Abstract
The development of an effective amebiasis vaccine could improve child health in the developing world, reducing cases of amebic colitis and liver abscess. An ideal vaccine would be comprised of a well-characterized parasite antigen and an adjuvant, which would have high potency while driving the immune response in a Th1 direction. This study describes a mucosal vaccine composed of the Entamoeba histolytica galactose/N-acetyl-d-galactosamine-inhibitable lectin (Gal-lectin) and CpG oligodeoxynucleotides (CpG-ODN). The Gal-lectin is a protein involved in parasite virulence and adherence and is known to activate immune cells, while CpG-ODN are known to be potent inducers of type 1-like immune responses. We demonstrated that intranasal administration of the vaccine resulted in strong Gal-lectin-specific Th1 responses and humoral responses. Vaccination induced the production of Gal-lectin-specific T cells and the production of the proinflammatory cytokine gamma interferon. Vaccinated animals had detectable serum anti-Gal-lectin immunoglobulin G (IgG) and stool anti-Gal-lectin IgA capable of blocking parasite adherence to target cells in vitro. One week after immunization, gerbils were challenged intrahepatically with live trophozoites. Vaccinated gerbils had no detectable abscesses after day 5, whereas control gerbils developed larger abscesses. These results show that mucosal vaccination with Gal-lectin and CpG-ODN can induce both systemic and humoral immune responses.
Entamoeba histolytica is the enteric protozoan parasite which causes amoebic colitis and liver abscess in humans. This parasite is estimated to infect 8% of the world's population, leading to 50,000 deaths annually (36). Amebic colitis is the most common clinical manifestation of disease as the parasite must first colonize the colon. Intestinal infection by E. histolytica and progression to disease could theoretically be prevented by vaccine intervention targeted to block parasite colonization (26, 35).
E. histolytica trophozoites are able to colonize the human intestine by adhering to colonic mucins and subsequently to epithelial cells via a cell surface lectin (3). This galactose/N-acetyl-d-galactosamine-inhibitable lectin (Gal-lectin) is a heterodimer containing disulfide-linked light and heavy subunits, the latter of which has high binding specificity for galactose and N-acetyl-d-galactosamine residues (29). The Gal-lectin is a prime vaccine candidate as it is an immunogenic molecule able to induce protection against amebic liver abscess (ALA) in rodent models of the disease (16, 27, 30). Recently, it has also been reported that there is a correlation between the presence of anti-lectin fecal immunoglobulin A (IgA) antibodies and protection from parasite colonization in humans (12, 13). Mucosal immunity against the parasite seems to be required to prevent infection, and there is substantial evidence suggesting that a colonization-blocking vaccine targeting the parasite Gal-lectin could prevent trophozoite adherence and thus provide protection against subsequent invasive disease.
The development of mucosal vaccines for use in humans has been hindered by the lack of safe yet effective mucosal adjuvants. The “gold standard” mucosal adjuvants in animals are bacterial toxins, such as cholera toxin; however, these adjuvants are too toxic for use in humans. A novel class of adjuvants is the CpG oligodeoxynucleotides (CpG-ODN), synthetic oligodeoxynucleotides containing immunostimulatory CpG motifs. These motifs are recognized by the innate immune system via Toll-like receptor 9 (TLR9) and can induce broad adjuvant effects, such as the direct activation of B cells, macrophages, and dendritic cells, as well as interleukin-6 (IL-6) and IL-12 cytokine secretion (1, 5, 22). CpG-ODN contain a nuclease-resistant phosphorothioate backbone, which can be coadministered with the vaccine antigen to induce specific immunity. It has been demonstrated that CpG-ODN are safe and effective adjuvants for both parenteral and mucosal vaccine administration (4, 6, 8). Recent studies have reported the ability of CpG-ODN to induce both systemic and humoral immunity upon mucosal application (8). CpG motifs have Th1-biased immune effects due to TLR9 signaling, which can be used to augment cell-mediated immunity. Here we evaluated CpG-ODN with intranasal delivery of purified E. histolytica Gal-lectin. We utilized the gerbil model of ALA and the C3H mouse model for amebic colitis. We found that vaccination induced both systemic and mucosal immunity against the Gal-lectin and prevented disease in the ALA model.
MATERIALS AND METHODS
Animals.
Male Mongolian gerbils (Meriones unguiculatus) that were 6 to 9 weeks old were purchased from Charles River (St. Constant, Canada). Female C3H/Hej mice that were 3 to 5 weeks old were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained in pathogen-free facilities at the University of Calgary. The University of Calgary Animal Care Committee approved all animal protocols for this study.
Parasites and vaccine antigens.
E. histolytica strain HM1:IMSS trophozoites were grown in axenic culture in TYI-S-33 medium. Parasites were grown for 72 h (log phase) for use in all experiments. The native Gal-lectin was purified from log-phase amebae on an immunoaffinity column as previously described (28). CpG-ODN 10103 (TCG TCG TTT CGT CGT TTT GTC GTT) with a full phosphorothioate backbone was purchased from Coley Pharmaceutical Group (Kanata, Canada).
Vaccinations and challenge infections.
Gerbils and C3H mice were immunized with an intranasal and intraperitoneal regimen over a 5-week period. Intranasal immunizations were given in weeks 1,3, and 5 to animals under light isoflurane anesthesia. The vaccine consisted of 10 μg of Gal-lectin and 20 μg of CpG-ODN administered to the nares in 20 μl of phosphate-buffered saline (PBS). Intraperitoneal injections containing 10 μg of Gal-lectin and 50 μg of CpG-ODN in 200 μl of PBS were delivered along with the intranasal immunization in week 5. Control animals received intranasal delivery of only 20 μg CpG-ODN in PBS and intraperitoneal delivery of 50 μg CpG-ODN in PBS. One week following the last immunization, gerbils were anesthetized with isoflurane and challenged via intrahepatic injection of 106 E. histolytica trophozoites into the left liver lobe as previously described (2). Gerbils were sacrificed postchallenge (days 2, 5, 10, and 15), and their spleens and sera were collected. Livers were excised, and the ALA weight was measured. Prechallenge samples of serum, spleen, and mesenteric lymph nodes (MLN) were taken from gerbils and C3H mice.
Immunoblotting.
Sera from vaccinated or control animals were tested for anti-Gal-lectin-specific IgG. The native Gal-lectin was run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto a nitrocellulose membrane. Membranes were probed either with 1:250 pooled sera from vaccinated or control animals or with the 1G7 anti-Gal-lectin monoclonal antibody (MAb). The blots were washed with Tween—Tris-buffered saline and incubated with 1:10,000 anti-gerbil horseradish peroxidase-conjugated antibody (Immunology Consultants Laboratory Inc.) or with 1:3,000 anti-mouse IgG (Amersham). Bound serum IgG antibodies were visualized using enhanced chemiluminescence (ECL Plus reagent; Amersham). Stool antigens and antibodies were isolated from fresh pellets dissolved in PBS with protease inhibitors (Roche) using 1 ml per 0.1-g pellet. Lectin-specific IgA antibodies in the stools were detected by immunoblotting against the native Gal-lectin with goat anti-mouse IgA antibodies (Sigma).
CHO cell adherence assay.
As previously described (23), serum and stool antibodies against the Gal-lectin were used in an adherence-blocking assay. Briefly, log-phase amebae were preincubated with a 1:100 dilution of prechallenge sera or stool lysate for 1 h at 4°C. Chinese hamster ovary (CHO) cells were added to the amoebae, and the tubes were centrifuged and incubated on ice for 2 h undisturbed. Amebic adherence was determined by light microscopy by identifying positive rosette formation (amoebae with three or more CHO cells attached). The percent rosette formation was determined relative to the positive control for adherence consisting of amoebae and CHO cells without inhibitory serum.
Intracellular cytokine staining.
MLN cells were harvested from control and vaccinated C3H mice 5 days after the last immunization. Cells were cultured at a concentration of 2 × 106 cells/ml and stimulated with 10 μg/ml Gal-lectin, 1 μg/ml ionomycin, and 20 μg/ml brefeldin A (Sigma) for 6 h. Cells were washed in PBS, resuspended at a concentration of 1 × 106 cells/ml in PBS, and stained with phycoerythrin-conjugated anti-CD4 antibody (BD Pharmingen) for 30 min at 4°C. Cells were then washed three times, fixed using a Fix & Perm cell permeabilization kit (Caltag Laboratories), and stained with either fluorescein isothiocyanate-conjugated anti-mouse IL-4 MAb or anti-mouse gamma interferon (IFN-γ) MAb for 30 min at 4°C. Cells were washed and analyzed by using FACScan cytometry and Cell Quest software.
Real-time PCR.
MLN cells were harvested from immunized or control animals and pelleted by centrifugation at 12,000 × g for 5 min at 4°C. The supernatants were decanted, and the pellets were vortexed to resuspend the cells. mRNA was extracted with Trizol (Invitrogen) used according to the manufacturer's instructions. cDNA was generated from 2.5 μg mRNA using random hexamer primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Gerbil cytokines were analyzed with Taqman probes as previously described (17), and mouse cytokines were measured using SYBR green reagent and mouse-specific primers (10). Gerbil cytokine expression was normalized to 18S rRNA, and mouse cytokine expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All real-time PCR experiments were performed using a Rotor Gene 3000 (Corbett Research), and results were analyzed using the 2−ΔΔCT method (24).
Lymphoproliferation assay.
MLN cells were harvested and grown at a concentration of 5 × 10 5 cells/well in a 96-well plate. Cells were stimulated with either RPMI medium alone, concanavalin A (ConA) (2.5 μg/ml), Gal-lectin (10 μg/ml), or amebic proteins (20 μg/ml) for 72 h at 37°C. To measure proliferation, [methyl-3H]thymidine (1 μCi/well) was added for the last 18 h of incubation. Cells were harvested onto filter paper, and [methyl-3H]thymidine incorporation was measured by scintillation counting. Lymphoproliferation was expressed as a stimulation index indicating the fold increase in proliferation compared with the proliferation of cells receiving medium alone.
Statistical analysis.
All animal experiments were repeated at least twice with similar results. Results are expressed as means ± standard errors of the means of triplicate experiments. Data were analyzed using one-way analysis of variance or paired-sample t tests. A P value of <0.05 was considered significant for all tests.
RESULTS
Intranasal vaccination with CpG-ODN and Gal-lectin generates adherence-blocking antibodies.
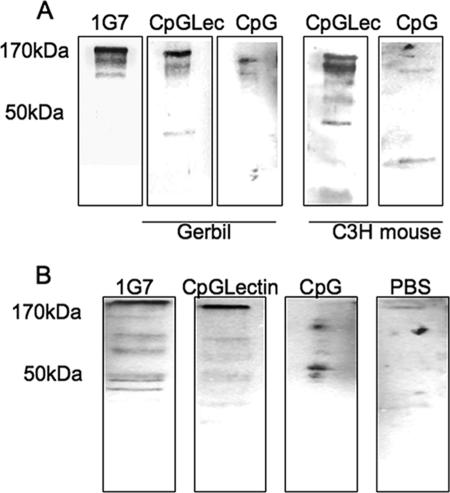
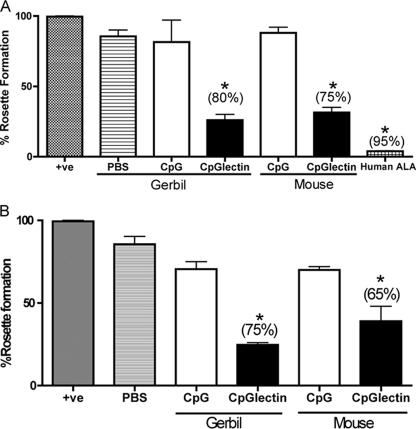
Prechallenge serum was collected from immunized and control gerbils and C3H mice and tested for the presence of Gal-lectin-specific antibodies. Native Gal-lectin was run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to a nitrocellulose membrane, and probed with serum (1:250) in a Western blot. Blots with sera from gerbils and mice immunized with CpG-ODN and Gal-lectin had a strong band at 170 kDa corresponding to the Gal-lectin heavy subunit (Fig. 1A). This band was also present in the blot with MAb 1G7 against the Gal-lectin, whereas serum from CpG-ODN control animals did not recognize the Gal-lectin. In order to test the ability of serum antibodies to block Gal-lectin-mediated parasite adherence, we performed a CHO cell adherence assay. Anti-Gal-lectin antibodies in the sera of vaccinated gerbils and C3H mice were capable of significantly (P < 0.05) inhibiting parasite adherence by 80 and 75%, respectively (Fig. 2B). An adherence assay was also performed with stool antibodies, and similar inhibition was observed. Gerbils and C3H mice vaccinated with CpG-ODN plus Gal-lectin had stool antibody titers capable of blocking parasite adherence by 75 and 65%, respectively (Fig. 2B). Stool and serum preparations from PBS-vaccinated and CpG-ODN control animals did not significantly inhibit parasite adherence. To verify the presence of anti-Gal-lectin IgA antibodies in the stool preparations, the native Gal-lectin protein was probed in a Western blot with a stool preparation (1:250) and detected with anti-mouse IgA antibody. A strong band at 170 kDa appeared in the blots for vaccinated animals but not in the blots for control animals (Fig. 1B). These results suggested that vaccination induced the production of both Gal-lectin-specific serum IgG and mucosal IgA antibodies.
FIG. 1.
(A) Immunoblotting with immune or control gerbil and C3H mouse sera against purified Gal-lectin. Gal-lectin-specific serum IgG (1:250) from immunized gerbils recognizes the 170-kDa heavy-subunit band. (B) Stool IgA from immunized C3H mice recognizes the Gal-lectin. The Gal-lectin was probed with stool antigen preparations (1:250) and detected with anti-mouse IgA antibody. The data shown are representative of three independent experiments. CpGLec and CpGLectin, CpG-ODN plus Gal-lectin; CpG, CpG-ODN.
FIG. 2.
(A) Inhibition of amebic adherence to target CHO cells by immune gerbil and C3H mouse sera. Amebae were preincubated with 1:100 immune or control serum dilutions, and subsequent amebic adherence to CHO cells was determined by an adherence assay. CpG-ODN-Gal-lectin immune serum significantly inhibited amebic adherence (asterisk, P < 0.05). (B) Inhibition of amebic adherence to CHO cells by gerbil and C3H mouse stool IgA. Compared to controls, immunized animals had Gal-lectin-specific stool IgA capable of significantly blocking parasite adherence (asterisk, P < 0.05). The data shown are from triplicates of three independent experiments. The bars indicate the percent amebic adherence to target cells. The values in parentheses are the percentages of inhibition. +ve, positive control; CpGlectin, CpG-ODN plus Gal-lectin; CpG, CpG-ODN.
CpG-ODN and Gal-lectin vaccination induces a cell-mediated immune response.
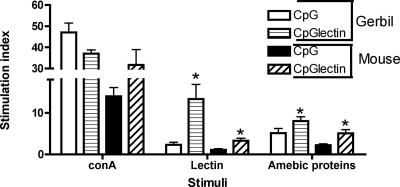
We evaluated the cellular immune response in both gerbils and C3H mice 30 days after the first immunization. Cell proliferation was measured by determining [methyl-3H]thymidine incorporation in spleen lymphocytes cultured in the presence of medium, ConA, native Gal-lectin, and whole amebic proteins. Animals immunized with CpG-ODN and Gal-lectin had a greater proliferation response to amebic Gal-lectin (P < 0.05) and amebic proteins than control animals (Fig. 3). Proliferation responses were greater in gerbils than in mice, but both cell types demonstrated viability with strong proliferation in response to the T-cell mitogen ConA.
FIG. 3.
Lymphoproliferation of gerbil and C3H mouse splenocytes in response to Gal-lectin stimulation. Immunized or control spleens were collected before challenge infection, and cells were restimulated in vitro for 72 h with either ConA (2.5 μg), Gal-lectin (10 μg), or 50 μg of soluble amebic proteins. Proliferation is expressed as a stimulation index (cpm of cells with antigen/cpm of cells without antigen). The asterisks indicate that there was significantly higher proliferation in response to amebic antigen or Gal-lectin than in the matched controls (P < 0.05). CpG, CpG-ODN; CpGlectin, CpG-ODN plus Gal-lectin.
Induction of Th1 cytokines.
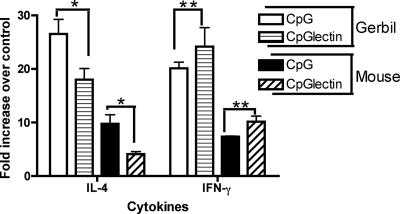
Five days after the last immunization, animals were sacrificed and MLN were collected. Cell suspensions were prepared from the MLN and restimulated in vitro with medium or native Gal-lectin. Cytokine mRNA levels were measured by real-time PCR for gerbils and C3H mice using species-specific primers and probes. Compared to cells stimulated with medium alone, vaccinated animals showed an increase in IFN-γ mRNA (P < 0.05) when they were stimulated with Gal-lectin (Fig. 4). Control animals did not demonstrate a specific response, having similar levels of IL-4 and IFN-γ mRNA. Control animals, however, had higher levels of IL-4 than vaccinated animals (P < 0.05).
FIG. 4.
Real-time PCR analysis of MLN cytokine gene expression. Gene expression was normalized to housekeeping genes and expressed as the fold increase compared with normal nontreated mRNA. There were substantially higher IFN-γ levels in the vaccinated animals (two asterisks, P < 0.05), whereas IL-4 was more prevalent in CpG-ODN control animals (one asterisk, P < 0.05). The data are the means ± standard errors of the means of the results from three independent PCRs. CpG, CpG-ODN; CpGlectin, CpG-ODN plus Gal-lectin.
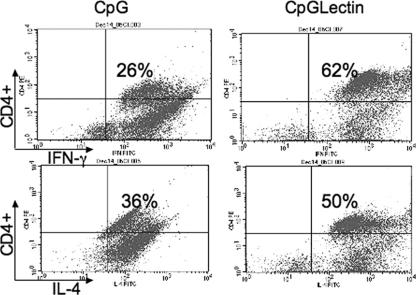
To evaluate the production of Th1 cytokines in vaccinated animals, we performed intracellular staining with restimulated MLN cells from C3H mice. Cells were stained for CD4+ T cells and either IL-4 or IFN-γ protein and analyzed with a FACScan. Consistent with the results of real-time PCR, vaccinated animals had more (P < 0.05) IFN-γ-producing CD4 T cells (62%) than animals receiving only CpG-ODN (26%). The Th2 cytokine IL-4 was detected in both groups, but the level was not significantly higher in vaccinated animals (Fig. 5). These data clearly suggest that mucosal immunization with CpG-ODN and Gal-lectin specifically induces IFN-γ production.
FIG. 5.
Intracellular cytokine staining in MLN cells. Control and vaccinated C3H mice (three mice per group) were sacrificed after the last immunization, and MLN cells were restimulated in vitro with Gal-lectin. Cells were stained for CD4+ T cells and either IFN-γ or IL-4. The values in the quadrants indicate the percentages of cells positive for CD4+ and the cytokine. The data show the results obtained from one mouse from each group and are representative of the whole group. Similar results were obtained in two separate experiments. CpG, CpG-ODN; CpGLectin, CpG-ODN plus Gal-lectin.
Mucosal immunization protects against intrahepatic challenge infection.
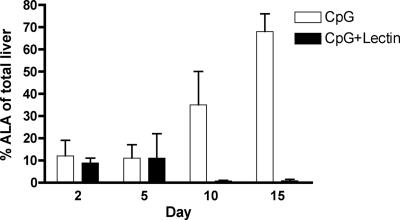
Next, we evaluated the protective effects of mucosal vaccination with CpG-ODN and Gal-lectin on ALA development. Five days after the last immunization, gerbils were challenged by direct intrahepatic inoculation of virulent trophozoites. Gerbils were sacrificed on different days postchallenge (days 2, 5, 10, and 15), and their livers were excised and examined for ALA formation. At day 2 postchallenge there were no detectable differences in ALA size between vaccinated and control animals (Fig. 6). However, at 5 days postchallenge no abscesses could be observed in vaccinated gerbils, while CpG-ODN control animals developed larger abscesses and exhibited greater hepatic damage. These results indicate that intranasal administration of CpG-ODN and Gal-lectin is sufficient to protect animals against ALA formation.
FIG. 6.
Progression of ALA formation. Gerbils were sacrificed on days 2, 5, 10, and 15 postchallenge, and their livers were examined for ALA formation. Abscess size is expressed as a percentage of the total liver weight. Gerbils vaccinated with CpG-ODN plus Gal-lectin had no detectable abscesses after day 5 postchallenge (n = 6 animals per group per day postchallenge). CpG, CpG-ODN; CpG+Lectin, CpG-ODN plus Gal-lectin.
DISCUSSION
In this work we studied the use of CpG-ODN as adjuvant in a mucosal Gal-lectin-based vaccine against E. histolytica. Here we report safe and effective use of a combination of CpG-ODN with native Gal-lectin to induce protective immunity in animal models of amebiasis. Intranasal immunization with this vaccine induced both anti-Gal-lectin IgG and IgA, both of which are capable of blocking parasite adherence in vitro. Cellular immunity was mediated by an IFN-γ response, and immunization protected against systemic challenge infection in gerbils.
Several properties of CpG-ODN and the Gal-lectin protein contribute to the efficacy of this vaccine. First, CpG-ODN are recognized by TLR9 and are known to function as a powerful adjuvant by activating antigen-presenting cells and B cells. CpG-ODN have been shown to induce B-cell proliferation in a non-antigen-specific way and to synergize with B-cell signaling through the B-cell antigen receptor (22, 31). Specially designed oligodeoxynucleotides can elicit both innate and acquired immune responses predominantly of the Th1 type, while potentiating the specific immune response to a coinjected antigen. Since the type of immune response induced by a vaccine is crucial to its efficacy, using CpG-ODN adjuvant can direct the response generated towards Th1. However, the hallmark of mucosal vaccination is the production of secretory IgA antibodies, which can be synthesized in the context of Th1 or Th2 milieus (25). In this study we report the production of both anti-Gal-lectin IgG and IgA following intranasal vaccination with CpG-ODN adjuvant. Second, the Gal-lectin antigen is also a potent immunogen capable of activating dendritic cells and macrophages (18, 33, 34). The Gal-lectin is known to induce Th1 cytokine production in immune cells and can induce protective immunity in animal models of amebiasis (16, 27, 30). Not only is the Gal-lectin immunogenic, but it is also the major surface lectin of the parasite essential for biological processes, including colonization, cytotoxicity, and complement resistance. Combining the effectiveness of CpG-ODN and Gal-lectin to activate innate immune cells and the antigenicity of the Gal-lectin resulted in a potent vaccine.
We have previously shown that CpG-ODN and the Gal-lectin can protect against amebic liver challenge infection when they are administered parenterally (17). In general, parenteral injections induce systemic immunity, whereas mucosal immunizations can induce mucosal immune responses at local and distant sites, as well as systemic immunity (9, 11). The majority of infectious diseases, including that caused by E. histolytica, are acquired through mucosal surfaces; therefore, mucosal vaccination against the parasite could theoretically prevent colonization and protect against invasive disease. Here we report the production of mucosal IgA and systemic immunity after intranasal administration of a vaccine containing CpG-ODN and Gal-lectin. Stool anti-Gal-lectin IgA was detected in immunized mice and could effectively block parasite adherence to target cells in vitro. Gerbils were protected from systemic infection with live trophozoites, demonstrating that an effective systemic response was produced after mucosal vaccination. Although we did not correlate anti-Gal-lectin IgA with protection in the C3H mouse model in this study, it has been reported that acquired resistance to infection in humans is linked to intestinal IgA against the carbohydrate recognition domain of the Gal-lectin (14). Protection could be correlated, however, with elevated levels of IFN-γ and high proliferative responses. IFN-γ is a signature Th1 cytokine and has been shown to stimulate immune cell amebicidal activity in vitro (7, 32). In fact, recent reports from a study in Bangladesh correlated higher IFN-γ production with reduced risk of amebic disease (15). Antigen-specific IFN-γ, as well as local release of IFN-γ in both mice and gerbils, could account for the protective responses against amoebic challenge infection.
In summary, the production of a Th1-type immune response characterized by IFN-γ production and a humoral response characterized by IgA antibodies is a consequence of a stimulating CpG-ODN adjuvant and a potent vaccine antigen. The adjuvant used in this vaccine formulation is an attractive candidate for potential use in a human vaccine against E. histolytica. Intranasal immunization represents a noninvasive, fast, and easy method to induce both humoral and systemic immunity in laboratory animals. In humans, however, nasal immunizations seem to result in antibody responses in the upper airway mucosa but not in intestinal responses (19, 20). At present, it is difficult to predict the efficacy of vaccines in protecting against amebiasis in humans, as there is a lack of adequate animal models and E. histolytica has been reported to be able to degrade human IgA in vitro (21). Future studies should therefore examine the efficacy of this vaccine in an intestinal amebiasis model and should elucidate the specific role of anti-Gal-lectin IgA in protection.
Acknowledgments
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. C. Ivory is a recipient of a Ph.D. scholarship from McGill University.
We thank Bill Petri from the University of Virginia for providing the immunoaffinity column for Gal-lectin purification and Elaine deHeuvel for her assistance with animal handling.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Askew, D., R. S. Chu, A. M. Krieg, and C. V. Harding. 2000. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 165:6889-6895. [DOI] [PubMed] [Google Scholar]
- 2.Chadee, K., and E. Meerovitch. 1984. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus). Am. J. Pathol. 117:71-80. [PMC free article] [PubMed] [Google Scholar]
- 3.Chadee, K., W. A. Petri, Jr., D. J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J. Clin. Investig. 80:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, C. L., H. L. Davis, M. L. Morris, S. M. Efler, A. M. Krieg, Y. Li, C. Laframboise, M. J. Al Adhami, Y. Khaliq, I. Seguin, and D. W. Cameron. 2004. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 22:3136-3143. [DOI] [PubMed] [Google Scholar]
- 5.Cornelie, S., J. Hoebeke, A. M. Schacht, B. Bertin, J. Vicogne, M. Capron, and G. Riveau. 2004. Direct evidence that Toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognition. J. Biol. Chem. 279:15124-15129. [DOI] [PubMed] [Google Scholar]
- 6.Corral, R. S., and P. B. Petray. 2000. CpG DNA as a Th1-promoting adjuvant in immunization against Trypanosoma cruzi. Vaccine 19:234-242. [DOI] [PubMed] [Google Scholar]
- 7.Denis, M., and K. Chadee. 1988. In vitro and in vivo studies of macrophage functions in amebiasis. Infect. Immun. 56:3126-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 9.Gallichan, W. S., and K. L. Rosenthal. 1995. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine 13:1589-1595. [DOI] [PubMed] [Google Scholar]
- 10.Giulietti, A., L. Overbergh, D. Valckx, B. Decallonne, R. Bouillon, and C. Mathieu. 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386-401. [DOI] [PubMed] [Google Scholar]
- 11.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 62:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 13.Haque, R., P. Duggal, I. M. Ali, M. B. Hossain, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed] [Google Scholar]
- 14.Haque, R., D. Mondal, P. Duggal, M. Kabir, S. Roy, B. M. Farr, R. B. Sack, and W. A. Petri, Jr. 2006. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect. Immun. 74:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haque, R., D. Mondal, J. Shu, S. Roy, M. Kabir, A. N. Davis, P. Duggal, and W. A. Petri, Jr. 2007. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am. J. Trop. Med. Hyg. 76:340-344. [PubMed] [Google Scholar]
- 16.Houpt, E., L. Barroso, L. Lockhart, R. Wright, C. Cramer, D. Lyerly, and W. A. Petri. 2004. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22:611-617. [DOI] [PubMed] [Google Scholar]
- 17.Ivory, C. P., K. Keller, and K. Chadee. 2006. CpG-oligodeoxynucleotide is a potent adjuvant with an Entamoeba histolytica Gal-inhibitable lectin vaccine against amoebic liver abscess in gerbils. Infect. Immun. 74:528-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivory, C. P., and K. Chadee. 2007. Activation of dendritic cells by the Gal-lectin of Entamoeba histolytica drives Th1 responses in vitro and in vivo. Eur. J. Immunol. 37:385-394. [DOI] [PubMed] [Google Scholar]
- 19.Johansson, E. L., L. Wassen, J. Holmgren, M. Jertborn, and A. Rudin. 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson, E. L., C. Bergquist, A. Edebo, C. Johansson, and A. M. Svennerholm. 2004. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine 22:984-990. [DOI] [PubMed] [Google Scholar]
- 21.Kelsall, B. L., and J. I. Ravdin. 1993. Degradation of human IgA by Entamoeba histolytica. J. Infect. Dis. 168:1319-1322. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 23.Li, E., A. Becker, and S. L. Stanley, Jr. 1989. Chinese hamster ovary cells deficient in N-acetylglucosaminyltransferase I activity are resistant to Entamoeba histolytica-mediated cytotoxicity. Infect. Immun. 57:8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 25.McCluskie, M. J., R. D. Weeratna, P. J. Payette, and H. L. Davis. 2001. The potential of CpG oligodeoxynucleotides as mucosal adjuvants. Crit. Rev. Immunol. 21:103-120. [PubMed] [Google Scholar]
- 26.Miller-Sims, V. C., and W. A. Petri, Jr. 2002. Opportunities and obstacles in developing a vaccine for Entamoeba histolytica. Curr. Opin. Immunol. 14:549-552. [DOI] [PubMed] [Google Scholar]
- 27.Petri, W. A., Jr., and J. I. Ravdin. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect. Immun. 59:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin, J. I., C. F. Murphy, R. A. Salata, R. L. Guerrant, and E. L. Hewlett. 1985. N-Acetyl-d-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J Infect. Dis. 151:804-815. [DOI] [PubMed] [Google Scholar]
- 29.Ravdin, J. I., and C. F. Murphy. 1992. Characterization of the galactose-specific binding activity of a purified soluble Entamoeba histolytica adherence lectin. J. Protozool. 39:319-323. [DOI] [PubMed] [Google Scholar]
- 30.Ravdin, J. I., D. C. Shain, and B. L. Kelsall. 1993. Antigenicity, immunogenicity and vaccine efficacy of the galactose-specific adherence protein of Entamoeba histolytica. Vaccine 11:241-246. [DOI] [PubMed] [Google Scholar]
- 31.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 32.Schain, D. C., R. A. Salata, and J. I. Ravdin. 1992. Human T-lymphocyte proliferation, lymphokine production, and amebicidal activity elicited by the galactose-inhibitable adherence protein of Entamoeba histolytica. Infect. Immun. 60:2143-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Séguin, R., B. J. Mann, K. Keller, and K. Chadee. 1995. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc. Natl. Acad. Sci. USA 92:12175-12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Séguin, R., B. J. Mann, K. Keller, and K. Chadee. 1997. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect. Immun. 65:2522-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley, S. L., Jr. 1997. Progress towards development of a vaccine for amebiasis. Clin. Microbiol. Rev. 10:637-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 1997. Amoebiasis. WHO Wkly. Epidemiol. Rec. 72:97-99. [Google Scholar]