SDS gel showing the Mr shift (from 88 to 97 kDa) during activation by MAF. Top right: MARK2 alone shows no Mr shift, despite some autophosphorylation. Bottom left: Autoradiogram of gel showing the increase in phosphate bound to MARK2, mostly at T208 (see below). Bottom right: MARK2 alone shows little autophosphorylation (outside the catalytic domain, data not shown). (D) Lanes 1 and 2, autoradiograms of MARK2 in active and inactive states. Activity was blocked by covalent modification of ATP binding site by FSBA. Lanes 3 and 4, incorporation of phosphate into active and inactive MARK2 by MAF, illustrating that MAF contains a kinase independently of the activity of MARK2. Lane 5, control, MAF alone shows no phosphate incorporation at the position of MARK2.
Thus far, the activating factor in the mixture of fraction 14 (Figure 2A) could be a kinase phosphorylating MARK2, or a factor binding to MARK2 and enhancing its kinase activity. To prove that the activating factor indeed contains a kinase, the fractions were tested for activity after incubation of MARK2 with FSBA (Figure 2D) which binds to ATP sites of proteins and blocks kinase activity (Anostario et al. 1990). The autoradiogram of Figure 2D shows that untreated MARK2 becomes autophosphorylated (lane 1, note that this affects sites not crucial for activation, e.g. T58, S275, T276, outside the catalytic site, as judged by a peptide array; data not shown). There is no autophosphorylation when MARK2 is incubated with FSBA prior to the kinase assay, and no incorporation of radioactivity (lane 2), indicating that the kinase is no longer active.
When MARK2 not treated with FSBA is exposed to MAF there is strong incorporation of phosphate into MARK2 (Figure 2D, lane 3), including the activation loop (see below). The activating factor can phosphorylate MARK2 even when MARK2 is inactive due to FSBA binding (lane 4). This rules out the possibility that MAF is a cofactor enhancing the autophosphorylation of MARK2 and argues that MAF indeed contains a kinase phosphorylating MARK2, which is therefore termed MARKK (MARK kinase).
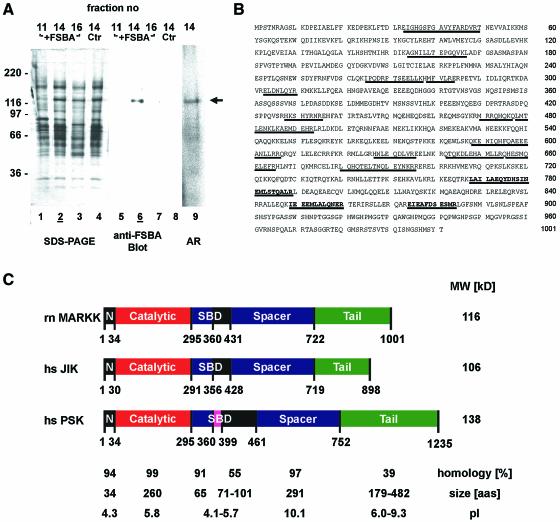
We next asked which protein in the MARK2-activating fraction was the kinase. We labeled fractions 11–16 of the G200 eluate with FSBA. The proteins were separated on an SDS gel, blotted and probed with an antibody against FSBA (Figure 3A). This revealed a single band at Mr ∼134 kDa, occurring only in the highly active fraction 14 (lane 6), and only when FSBA was present (compare with lane 8). This fraction also showed some autophosphorylation (Figure 3A, lane 9).
Fig. 3. Sequence and domain structure of MARKK. (A) Identification of MARKK. Lanes 1–4 show SDS gel of fractions 11, 14, 16 (with FSBA), and 14 (control without FSBA) from G200 column (fraction 14 contains the highest activity, see Figure 2A). Lanes 5–8 are blots with an antibody against FSBA. Only one band at 134 kDa is labeled in the active fraction (lane 6, fraction 14); the control without FSBA shows no signal (lane 8). For comparison, lane 9 shows an autoradiogram of an active fraction (same sample as in lane 4) with a band of active MARKK at Mapp= 134 kDa, labeled because of autophosphorylation. (B) Sequence of MARKK from a rat brain cDNA library. Peptides identified by sequencing and mass spectrometry are underlined. The sequence is equivalent to that of TAO-1 (DDBJ/EMBL/GenBank accession no. AF084205) or the human sequence KIAA1361 (AB037782). The three bold peptides are unique for TAO-1 and distinguish the kinase from PSK/TAO-2 and JIK. (C) Domain structure of MARKK/TAO-1 and comparison with other kinases of high homology (JIK and PSK) in the Ste20 family. N = N-terminal header domain, C = catalytic domain, SBD = substrate binding domain, S = spacer domain, T = tail domain. Extended coiled-coil sequences are predicted in the spacer and tail domains (S430-A630, K730-F900). The substrate binding domain of PSK/TAO-2 binds to the N-terminal header domain of its substrate MKK3, the kinase upstream of p38 MAP kinase. However, it is unclear whether an analogous interaction occurs with MARKK and MARK since the corresponding domains are poorly conserved.
Cloning and expression of MARKK
The 134 kDa band was isolated by preparative SDS–PAGE, digested with trypsin, and 16 peptides were identified by mass spectrometry and sequencing as described (Gevaert et al., 2001; Figure 3B). A search showed that all peptides were contained in the sequence AF084205, a protein kinase named TAO-1 (Hutchison et al., 1998), indicating that MARKK must be highly homologous to that kinase, a member of the Ste20 kinase family (Dan et al., 2001). Other kinases with high homology include PSK/TAO-2 and JIK/TAO-3; however, several peptides of MARKK (bold in Figure 3B) do not fit, thus excluding these kinases. We cloned the kinase from rat brain cDNA bank using primers derived from the 5′- and 3′-ends of the TAO-1 sequence (Figure 3B and C). The arrangement of domains is similar to that of MARK, with an N-terminal kinase catalytic domain preceded by a header and followed by spacer and tail domains. Secondary structure predictions reveal extended coiled-coil motifs in the spacer and tail domains (S430-A630, K730-F900).
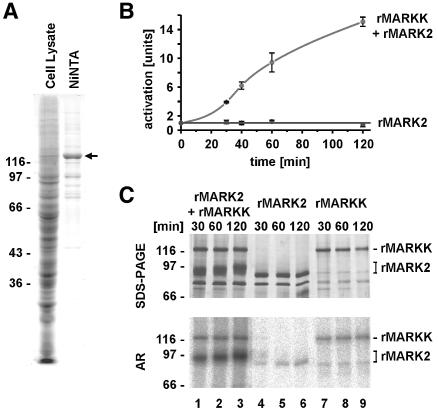
Expression of MARKK in E.coli yielded only degraded and insoluble material, but expression in Sf9 cells resulted in functional protein with an Mr value of 134 kDa, as expected (Figure 4A). The activation of MARK2 by recombinant MARKK was demonstrated by the same procedures as those used before for MARK-activating factor (Figure 4B, see Figure 2B). MARK2 alone showed a low basal activity of 55 nmol/min/mg, rising to 233 nmol/min/mg at TR1 concentrations >1.5 mM. Treatment of MARK2 with MARKK lead to a 10–15-fold activation (∼800 nmol/min/mg; Figure 4B, circles), whereas MARKK alone showed no activity towards TR1 at all. In the SDS gel, recombinant MARK2 appears at ∼88 kDa (Figure 4C, top panel, lanes 4–6) and recombinant MARKK runs at 134 kDa (lanes 7–9). In combination, MARKK shows the same Mr value, while that of MARK2 is shifted up to ∼97 kDa due to phosphorylation by MARKK (lanes 1–3). In the autoradiogram (Figure 4C, bottom panel), MARK2 reveals little autophosphorylation, indicating that this is not responsible for the basal activity of MARK2 (lanes 4–6). MARKK alone also shows only slight autophosphorylation (lanes 7–9; note that the 88 kDa band is probably a degradation product of MARKK). By contrast, combining MARK2 and MARKK (lanes 1–3) leads to a strong incorporation of phosphate into MARK2, but only minor phosphorylation of MARKK. This confirms that MARKK can phosphorylate (and activate) MARK2, but the reverse does not hold.
Fig. 4. Activity of recombinant MARKK. (A) Expression of recombinant MARKK (His10-tagged at the N-terminus) in Sf9 cells. Lane 1, SDS gel of Sf9 cell lysate, lane 2, protein retained on Ni-NTA column, showing enrichment of MARKK at Mapp = 134 kDa (slightly larger than the predicted 119 kDa). (B) Activation of MARK2 by recombinant MARKK, measured by the phosphorylation of the TR1 peptide by MARK2. Bottom curve (squares), recombinant MARK2 alone phosphorylates the peptide at a constant low rate. MARKK activates MARK2 >16-fold after 2 h (top curve, circles). (C) Phosphorylation of MARK2 increases with activation by MARKK. Top left (lanes 1–3), SDS gel showing the gradual shift of MARK2 in Mr (from 88 to 97 kDa) during activation by MAF. Top middle (lanes 4–6), MARK2 alone shows no shift in Mr, despite some autophosphorylation. Top right (lanes 7–9), MARKK alone shows no shift in Mr either. Bottom left, autoradiogram of gel showing the increase in phosphate bound to MARK2, mostly at T208 (see below). Bottom middle, MARK2 alone shows little autophosphorylation (at sites outside the catalytic domain; data not shown). Bottom right, MARKK shows some autophosphorylation (note that the lower band comigrating with MARK2 is a coincidence and probably due to degradation of MARKK).
Regulation of MARK2 by MARKK
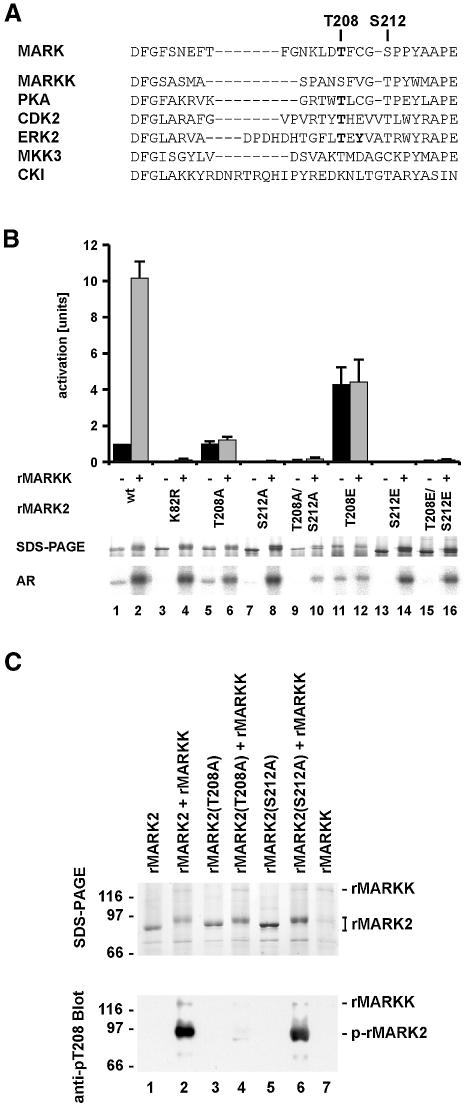
Previous experiments (Drewes et al., 1997) revealed two phosphorylation sites in the activation loop of MARKs; T208 and S212 (Figure 5A). When MARK was purified from brain, the removal of phosphates by PP-2a lead to inactivation, but it was not clear what the interplay between the sites was. This issue was addressed by site-directed mutagenesis. Figure 5B illustrates the activity of MARK2 carrying mutations in the activation loop or at K82, without or with activation by recombinant MARKK. Lanes 1 and 2 show the 10-fold activation by MARKK. Disturbing the ATP site by the K82R mutation destroys the activity (top panel, bars 3 and 4). When T208 was mutated into Ala, the activation by MARKK was lost and only basal activity remained (bars 5 and 6). This means that T208 is important for activation, and that the basal activity does not depend on it. By contrast, when S212 or both T208 and S212 were mutated into Ala, the intrinsic and the activatable activities of MARK2 were completely abolished (bars 7–10). When T208 was mutated into Glu, the basal activity increased 4-fold but no further activation by MARKK took place. By changing S212 of MARK into Glu the basal activity and the activation by MARKK was lost, independently of whether T208 was mutated to Glu or not. This means that S212 must be present for basal activity, and that it is required for the activation via the phosphorylation of T208 (by MARKK). Since E often mimicks phospho-serine or -threonine, this also suggests that phosphorylation of S212 is not essential for activity, but on the contrary is inhibitory. This can be understood by the structural role of S212 (see below).
Fig. 5. Regulation of MARK. (A) Activation loop of MARK2 and other kinases. Known phosphorylation sites are in bold. (B) Influence of activation loop on MARK2 activity. Top panel, bar 1, MARK2 alone has a low basal activity of 55 nmol/min/mg. Bar 2, activation by MARKK is 10-fold. Bars 3 and 4, the K82R mutant has no activity and cannot be activated. Bars 5 and 6, the T208A mutant has the same activity as wild type, irrespective of MARKK, indicating that the phosphorylation of T208 is important for activation. Bars 7–10, the S212A mutant and the T208A/S212A mutant of MARK2 show no activity at all, indicating that S212 is important for basal activity, independently of whether T208 is phosphorylated by MARKK or not. Bars 11 and 12, the T208E mutant has a 4-fold higher activity than the wild type but cannot be activated further by MARKK. Bars 13–16, the S212E mutant and the T208E/S212E mutant of MARK2 show no activity. Middle panel, SDS gel shows the shift in Mr of all MARK2 mutants after phosphorylation with MARKK as well as the wild-type MARK2. The T208E mutant has a lower electrophoretic mobility even without phosphorylation (lane 11). Bottom panel, autoradiograms showing that autophosphorylation corresponds to activity (odd lanes). All proteins become phosphorylated upon incubation with MARKK (even lanes). This indicates at least one phosphorylation site outside the regulatory loop but with no influence on activation. (C) Phosphorylation of MARK2 and mutants (T208A or S212A) during activation by MARKK. Top, SDS gel; bottom, blot with antibody against phospho-Thr. Odd lanes without MARKK; even lanes with activation by MARKK. Lane 1, MARK2 alone. Lane 2, MARKK phosphorylates MARK2 with a shift in Mr (top), and with a prominent phospho-Thr site recognized by the pT208 antibody. Lane 3, T208A mutant of MARK2. Lane 4, MARKK causes an Mr shift of MARK2-T208A (top), but there is no phospho-Thr reaction, concomitant with the loss of activation [see (B) above]. It also shows that T208 is not responsible for the Mr shift. Lane 5, S212A mutant of MARK2. Lane 6, phosphorylation by MARKK of MARK2-S212A creates the phospho-Thr epitope at T208 (but without activity). There is an Mr shift that occurs independently of S212. Lane 7, Control, MARKK alone shows no signal with the antibody against phospho-T208.
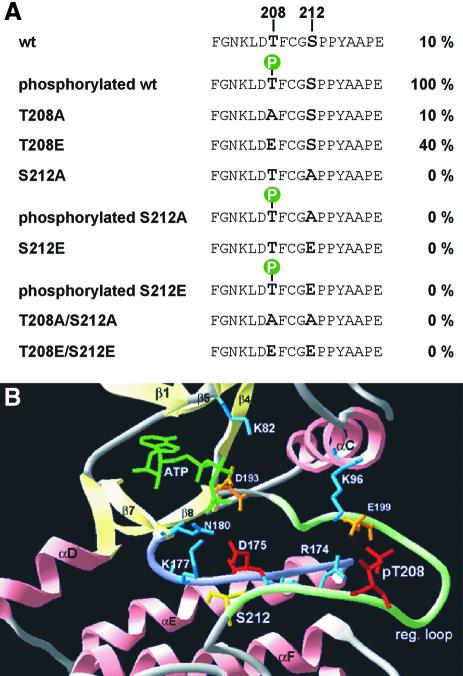
To determine whether the mutation of S212 affects the ability of MARKK to phosphorylate MARK we raised antibodies against the regulatory peptide (phosphorylated specifically at T208 or S212). MARK2wt or the T208A- or S212A-mutants all become phosphorylated only in the presence of MARKK (as indicated by an Mr shift; Figure 5C top panel, lanes 2, 4 and 6). The pT208-specific antibody recognises MARK2wt and MARK2/S212A treated with MARKK (Figure 5C bottom panel, lanes 2 and 6) but not phosphorylated MARK2/T208A (lane 4). This verifies that MARKK phosphorylates MARK2 at T208 even when the S212 is mutated to alanine. The phospho-S212-specific antibody could only detect MARK purified from brain but not recombinant MARK2 or its mutants, showing that the S212 is not a target for MARKK (data not shown). The data are summarized in Figure 6A: p-T208 plus S212 causes maximal activity, Thr or Ala at 208 plus S212 suffices for basal activity, and absence of S212 destroys the basal activity, even when T208 is phosphorylated (Figure 5C, lane 6). These features can be explained in terms of known structures of kinases (Figure 6B). The Mr shift of MARK2 is due to other sites phosphorylated by MARKK but unrelated to the activity of MARK2 (Figure 5C, lane 5), and serves as an indicator for active MARKK.
Fig. 6. Regulation of MARK. (A) Sequence F202-E219 around the activation loop of MARK2, with T208 and S212 highlighted. The wild-type sequence allows only basal activity (10%) which rises 10-fold upon phosphorylation of T208 by MARKK. Mutant T208A allows only basal activity, T208E shows partial activation. Phosphorylation of S212 is not required for activation, but any mutation of S212 (A or E) destroys activity, showing that S212 must be present for activity while mutants of S212 are inactive even when T208 is phosphorylated. (B) Structural model of the regulatory loop of MARK2 using PKA as a template (SWISSMODEL, http://www.expasy.ch/swissmod/SWISS-MODEL.html). Catalytic (violet) and activation loops (light green) lie between the small lobe (mainly β-sheets, light yellow) and the large lobe (mainly α-helices, pink). Phospho-T208 (red) contacts K96, R174 (light blue) and E199 (orange) via hydrogen bonds stabilizing the regulatory loop. S212 (yellow) forms a hydrogen bond with K177 (light blue) in the catalytic loop which also stabilizes the γ-phosphate of ATP (green) during the phospho-transfer and is conserved in S/T-kinases. The catalytic D175 (red) is stabilized by N180 (light blue). K82 (light blue) fixes the α- and β-phosphates of ATP, together with the Mg2+-binding D193 (orange). Note that in PKA and MAP kinase the phosphorylation corresponding to T208 stabilizes the activation loop and opens the substrate pocket (by interacting with the residues corresponding to K96, R174 and E199 in MARK, Johnson et al., 1996), whereas the conserved DFG motif (193–195) and S212 form fixed points for anchoring this flexible loop (contributing a hydrogen bond with K177). In this scheme, the phosphorylation of S212 would be inhibitory because it would disrupt the fixation of the loop. It is also possible that the Glu in this postion interferes with the binding of the substrate to the catalytic cleft (Scott et al., 2002). Mutation of the equivalent to S212 in JIK results in a dramatic decrease of activity (Tassi et al., 1999). The doubly phosphorylated peptide in brain-derived MARK2 probably come from an inactive fraction. The kinase responsible for phospho-S212 is unknown, but we note that none of the kinases applied in combination with MARKK was able to phosphorylate S212 in MARK2, and there was no change in activity.
The specificity of MARKK for T208 in the activation loop of MARK was demonstrated by phosphorylating the lip peptide 203GNKLDTFCGSPPYAAPELQGKK224. It contains three phosphorylatable residues, but phosphate is incorporated almost exclusively at T208 (see Supplementary figure 1, available at The EMBO Journal Online). Since S212 is followed by a proline we considered whether a proline-directed kinase might contribute to the regulation of activity. Several kinases were tested (p38 stress activated kinase, p42/ERK2, p44/ERK1, Cdc2, Cdk5, GSK3β, CKI), but they had no effect on the basal activity of MARK, its activation by MARKK, and did not induce the antibody reaction against phospho-S212 (data not shown). Finally, we tested whether other MARK isoforms behaved similarly. This was indeed the case; MARK1, MARK2, and MARK3 had similar basal activities and were activated by MARKK, indicating some promiscuity in the activation cascade.
The MARKK–MARK–MAP cascade in cells
One aim of our studies was to show that MARKK stimulates MARK2 in cells. We transfected Sf9 cells with baculovirus-encoded MARK2 or MARK2 plus MARKK. Cell extracts were immunoblotted to demonstrate the expression of MARK2 and MARKK (details in Supplementary figure 2). Immunoprecipitation of the HA-tagged MARK2 from the cell lysates shows that co-expression of MARKK does not alter the level of MARK2. This provides a basis for comparing the MARK2 activity in Sf9 cells alone or with MARKK. Judging by the phosphorylation of the tau peptide TR1, MARK2 expressed in Sf9 cells becomes ∼3-fold more active when it is coexpressed with MARKK. Next we asked whether MARK2 interacts directly with MARKK in Sf9 cells, using immunoprecipitation of transfected MARK2 with the HA-tag antibody. In the case of coexpression of HA-MARK2 with MARKK no detectable amounts of MARKK were found by immunoprecipitation. However, when the dominant-negative mutant MARK2 (T208A/S212A) was coexpressed with MARKK both proteins were immunoprecipitated. This is consistent with the enrichment of MARKK during the preparation from brain where the inactive catalytic domain of MARK2 (T208A/S212A) was used for affinity chromatography (Table I).
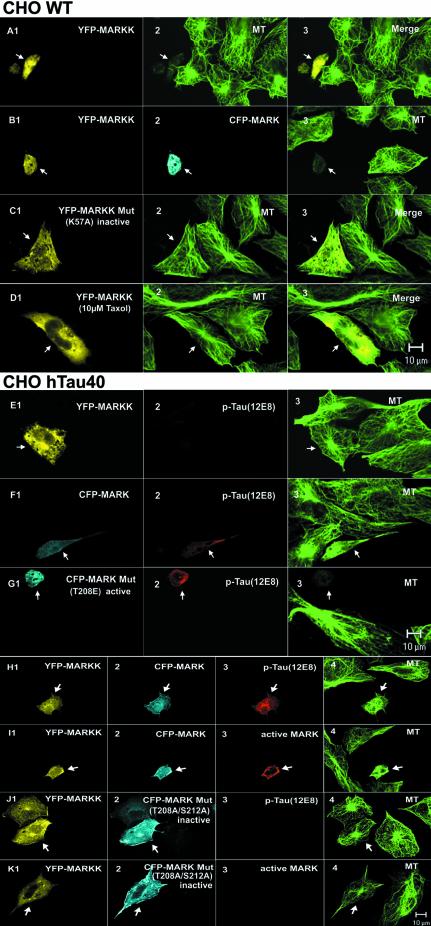
It was shown earlier that overexpression of MARK leads to phosphorylation of MAPs at the KXGS motifs, their detachment from microtubules, and increased microtubule dynamics. In CHO cells this results in microtubule breakdown and cell death, which can be compensated by increasing MAPs and thus stabilizing microtubules (Drewes et al., 1997). In neuronal cells (N2a) the consequence of increased microtubule dynamics is the stimulation of neurite outgrowth (Biernat et al., 2002). If MARKK indeed activated MARK one would expect similar consequences of MAP phosphorylation and increased microtubule dynamics when MARKK is overexpressed in cells. Figures 7 and 8 illustrate the operation of the MARKK–MARK cascade in cells. One set of experiments was performed in CHO cells which contain endogenous MAP4 (phosphorylatable at KXGS motifs in the repeat domain). Figure 7A shows CHO cells transfected with MARKK (labeled with YFP). While normal cells have an extended shape with a clear microtubule network, transfection by MARKK leads to loss of microtubules, shrinkage of the cells and eventually cell death (see Figure 7A1 and A2, arrows). As a control, transfection with inactive MARKKK57A does not perturb microtubules, demonstrating that kinase activity is required for the effect (Figure 7C). Microtubule perturbation is also observed when MARKK (YFP) and MARK (CFP) are cotransfected in CHO cells (Figure 7B). If our interpretation is correct, then stabilizing microtubules, for example by taxol, should prevent the catastrophic effect of MARKK. This is indeed the case. Figure 7D shows that 10 µM taxol prevents microtubule destruction and cell death. Note that MARKK is well expressed (Figure 7D1, arrow), but the cell has a normal microtubule network and size (Figure 7D2, arrow). To verify the relationship between MARKK, MARK, MAPs and microtubules, we transfected CHO cells stably with htau40 in order to increase the stability of microtubules (analogous to the taxol experiment). Figure 7E demonstrates that transfection of MARKK leads to expression of the kinase but cannot destroy the microtubules because they are stabilized by excess tau (Figure 7E, arrows). Apparently the activity of the endogenous MARK is too low (also supported by the absence of tau staining by the 12E8 antibody). A similar result is obtained when we transiently transfect MARK in the tau-stable CHO cells. Although there is now a higher concentration of active MARK (as seen by phospho-tau staining with 12E8 antibody, Figure 7F2, arrow), the MARK activity still cannot counteract the stabilization by tau, and the cell nearly retains its microtubule network. However, when the transfected MARK is constitutively active (mutant MARKT208E), even the presence of tau cannot protect the microtubule network (Figure 7G). Similarly, when we co-transfect the cells with MARKK and MARK the microtubule-stabilizing effect of tau can be overcome by the phosphorylation of tau at the KXGS motifs (strong staining by 12E8 antibody, Figure 7H3, arrow), and the detrimental effect of this phosphorylation is seen by the collapse of microtubules and the rounding up of cells (Figure 7G and 7H). At the same time the antibody diagnostic for active MARK shows strong staining (Figure 7I, arrow). To verify that MARK plays an intermediary role in the cascade we co-transfected MARKK with an inactive MARK mutant (T208A, S212A) (Figure 7J2 and K2). As anticipated, tau shows no phosphorylation, the cells remain healthy (Figure 7J3 and J4, arrow), and there is no detectable activated MARK (Figure 7K3).
Fig. 7. Active MARKK in cells destroys microtubules via activation of MARK and phosphorylation of Tau. (A) CHO cell transfected with YFP-MARKK, fixed and observed by confocal microscopy (A1, arrow), staining of microtubules with antibody YL1/2 and Cy5-secondary antibody (A2), merged images (A3). Note that the transfected cell (arrow) lost its microtubule network, rounded up and appears smaller. (B) Cell co-transfected with YFP-MARKK (B1, arrow) and CFP-MARK (B2) or staining of microtubules with YL1/2 (B3). Note the shape change and destruction of microtubules in the co-transfected cell, similar to (A). (C) CHO cell transfected with inactive YFP-MARKKK57A imaged as above (C1, YFP fluorescence, C2 microtubules, C3 merged). The transfected cell (left, arrow) retains its microtubule network. (D) Cell transfected with YFP-MARKK (D1, arrow), and microtubules stabilized by 10 µM taxol (D2), merged images (D3). Note that taxol prevents the destabilization of microtubules by MARKK. (E) Cell stably transfected with tau and transiently transfected with YFP-MARKK. Staining of YFP-MARKK (E1, arrow), phospho-tau (KXGS motifs) by antibody 12E8 (TRITC) (E2), microtubules (E3). Note that elevated tau stabilizes microtubules against the effect of MARKK. (F) Cell stably transfected with tau and transiently transfected with CFP-MARK. Staining of CFP-MARK (F1, arrow), phospho-tau (KXGS motifs) (F2), microtubules (F3). Note that elevated tau stabilizes microtubules against the effect of MARK. (G) Cell stably transfected with tau and transiently transfected with CFP-MARKT208E. Constitutively active MARK (G1, arrow) phophorylates tau at KXGS motifs (G2), and destroys microtubules (G3). (H) Cell stably transfected with tau and transiently co-transfected with YFP-MARKK and CFP-MARK. Staining of YFP–MARKK (H1, arrow), CFP-MARK (H2), phospho-tau (KXGS motifs) (H3), and microtubules (H4). Note that the combined effect of MARKK and MARK destabilizes microtubules in spite of tau. (I) Experiment similar to (H) but highlighting active MARK by the antibody SA6941-TRITC against the phosphorylated activation loop (I3, arrow). YFP–MARKK (I1), CFP–MARK (I2), active MARK (TRITC) (I3), and microtubules (Cy5) (I4). (J) Cell stably transfected with tau and transiently co-transfected with YFP-MARKK and CFP-MARK-mutant (T208A, S212A). Staining as in (H). Microtubules remain intact because the MARK mutant cannot be activated. (K) Experiment similar to (J), i.e. cotransfection with YFP-MARKK (K1, arrow), CFP-MARK mutant (K2), and staining with antibody against active MARK (K3).
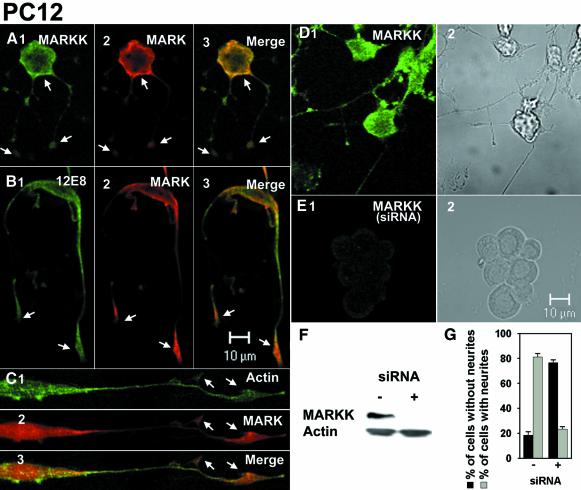
Fig. 8. (A) PC12 cells differentiated with NGF (72 h, 100 ng/ml) and immunostained for MARKK (A1, antibody TAO-1), MARK (A2, antibody SA2118), and merged image (A3). Note that endogenous MARKK and MARK colocalize, notably beneath the plasma membrane and at growth cones (arrows). (B) PC12 cells differentiated as above, immunostained for phospho-KXGS-tau (B1, antibody 12E8), MARK (B2), and merge (B3). Note that endogenous phospho-KXGS-tau colocalizes with MARK, especially near the plasma membrane and the growth cones. (C) PC12 cells differentiated as above and immunostained for actin (C1, oregon-green-phalloidin), MARK (C2), and merge (C3). Note that endogenous MARK colocalizes with actin at growth cones. (D) PC12 cells differentiated with NGF (48 h, 100 ng/ml). D1, mock transfected cell, stained for endogenous MARKK. D2, phase image. (E) PC12 cells transfected with RNAi against MARKK exposed to NGF as above. Note that MARKK is largely suppressed (E1), and the cells cannot differentiate (phase image, E2). (F) Western blot of PC12 cells after NGF-differentiation, mock-transfected (left) or with RNAi against MARKK (right). Note that expression of MARKK is suppressed. (G) Quantification of effects of RNAi against MARKK (see D and E). The fraction of cells with neurites decreases from ∼80% to 20% upon treatment with RNAi.
In another set of experiments we studied the expression of endogenous MARKK, MARK, and the phosphorylation of tau in PC12 cells differentiated with NGF (Figure 8). We had found previously that transfection of MARK induced tau phosphorylation concomitant with neurite outgrowth, and that MARK, activated MARK, phospho-KXGS-tau and actin colocalized (Biernat et al., 2002). The effect was abolished if the KXGS motifs of tau were mutated into KXGA, or if a dominant-negative mutant of MARK was expressed. Figure 8 illustrates PC12 cells differentiated with 100 ng/ml NGF and then immunostained for endogenous MARKK and MARK. Differentiation causes upregulation of MARK (and to a lesser extent MARKK; data not shown), and both kinases colocalize—together with phospho-tau and the actin network—near the plasma membrane and at the growth cones (Figure 8A–C). As a control, the ability of MARKK to promote neurite outgrowth after the NGF trigger can be suppressed by downregulating MARKK with RNAi (Figure 8E and F). While mock-transfected PC12 cells can be differentiated with high efficiency (Figure 8D and G), those transfected with MARKK-RNAi show only negligible amounts of endogenous MARKK and their differentiation is suppressed (20%; Figure 8E and G). The result is corroborated by western blotting showing that after MARKK-RNAi transfection into PC12 cells the protein was suppressed (Figure 8F). Thus, all experiments are consistent with a cascade connecting MARKK, MARK, tau (or related MAPs), microtubule plasticity, and cell shape.
Discussion
Control of MARK2 by phosphorylation of the activation loop
This study is part of our efforts to understand the regulation of tau–microtubule interactions and pathological phosphorylation of tau protein in the neurofibrillary pathology of Alzheimer’s disease (AD). Tau contains many potential phosphorylation sites. Some are particularly interesting, such as the KXGS motifs in the repeat domain whose phosphorylation inhibits the binding to microtubules (Biernat et al., 1993) and is elevated in AD. A search for the kinase led to the identification of MARK which causes the detachment of tau and related MAPs and destabilizes microtubules (Drewes et al., 1997). The human genome contains four genes of the MARK family (MARK1–4; Manning et al, 2002).
When MARK1 or 2 from brain is analyzed by phosphopeptide mapping one finds two phosphorylated residues in the activation loop (T208 and S212 in MARK2). This region controls kinase activity by blocking access to the substrate binding site and is itself regulated by phosphorylation in many kinases (Johnson et al., 1996). The control can be achieved by single or dual phosphorylation. Single phosphorylation occurs at an S or T residue corresponding to T208 of MARK2. In the case of dual phosphorylation, the first site also aligns with T208 (e.g. T183 in MAP kinase/ERK2) and the second site is close to S212 (e.g. Y185 in MAP kinase; Chen et al., 2001). Since we found both T208 and S212 phosphorylated in the same peptide, we initially assumed an activation mechanism by dual phosphorylation.We therefore searched for activating kinase(s) that would phosphorylate the activation loop of MARK2 and enable it to phosphorylate the tau peptide TR1, containing the first KXGS motif. The activating factor MAF had an Mapp of ∼330 kDa, suggesting a complex of several subunits. Peptide analysis revealed 16 sequences nearly identical to the kinase TAO-1 (DDBJ/EMBL/GenBank accession no. AF084205; Hutchison et al., 1998). With this information we cloned rat brain MARKK, expressed the His-tagged kinase in Sf9 cells, and used it to phosphorylate MARK2. Unlike E.coli, the Sf9 cells contain the machinery for posttranslational modifications, including kinases for keeping MARKK active.
The activation of MARK2 and the roles of the phosphorylation sites turned out to be unexpectedly complex (Figure 5B, Figure 6A). Full activation requires only the phosphorylation of T208; mutation of T208 into A abolishes activity; and replacement by E mimicks it partially (40%). MARKK does not phosphorylate S212 at all, so that the phosphorylation of this residue in brain-derived MARK must stem from another kinase. All mutations of S212 destroyed the activity of MARK, both S212A (designed for constitutive inactivation) and S212E (intended to increase activity). Even the phosphorylation at T208 was not able to override the negative effect of S212 mutations. Thus the presence of S212 is essential for the activation of MARK2, even though its phosphorylation is not, and indeed appears to be inhibitory. It is likely that the hydroxyl group of S212 is needed for stabilizing the activation loop, as suggested by analogy with known kinases (Figure 6A and B).
Can MARKK also activate MARK in cells and trigger responses of the microtubule cytoskeleton? This was demonstrated in several ways. First, the elevation of MARKK in Sf9 cells increases the activity of MARK and enhances the phosphorylation of the KXGS motifs in tau. MARKK and MARK can be isolated in a complex if the activation loop is made non-phosphorylatable so that the interaction becomes long-lived, in analogy to kinase cascade complexes (Müller et al., 2001; Ge et al., 2002). In other cell types suitable for observing the cytoskeleton (e.g. CHO) it can be shown that an increase in MARK activity by overexpression causes the phosphorylation and detachment of tau or related MAPs, microtubule breakdown and cell death (Drewes et al., 1997). The same chain of events can be triggered by elevating MARKK (Figure 7), and conversely the chain can be interrupted at different levels, either by stabilizing microtubules (via more MAPs or taxol), or by making MARK or MAPs non-phosphorylatable at the critical residues. Thus MARKK acts as the activating kinase of MARK and the MARKK cascade is operational in cells.
In contrast to CHO cells, neuronal cells (PC12) have higher levels of endogenous MARKK and MARK which can be seen by immunostaining (Figure 8). This allows observation of the endogenous functions of the kinases without the risk of artefacts due to overexpression. MARKK, MARK and phospho-KXGS-tau colocalize, particularly near the plasma membrane and in growth cones where actin is enriched (Figure 8A–C), and the cascade is upregulated by differentiation and the development of neuronal cell polarity. Conversely, suppression of endogenous MARKK (by RNAi; Figure 8E) abrogates the cell’s ability to develop neurites in spite of an NGF differentiation signal. These results fit well with observations that neurite outgrowth requires active MARK and tau phosphorylatable at KXGS motifs (Biernat and Mandelkow, 1999; Biernat et al., 2002). This is consistent with the interpretation that neurite outgrowth requires dynamic microtubules, short enough to enter the neurite shaft but ready to elongate and stabilize it. The role of MARKK and MARK would be to transiently subdue microtubule stabilization by tau.
Properties of the activating kinase MARKK
MARKK belongs to the Ste20 group of kinases which can be divided into the p21-activated protein kinases (PAKs, with an N-terminal p21-binding domain and a C-terminal kinase domain) and the germinal center kinases (GCKs; N-terminal kinase domain, no p21-binding domain). About 31 Ste20-related kinases are known (two PAK-, and eight GCK-subfamilies; Manning et al., 2002). The kinases have various effects including the regulation of apoptosis and the rearrangement of the cytoskeleton, and many play a role in the activation of MAP kinases. Regulation of Ste20 kinases is achieved through the Ras family of GTPases, additional protein kinases, adapter proteins coupled to cytokine receptors, and via dimerization or association with inhibitors combined with autophosphorylation after stimulation (Dan et al., 2001). MARKK/TAO-1 is part of the kinase subfamily GCK-VIII, together with the kinases PSK/TAO-2 (Chen et al., 1999) and JIK/TAO-3 (Tassi et al., 1999). The sequence of MARKK and its two relatives in humans (Manning et al., 2002) is unusually long, suggesting several functions besides the kinase activity. The active complex from brain has a mass of ∼330 kDa which would be compatible with a complex of two or three MARKK molecules, or with a complex between MARKK, scaffolding proteins, and other cofactors. An example is the complex of c-raf, the scaffold proteins 14-3-3, KSR-1 and other factors important for the MAP kinase cascade (Müller et al., 2001), or the complex between PAR-1 and 14-3-3 (Benton et al., 2002). MARKK contains predicted amphipathic helices in the spacer and tail domains which would favor protein–protein interactions.
PSK/TAO-2 has been reported to be a regulator of stress activated MAP kinase pathway (Chen et al., 2003), but this is not the case for MARKK/TAO-1, although it has the ability to phosphorylate MKK3 and MKK6, the activators of p38 stress activated kinases in vitro (Hutchison et al., 1998). Endogenous MKK3 can be copurified with transfected TAO-1 and TAO-2 from Sf9 cells (Hutchison et al., 1998; Chen et al., 1999). This involves the binding of the substrate binding domain of TAO to the N-terminal header domain of its substrate MKK3 (Chen et al., 1999). At present, the relationship between MAP kinase signalling and MARK signalling is unclear, but it is interesting to note that cellular stress can lead to the phosphorylation of KXGS motifs in tau through the activation of MARK (Jenkins and Johnson, 2000). Furthermore, the transcripts of MARK1 and MKK3 are upregulated after differentiation of PC12 cells (Brown et al., 1999; and our results). Thus far, upstream effectors or scaffolding proteins for MARKK are unknown.
Implications for the functions of MARK
A striking link exists between MARK and kinases involved in establishing cell polarity during development, notably PAR-1 (Guo and Kemphues, 1995; Böhm et al., 1997; Tomancak et al., 2000; Sun et al., 2001). The common theme is that cell polarization requires an asymmetric distribution of cell components whose transport often involves microtubules. This suggests a link between microtubules and cell polarity. For example, polarized layers of MDCK cells were maintained only when PAR-1 (equivalent to MARK2) was localized at the tight junction (Böhm et al., 1997). In C.elegans, PAR-1 is required for the migration of P-granules to the posterior pole during embryonic development (Guo and Kemphues, 1995). In fruit flies, PAR-1 is required for the asymmetric distribution of oocyte factors and the organization of microtubules (Shulman et al., 2000; Cox et al., 2001), including the local destabilization of microtubules (Cha et al., 2002), and it increases the stability of oskar protein at the posterior pole by phosphorylation (Riechmann et al., 2002). A relationship with Wnt signalling is emphasized by the fact that PAR-1 causes the phosphorylation of dishevelled and hence activates gene transcription through β-catenin (Sun et al., 2001). We showed earlier that the outgrowth of cell processes from Sf9 or neuronal cells depended on active MARK phosphorylating the KXGS motifs of tau protein (Biernat and Mandelkow, 1999; Biernat et al., 2002). The link to the problem of AD is that neurons suffer from an early defect in their polarity which they attempt to compensate by aberrant sprouting. Since this involves microtubules, tau protein, and ‘erroneous’ signalling this might explain the higher level of tau phosphorylation at KXGS and other sites. By implication, one would expect a higher level of MARK activation and a higher activity of MARKK in AD brains.
Materials and methods
Purification of MARK-activating factor (MAF)
The homogenate from porcine brain was fractionated by several chromatographic steps (cationic, anionic, affinity), and the fractions were selected on the basis of their ability to phosphorylate and activate MARK2. A detailed description is given in the Supplementary data.
Labeling of proteins with FSBA
Proteins were transferred into PBS using a FastDesalting™ column (Pharmacia) and incubated overnight with FSBA (5′-p-fluorosulfonylbenzoyl adenosine, ATP-Binding Protein Detection Kit, Boehringer Mannheim) at a final concentration of 1 mM. The reaction was stopped with 5 mM β-mercaptoethanol at RT for 1 h and dialysed against 50 mM Tris–HCl pH 7.5, 200 mM NaCl, 1 mM benzamidine, 1 mM β-mercaptoethanol, 1 mM PMSF, 50% glycerol.
Kinase assay
The activity of MARK2 was assayed as described (Drewes et al., 1997), using a substrate peptide from the first repeat of tau containing S262 in the KXGS motif (TR1-peptide NVKSKIGSTENLK, for details see Supplementary data).
cDNA Cloning
The sequences obtained from peptide analysis were used for data base screening. Seventeen of the tryptic peptides were found to be part of a kinase named TAO1 (Thousand And One amino acids, DDBJ/EMBL/Genbank accession no. AF084205) of rat brain origin. The cDNA coding for this kinase was amplified with PCR (details in Supplementary data).
MARK, MARKK preparation
The preparation of the kinases was performed in analogy with Drewes et al. (1997); for details see Supplementary data.
Coimmunoprecipitation
MARK2 and MARKK expressed in Sf9 cells were coimmunoprecipitated by standard procedures (details in Supplementary data).
Antibodies
Rabbit antisera were raised against the N-terminal peptide of MARK, the lip peptide of MARK phosphorylated at T208, or phosphorylated at S212 (pT208-lip antibody and pS212-lip antibody). Secondary antibody for blotting was HRP-conjugated goat anti-rabbit (Dianova). Antibody 12E8 against phosphorylated S262 of tau was a gift of P.Seubert (Elan Pharma). Rat monoclonal antibody YL1/2 against tubulin was from Serotec, antibody against MARKK/TAO-1 was from BD Biosciences (Palo Alto, CA), anti-β-actin was from Sigma, and rabbit polyclonal antibody against FSBA was from Boehringer Mannheim.
Cell culture and immunofluorescence
Cell culture and immunofluorescence were performed with CHO wt, stably transfected with hTau40, and PC12 cells following the standard protocols (details in Supplementary data).
RNAi preparation and transfections
21-nucleotide RNAs were designed as recommended (Elbashir et al., 2001) and chemically synthesized by Qiagen-Science (Germantown, MD). The RNAi targeting sequences of MARKK/TAO-1 corresponded to the coding regions 182–202 and 1699–1719. Transfections of RNAi for endogenous MARKK/TAO1 gene silencing were carried out with Oligofectamine (Invitrogen) using simultaneously both RNAi complexes at final concentrations of 100 nM. 24 h after transfection, cells were differentiated for 24–48 h with 100 ng/ml NGF before IF analysis or western blots were performed. Neurite extension was quantified by analyzing three experiments with 200 cells each.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Matthias Bilang and Alice Grabbe for expert help with protein preparations and cell culture. This project was supported by the Deutsche Forschungsgemeinschaft.
References
- Anostario M., Harrison,M.L. and Geahlen,R.L. (1990) Immunochemical detection of adenine nucleotide-binding proteins with antibodies to 5′-p-fluorosulfonylbenzoyladenosine. Analyt. Biochem., 190, 60–65. [DOI] [PubMed] [Google Scholar]
- Benton R., Palacios,I. and St Johnston,D. (2002) Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev. Cell, 3, 659–671. [DOI] [PubMed] [Google Scholar]
- Biernat J. and Mandelkow,E.-M. (1999) The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol. Biol. Cell, 10, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J., Gustke,N., Drewes,G., Mandelkow,E.-M. and Mandelkow,E. (1993) Phosphorylation of Ser 262 strongly reduces the binding of tau protein to microtubules. Neuron, 11, 153–163. [DOI] [PubMed] [Google Scholar]
- Biernat J., Wu,Y.Z., Timm,T., Zheng-Fischhöfer,Q., Mandelkow,E., Meijer,L. and Mandelkow,E.-M. (2002) Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol. Biol. Cell, 13, 4013–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm H., Brinkmann,V., Drab,M., Henske,A. and Kurzchalia,T.V. (1997) Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr. Biol., 7, 603–606. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., van der Geer,P. and Hunter,T. (1991). Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin layer cellulose plates. Methods Enzymol., 201, 110–149. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Hutchings,C., Burke,J.F. and Mayne,L.V. (1999) Application of a rapid method (targeted display) for the identification of differentially expressed mRNAs following NGF-induced neuronal differentiation in PC12 cells. Mol. Cell. Neurosci., 13, 119–130. [DOI] [PubMed] [Google Scholar]
- Cha B.-J., Serbus,L., Koppetsch,B. and Theurkauf,W.E. (2002) Kinesin-I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat. Cell Biol., 4, 592–598. [DOI] [PubMed] [Google Scholar]
- Chen Z., Hutchison,M. and Cobb,M.H. (1999) Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J. Biol. Chem., 274, 28803–28807. [DOI] [PubMed] [Google Scholar]
- Chen Z., Gibson,T.B., Robinson,F., Silvestro,L., Pearson,G., Xu Be,B.E., Wright,A., Vanderbilt,C. and Cobb,M.H. (2001) MAP Kinases. Chem. Rev., 101, 2449–2476. [DOI] [PubMed] [Google Scholar]
- Chen Z., Raman,M., Chen,L., Lee,S., Gilman,A. and Cobb,M. (2003) TAOs (thousand-and-one amino acid) protein kinases mediate signaling from carbachol to p38 MAP kinase and ternary complex factors. J. Biol. Chem., 278, 22278–22283. [DOI] [PubMed] [Google Scholar]
- Cox D.N., Lu,B., Sun,T.Q., Williams,L.T. and Jan,Y.N. (2001) Drosophila par-1 is required for oocyte differentiation and microtubule organization. Curr. Biol., 11, 75–87. [DOI] [PubMed] [Google Scholar]
- Dan I., Watanabe,N.M. and Kusumi,A. (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol., 11, 220–230. [DOI] [PubMed] [Google Scholar]
- Drewes G., Ebneth,A., Preuss,U., Mandelkow,E.-M. and Mandelkow,E. (1997) MARK—a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell, 89, 297–308. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- Ge B., Gram,H., Di Padova,F., Huang,B., New,L., Ulevitch,R.J., Luo,Y. and Han,J. (2002) MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science, 295, 1291–1294. [DOI] [PubMed] [Google Scholar]
- Gevaert K., Demol,H., Martens,L., Hoorelbeke,B., Puype,M., Goethals,M., Van Damme,J., De Boeck,S. and Vandekerckhove,J. (2001) Protein identification based on matrix assisted laser desorption/ionization-post source decay-mass spectrometry. Electrophoresis, 22, 1645–1651. [DOI] [PubMed] [Google Scholar]
- Guo S. and Kemphues,K.J. (1995) Par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell, 81, 611–620. [DOI] [PubMed] [Google Scholar]
- Hanks S.K. and Hunter,T. (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase catalytic domain structure and classification. FASEB J., 9, 576–596. [PubMed] [Google Scholar]
- Hutchison M., Berman,K.S. and Cobb,M.H. (1998) Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem., 273, 28625–28632. [DOI] [PubMed] [Google Scholar]
- Jenkins S.M. and Johnson,G.V. (2000) Microtubule/MAP-affinity regulating kinase (MARK) is activated by phenylarsine oxide in situ and phosphorylates tau within its microtubule-binding domain. J. Neurochem., 74, 1463–1468. [DOI] [PubMed] [Google Scholar]
- Johnson L.N., Noble,M.E.M. and Owen,D.J. (1996) Active and inactive protein kinases: Structural basis for regulation. Cell, 85, 149–158. [DOI] [PubMed] [Google Scholar]
- Manning G., Whyte,D.B., Martinez,R., Hunter,T. and Sudarsanam,S. (2002) The protein kinase complement of the human genome. Science, 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- Müller J., Ory,S., Copeland,T., Piwnica-Worms,H. and Morrison,D.K. (2001) C-TAK1/MARK3 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell, 8, 983–993. [DOI] [PubMed] [Google Scholar]
- Riechmann V., Gutierrez,G.J., Filardo,P., Nebreda,A.R. and Ephrussi,A. (2002) Par-1 regulates stability of the posterior determinant Oskar by phosphorylation. Nat. Cell Biol., 4, 337–342. [DOI] [PubMed] [Google Scholar]
- Scott J.W., Norman,D.G., Hawley,S.A., Kontogiannis,L. and Hardie,D.G. (2002) Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J. Mol. Biol., 317, 309–323. [DOI] [PubMed] [Google Scholar]
- Shulman J.M., Benton,R. and St Johnston,D. (2000) The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell, 101, 377–388. [DOI] [PubMed] [Google Scholar]
- Sun T.Q., Lu,B., Feng,J.J., Reinhard,C., Jan,Y.N., Fantl,W.J. and Williams,L.T. (2001) PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol., 3, 628–636. [DOI] [PubMed] [Google Scholar]
- Tassi E., Biesova,Z., Di Fiore,P.P., Gutkind,J.S. and Wong,W.T. (1999) Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor. J. Biol. Chem., 274, 33287–33295. [DOI] [PubMed] [Google Scholar]
- Tomancak P., Piano,F., Riechmann,V., Gunsalus,K.C., Kemphues,K.J. and Ephrussi,A. (2000) A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat. Cell Biol., 2, 458–460. [DOI] [PubMed] [Google Scholar]