Abstract
Autophagy has been implicated in the intracellular destruction of Toxoplasma gondii by primed macrophages following gamma interferon (IFN-γ) activation of p47 GTPases. CD40 ligation has also been shown to trigger autophagic elimination of T. gondii independent of IFN-γ and p47 GTPases. Here we demonstrate that IFN-γ/p47 GTPase-dependent elimination of T. gondii by strain CPS vaccine-primed macrophages is independent of CD40/tumor necrosis factor signaling. Similar to wild-type controls, both CD40-deficient and tumor necrosis factor receptor 1/2 (TNFR1/2)-deficient macrophages can efficiently eliminate invaded strain GFP-PTG and restrain its replication following priming. In contrast, macrophages from mice lacking the IFN-γ receptor gene neither clear the parasites nor repress their proliferation. Thus, CD40 and IFN-γ-induced pathogen elimination might represent two independent resistance pathways, the latter of which plays a primary role in anti-Toxoplasma immunity in mice.
The protozoan Toxoplasma gondii is an obligate intracellular pathogen with a worldwide distribution. Tachyzoites of T. gondii infect any nucleated cell of the host and disseminate throughout the body during the acute phase of infection. It is well established that cell-mediated immunity is critical in both human and mouse resistance to infection caused by T. gondii (11). Exposure to gamma interferon (IFN-γ) and members of the tumor necrosis factor (TNF) cytokine family is thought to activate cell autonomous mechanisms that restrict parasite replication and/or promote pathogen clearance (27). However, the intracellular mechanisms that lead to parasite elimination have remained unclear. Recently, two independent reports revealed that invaded T. gondii can be eliminated in an autophagy-dependent fashion. Ling and colleagues showed that IFN-γ signaling induces expression of p47 GTPases, causing disruption of the parasitophorous vacuole membrane. The stripped parasites are then enveloped within autophagosomes and subsequently undergo lysosome-mediated degradation (16). A contemporaneous report by Andrade and colleagues demonstrated that CD40/TNF signaling also triggers pathogen-lysosome fusion via an autophagic mechanism (2). Using IFN-γ-deficient mice, they also previously showed that CD40 ligation triggers in vivo and in vitro elimination of T. gondii independent of IFN-γ and the p47 GTPases (1, 23). Instead, the CD40 pathway operates through autocrine TNF-α activation involving TNF receptor 2 (TNFR2) and TRAF6 (3). Nevertheless, it is still not clear whether IFN-γ-triggered autophagic elimination of T. gondii requires CD40/TNFR signaling in the intact host. Using our recently described ex vivo T. gondii killing assay (16) and CD40−/− and TNFR1/2−/− mice, we demonstrate here that rapid elimination of T. gondii by strain CPS vaccine-primed macrophages, which we previously reported to be IFN-γ/IGTP dependent, proceeds normally in the absence of CD40/TNF signaling.
MATERIALS AND METHODS
Experimental animals.
C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA), and CD40−/− and TNFR1 and TNFR2 double-knockout mice were obtained from the Jackson Laboratory (Bar Harbor, ME). These mice, along with mice lacking the IFN-γ receptor gene (IFN-γR−/− mice), were bred and maintained under specific-pathogen-free conditions at the Brown University animal care facility. Mice of both sexes between 5 and 12 weeks old were used for experiments. Studies were performed in accordance with guidelines of the Brown University Institutional Animal Care and Use Committee.
Parasites.
The uracil auxotrophic CPS strain of T. gondii was provided by D. Bzik (Dartmouth Medical School, Hanover, NH) (12). Strain GFP-PTG (ATCC 50941) was obtained from the American Type Culture Collection (14). CPS parasites were γ irradiated with 15,000 rads before use.
Priming of mice, ex vivo infection, and flow cytometry.
Mice were intraperitoneally primed on day 0 with 1 × 106 irradiated CPS cells and on day 4 with 5 × 105 irradiated CPS cells. Peritoneal exudate cells (PECs) were harvested from CPS-primed mice on day 7 by washing the peritoneum with 10 ml ice-cold RPMI 1640. PECs were then infected in vitro at a multiplicity of infection (MOI) of 0.5 to 1 in invasion medium (RPMI 1640 plus 1% fetal bovine serum) at 37°C for 20 min. After two washes with termination medium (RPMI 1640 plus 5% fetal bovine serum) at 700 rpm (Beckman Allegra 6R benchtop centrifuge; Beckman, Fullerton, CA) for 5 min to remove extracellular parasites, 2 × 106 cells per time point were further cultured in invasion medium for 0, 3, 6, 9, and 12 h individually. At the corresponding time points, cells were spun down and fixed for flow cytometry. Fluorescence-activated cell sorting staining for CD40, CD154, CD4, T-cell receptor β, CD19, and F4/80 markers was carried out using standard procedures with antibodies purchased from BD Biosciences (San Jose, CA). Intracellular IGTP staining was performed as described previously (16). The number of mice used in each group is indicated in the figure legends. Data are expressed as means ± standard errors.
Parasite enumeration by light microscopy.
Total PECs harvested from primed mice were infected ex vivo with freshly prepared GFP-PTG. At corresponding time points postinfection, Cytospin slides were prepared using a cytocentrifuge (Cytospin; Shandon, Pittsburgh, PA). Smears consisting of 0.5 × 106 to 1 × 106 cells per slide were then fixed in 100% methanol and stained in Hemacolor solution 2 and solution 3 (EM Science, Gibbstown, NJ). At least 400 macrophages randomly selected from each slide were observed by light microscopy, and the numbers of total macrophages, infected macrophages, total vacuoles, and total parasites were determined. The replication of parasites was represented by the number of parasites per vacuole in infected macrophages at 0, 12 (or 13), and 24 (or 26) h postinfection.
LAMP1 staining and confocal microscopy.
Before ex vivo infection, primed PECs were allowed to adhere to coverslips for 3 h, and nonadherent cells were washed away with phosphate-buffered saline (PBS). After a 10-min incubation with GFP-PTG in invasion medium at 37°C, adherent PECs were washed extensively with PBS to remove extracellular parasites. Infected cells were further incubated in invasion medium at 37°C until they were processed at different time points for immunofluorescent staining. Adherent PECs were washed with PBS twice and then fixed and permeabilized with a BD Cytofix/Cytoperm solution for 30 min at room temperature. After permeabilization and blocking (5% goat serum, 1% bovine serum albumin, and 0.05% Tween 20 in PBS for 1 h), cells were incubated with anti-rat lysosome-associated membrane protein 1 (LAMP1) antibody (1D4B; 0.335 μg/ml; diluted in blocking buffer; DSHB, University of Iowa) for 45 min. Rat immunoglobulin G was included as a negative control. After washing, cells were incubated with Alexa Fluor 568-conjugated goat anti-rat antibody (1:1,000, diluted in blocking buffer) for 30 min, followed by washing. Stained coverslips were mounted on slides with ProLong Gold antifade solution with 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA).
RESULTS
Primed CD40−/− macrophages eliminate T. gondii efficiently, in contrast to IFN-γR−/− macrophages.
To test if CD40−/− macrophages can clear parasites, we used a green fluorescent protein (GFP)-labeled type II avirulent strain of T. gondii designated GFP-PTG. After priming with irradiated strain CPS, peritoneal macrophages harvested from wild-type (WT), IFN-γR−/−, and CD40−/− mice were infected ex vivo with GFP-PTG. As shown in Fig. 1A, primed CD40−/− macrophages eliminated parasites as efficiently as WT cells. After 12 h of infection, more than 90% of GFP-PTG parasites were destroyed in both CD40−/− and WT macrophages. In contrast, nearly 50% of the GFP-PTG parasites persisted 12 h postinfection in primed IFN-γR−/− macrophages, demonstrating that there was impaired clearance of actively invaded T. gondii parasites in the absence of IFN-γ signaling (P = 0.013 for a comparison with the WT at 12 h postinfection [n = 4]) (Fig. 1A). Nevertheless, partial clearance of GFP-PTG was sometimes observed in IFN-γR−/− macrophages, especially at early time points following infection (Fig. 1A). This might have reflected the early destruction of a certain fraction of noninvasive parasites that had been phagocytosed and was not related to nitric oxide production, which was undetectable in cultures of primed IFN-γR−/− macrophages (data not shown). Consistent with the results described above, the replication of GFP-PTG, which was not eliminated in invaded macrophages, was restrained well in WT and CD40−/− macrophages but not in IFN-γR−/− macrophages (Fig. 1B). After 24 h of infection, most parasites had multiplied twice in IFN-γR−/− macrophages (P < 0.01 for a comparison with the zero-time data [n = 4]), but no replication was observed in either WT or CD40−/− macrophages (Fig. 1B). These results indicate that both toxoplasmacidal and toxoplasmastatic activities of primed macrophages require intact IFN-γ responsiveness, but CD40 signaling seems to be dispensable.
FIG. 1.
Ex vivo elimination of T. gondii GFP-PTG by WT and CD40−/− vaccine-primed peritoneal macrophages but not by IFN-γR−/− vaccine-primed peritoneal macrophages. CPS-primed PECs from WT, CD40−/−, and IFN-γR−/− mice were infected with GFP-PTG ex vivo at an MOI of 1. (A) Ex vivo killing of GFP-PTG by primed macrophages from WT, CD40−/−, and IFN-γR−/− mice (n = 4). A total of 2 × 106 primed PECs per time point were infected by GFP-PTG. The infection rates (percentages of GFP-positive macrophages [forward scatterhigh and side scatterhigh cells]) were individually determined by flow cytometry at 0, 3, 6, 9, and 12 h postinfection. The data are representative of five independent experiments. (B) Restriction of GFP-PTG replication by WT and CD40−/− primed macrophages but not by IFN-γR−/− primed macrophages. A total of 2 × 106 primed PECs were infected by GFP-PTG ex vivo. At 0, 12, and 24 h postinfection, the replication of GFP-PTG was monitored by light microscopy and expressed as the number of tachyzoites per vacuole. Over 400 randomly selected macrophages were counted per time point for each mouse for the primed WT, CD40−/−, and IFN-γR−/− mice (n = 4). The data are representative of three independent experiments. (C) Accumulation of LAMP1 staining around GFP-PTG in WT and CD40−/− macrophages but not in IFN-γR−/− macrophages. A total of 2 × 106 primed PECs were plated onto coverslips, and attached macrophages were infected by GFP-PTG ex vivo for 30 min. After further culturing for 1 h, infected cells were fixed and stained with anti-rat LAMP1 and subsequently with goat anti-rat Alexa Fluor 568 and then counterstained with DAPI prior to confocal microscopy. The arrows indicate GFP-PTG parasites (green) surrounded by LAMP1 staining (red). KO, knockout.
In macrophages, both IFN-γ-induced and CD40-triggered autophagic elimination of T. gondii occurs through a final step involving host lysosome fusion with invaded parasites (2, 16). Therefore, we examined whether the lysosome membrane marker LAMP1 accumulated around GFP-PTG within CD40−/− and IFN-γR−/− macrophages. As shown in Fig. 1C, LAMP1 immunoreactivity surrounding GFP-PTG parasites was readily observed in both WT and CD40−/− macrophages but not in IFN-γR−/− macrophages. Thus, elimination of GFP-PTG by CD40−/− primed macrophages is associated with lysosome-mediated degradation.
Expression levels of molecules involved in CD40 and IFN-γ signaling.
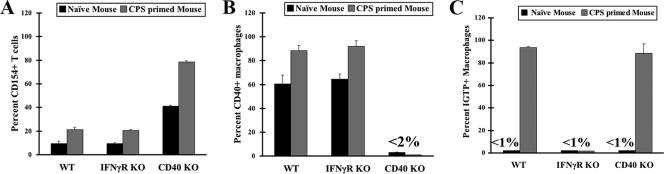
The lack of a requirement for CD40 might simply reflect the absence of significant CD154 induction during CPS priming. We therefore examined the percentages of CD154+ CD4+ T cells and CD40+ F4/80+ macrophages. As shown in Fig. 2A and 2B, in WT peritoneal cells, the frequencies of CD40+ and CD154+ cells were both increased 7 days after CPS priming. In CD40−/− mice, the basal and induced levels of CD154+ T cells were nearly fourfold higher than the WT levels. This may reflect the role of CD40-induced endocytosis of surface CD154 by T cells previously reported by Yellin et al. (28). Interestingly, the absolute change in the frequency of CD154+ CD4 cells increased by 40% in CD40−/− mice, while the increase observed in WT CD4+ T cells was only 10%. Thus, in WT mice, CD40-CD154 engagement probably occurred in vivo.
FIG. 2.
Expression of CD154, CD40, and IGTP in resident peritoneal cells obtained from naïve and primed WT, CD40−/−, and IFN-γR−/− mice. A total of 2 × 106 PECs obtained from WT, CD40−/−, and IFN-γR−/− naïve mice (n = 2) or primed mice (n = 4) were stained with the indicated antibodies. (A) Frequency of CD154 expression by CD4 T cells. (B) Frequency of CD40+ F4/80+ macrophages. (C) Frequency of macrophages expressing intracellular IGTP. Experiments were repeated at least three times with primed mice. KO, knockout.
We next examined the expression of CD40 and CD154 in peritoneal cell populations of IFN-γR−/− mice (Fig. 2A and 2B). Similar to the results for WT mice, the expression of both molecules increased upon CPS priming, indicating that induction of CD40 and CD154 is independent of IFN-γ signaling. Given that IFN-γR-deficient macrophages failed to eliminate and restrict parasites, prior in vivo CD40 engagement alone may not be sufficient for ex vivo macrophage resistance to T. gondii.
Since IFN-γ-mediated autophagic parasite elimination is critically dependent on the p47 GTPase IGTP while CD40-triggered macrophage resistance is not, we examined whether IGTP induction by IFN-γ signaling is normal in CD40−/− macrophages. Figure 2C shows that most macrophages acquired IGTP expression in the absence of CD40 signaling, but not when IFN-γ signaling was blocked. These observations, together with reports from the Subauste laboratory (1, 23), suggest that IFN-γ/IGTP- and CD40/TNF-dependent toxoplasmacidal activities can operate independently.
TNFR1 and TNFR2 are not necessary for ex vivo elimination of T. gondii.
TNF-α signaling via TNFR2 plays a pivotal role in CD40-mediated destruction of T. gondii (3). We therefore examined whether primed macrophages lacking both TNFR1 and TNFR2 can clear T. gondii ex vivo. As shown in Fig. 3A, TNFR1/2−/− macrophages eliminated invaded GFP-PTG as quickly as WT controls. Replication of residual intracellular parasites was also well restrained in TNFR1/2−/− cells (Fig. 3B). Thus, neither CD40 nor TNFRs play an obligatory role in ex vivo resistance to T. gondii in primed macrophages. Consistent with this conclusion, neutralization of endogenous TNF-α in CD40−/− mice did not impair parasite elimination (data not shown).
FIG. 3.
Ex vivo killing and restriction of parasite replication by TNFR1/2−/−macrophages. CPS-primed PECs from WT and TNFR1/2−/− mice were infected with GFP-PTG ex vivo at an MOI of 0.5. (A) Infection rate expressed as a percentage of GFP-positive macrophages, as monitored by flow cytometry. (B) Kinetics of GFP-PTG replication expressed as intracellular parasite growth (number of tachyzoites per vacuole), determined by light microscopy at 0, 13, and 26 h postinfection. Over 400 macrophages from three individual mice per group were counted per time point. (C) Comparison of frequencies of CD154+ T cells, CD40+ macrophages, and IGTP+ macrophages for naïve mice (n = 2) and primed WT and TNFR1/2−/− mice (n = 3). KO, knockout.
We also examined the effects of TNF signaling on the activation of macrophages and T cells during CPS priming. Similar to the results for WT mice, both CD40 expression on macrophages and CD154 up-regulation by CD4+ T cells were induced upon CPS priming in TNFR1/2−/− mice, although the extent of CD154 expression was somewhat less in TNFR1/2−/− mice (Fig. 3C). The frequency of IGTP+ macrophages in TNFR1/2−/− mice was tremendously increased after priming with CPS, indicating that TNF signaling is not required for induction of IGTP (Fig. 3C).
DISCUSSION
Taken together, our data clearly indicate that elimination of T. gondii by primed macrophages is IFN-γ dependent and functions in the absence of CD40/TNFR signaling. This is consistent with previously published data showing that IFN-γ-deficient mice are acutely susceptible to T. gondii challenge, while CD154−/− and TNFR1/R2−/− mice succumb only during the chronic phase of infection in the brain (10, 20, 24, 26). These observations underscore the primacy of IFN-γ signaling over CD40 function in mediating cellular resistance to T. gondii in the murine host.
IFN-γ mediates host resistance to T. gondii and other intracellular pathogens via induction of STAT-1-dependent expression of multiple antimicrobial genes. In the mouse, a family comprised of 23 p47 GTPases is expressed following IFN-γ exposure in both hemopoietic and nonhemopoietic cells (4). Three members of the p47 GTPase family, IGTP (25), LRG-47 (22), and IIGP (7), have been shown to mediate cell autonomous resistance to T. gondii, as well as a variety of intracellular bacterial pathogens (5, 9, 18, 24). In activated astrocytes, p47 GTPases were shown to accumulate around vacuoles containing T. gondii and initiate a process culminating in the disruption of the parasitophorous vacuole and finally disruption of the parasite itself (19). Similarly, we recently reported that in CPS-primed macrophages elimination of invaded parasites is preceded by parasitophorous vacuole membrane remodelling, vesiculation, disruption, and stripping of the parasite's plasma membrane. Denuded parasites were then enveloped in autophagosome-like vacuoles, which ultimately fused with lysosomes (16). Macrophage elimination of T. gondii was dependent on the IFN-γ-inducible p47 GTPase IGTP and required phosphatidylinositol 3-kinase activity, consistent with an autophagic mechanism for delivery of stripped parasites to the lysosome compartment. In addition, IFN-γ induces expression of inducible nitric oxide synthase (iNOS), which restrains parasite replication within activated macrophages (21). These toxoplasmacidal and toxoplasmastatic activities explain the pivotal role of IFN-γ signaling in resistance of T. gondii in vivo.
Interestingly, autophagic eradication of T. gondii was also observed in CD40/TNF-α signaling-induced macrophages. CD40 stimulation of human and mouse macrophages infected with T. gondii resulted in fusion of parasitophorous vacuoles and late endosomes/lysosomes and final degradation of parasites (2), stressing the potentially important role of CD40 signaling in cellular resistance to T. gondii. TNFR2 and TRAF6, but not IFN-γ, IGTP, and iNOS, are required for induction of toxoplasmacidal activity following CD40 ligation (1). However, our ex vivo killing data show that CPS vaccine-primed macrophages can efficiently eliminate and control T. gondii in either CD40-deficient or TNFR1/2-deficient mice, indicating the dispensable role of CD40/TNF-α signaling in mouse macrophage resistance. It seems questionable that CD40/TNF-α alone can efficiently control proliferation of invaded T. gondii. Mouse resident peritoneal macrophages stimulated by anti-CD40 agonistic antibodies, although mediating autophagic elimination, failed to effectively restrain intracellular replication of T. gondii in vitro (1). Furthermore, in the absence of IFN-γ signaling, CD40-activated macrophages exhibit only limited toxoplasmastatic activity in vivo (23). CD40 stimulation alone or in the presence of TNF-α is unable to induce reactive nitrogen intermediate (RNI) generation via the iNOS response (6). Considering the crucial role of RNI in repressing T. gondii proliferation, the failure to generate RNI and other toxic metabolites might explain the limited ability of the CD40/TNF-α signaling cascade to mediate cellular resistance to T. gondii.
Despite its apparent dispensability, CD40-dependent macrophage resistance may represent an important complementary “fail-safe” defense mechanism to counter evasion mechanisms that parasites have evolved to inhibit the primary IFN-γ/IGTP/iNOS-mediated pathway (15, 17). Further studies are needed to elucidate how these two major arms of cellular immunity interact to mediate host immunity to intracellular pathogens and how their roles might have shifted during evolution of the vertebrate lineage (4, 8, 13).
Acknowledgments
We thank Xin Wang for help with confocal microscopy and Pamela Gaddi for technique support.
This work was supported by grant AI-50618 from the NIH to G.S.Y.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 6 August 2007.
REFERENCES
- 1.Andrade, R. M., J. A. Portillo, M. Wessendarp, and C. S. Subauste. 2005. CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect. Immun. 73:3115-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade, R. M., M. Wessendarp, M. J. Gubbels, B. Striepen, and C. S. Subauste. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Investig. 116:2366-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade, R. M., M. Wessendarp, J. A. Portillo, J. Q. Yang, F. J. Gomez, J. E. Durbin, G. A. Bishop, and C. S. Subauste. 2005. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-alpha. J. Immunol. 175:6014-6021. [DOI] [PubMed] [Google Scholar]
- 4.Bekpen, C., J. P. Hunn, C. Rohde, I. Parvanova, L. Guethlein, D. M. Dunn, E. Glowalla, M. Leptin, and J. C. Howard. 2005. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 6:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein-Hanley, I., J. Coers, Z. R. Balsara, G. A. Taylor, M. N. Starnbach, and W. F. Dietrich. 2006. The p47 GTPases Igtp and Irgb10 map to the Chlamydia trachomatis susceptibility locus Ctrq-3 and mediate cellular resistance in mice. Proc. Natl. Acad. Sci. USA 103:14092-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingaman, A. W., T. C. Pearson, and C. P. Larsen. 2000. The role of CD40L in T cell-dependent nitric oxide production by murine macrophages. Transplant Immunol. 8:195-202. [DOI] [PubMed] [Google Scholar]
- 7.Boehm, U., L. Guethlein, T. Klamp, K. Ozbek, A. Schaub, A. Futterer, K. Pfeffer, and J. C. Howard. 1998. Two families of GTPases dominate the complex cellular response to IFN-gamma. J. Immunol. 161:6715-6723. [PubMed] [Google Scholar]
- 8.Casanova, J. L., and L. Abel. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 4:55-66. [DOI] [PubMed] [Google Scholar]
- 9.Collazo, C. M., G. S. Yap, G. D. Sempowski, K. C. Lusby, L. Tessarollo, G. F. Woude, A. Sher, and G. A. Taylor. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckert-Schluter, M., A. Rang, D. Weiner, S. Huang, O. D. Wiestler, H. Hof, and D. Schluter. 1996. Interferon-gamma receptor-deficiency renders mice highly susceptible to toxoplasmosis by decreased macrophage activation. Lab. Investig. 75:827-841. [PubMed] [Google Scholar]
- 11.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox, B. A., and D. J. Bzik. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926-929. [DOI] [PubMed] [Google Scholar]
- 13.Janssen, R., A. Van Wengen, E. Verhard, T. De Boer, T. Zomerdijk, T. H. Ottenhoff, and J. T. Van Dissel. 2002. Divergent role for TNF-alpha in IFN-gamma-induced killing of Toxoplasma gondii and Salmonella typhimurium contributes to selective susceptibility of patients with partial IFN-gamma receptor 1 deficiency. J. Immunol. 169:3900-3907. [DOI] [PubMed] [Google Scholar]
- 14.Kim, K., M. S. Eaton, W. Schubert, S. Wu, and J. Tang. 2001. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 113:309-313. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. K., A. E. Fouts, and J. C. Boothroyd. 2007. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J. Immunol. 178:5154-5165. [DOI] [PubMed] [Google Scholar]
- 16.Ling, Y. M., M. H. Shaw, C. Ayala, I. Coppens, G. A. Taylor, D. J. Ferguson, and G. S. Yap. 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 203:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luder, C. G., M. Algner, C. Lang, N. Bleicher, and U. Gross. 2003. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int. J. Parasitol. 33:833-844. [DOI] [PubMed] [Google Scholar]
- 18.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654-659. [DOI] [PubMed] [Google Scholar]
- 19.Martens, S., I. Parvanova, J. Zerrahn, G. Griffiths, G. Schell, G. Reichmann, and J. C. Howard. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 1:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichmann, G., W. Walker, E. N. Villegas, L. Craig, G. Cai, J. Alexander, and C. A. Hunter. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorace, J. M., R. J. Johnson, D. L. Howard, and B. E. Drysdale. 1995. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J. Leukoc. Biol. 58:477-484. [DOI] [PubMed] [Google Scholar]
- 23.Subauste, C. S., and M. Wessendarp. 2006. CD40 restrains in vivo growth of Toxoplasma gondii independently of gamma interferon. Infect. Immun. 74:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor, G. A., C. M. Collazo, G. S. Yap, K. Nguyen, T. A. Gregorio, L. S. Taylor, B. Eagleson, L. Secrest, E. A. Southon, S. W. Reid, L. Tessarollo, M. Bray, D. W. McVicar, K. L. Komschlies, H. A. Young, C. A. Biron, A. Sher, and G. F. Vande Woude. 2000. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc. Natl. Acad. Sci. USA 97:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor, G. A., M. Jeffers, D. A. Largaespada, N. A. Jenkins, N. G. Copeland, and G. F. Woude. 1996. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J. Biol. Chem. 271:20399-20405. [DOI] [PubMed] [Google Scholar]
- 26.Yap, G. S., T. Scharton-Kersten, H. Charest, and A. Sher. 1998. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J. Immunol. 160:1340-1345. [PubMed] [Google Scholar]
- 27.Yap, G. S., and A. Sher. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yellin, M. J., K. Sippel, G. Inghirami, L. R. Covey, J. J. Lee, J. Sinning, E. A. Clark, L. Chess, and S. Lederman. 1994. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J. Immunol. 152:598-608. [PubMed] [Google Scholar]