Abstract
Matrix metalloproteinases (MMPs) are a family of extracellularly acting proteolytic enzymes with well-recognized roles in plasticity and remodeling of synaptic circuits during brain development and following brain injury. However, it is now becoming increasingly apparent that MMPs also function in normal, nonpathological synaptic plasticity of the kind that may underlie learning and memory. Here, we extend this idea by investigating the role and regulation of MMP-9 in an inhibitory avoidance (IA) learning and memory task. We demonstrate that following IA training, protein levels and proteolytic activity of MMP-9 become elevated in hippocampus by 6 h, peak at 12–24 h, then decline to baseline values by ∼72 h. When MMP function is abrogated by intrahippocampal infusion of a potent gelatinase (MMP-2 and MMP-9) inhibitor 3.5 h following IA training, a time prior to the onset of training-induced elevation in levels, IA memory retention is significantly diminished when tested 1–3 d later. Animals impaired at 3 d exhibit robust IA memory when retrained, suggesting that such impairment is not likely attributed to toxic or other deleterious effects that permanently disrupt hippocampal function. In anesthetized adult rats, the effective distance over which synaptic plasticity is impaired by a single intrahippocampal infusion of the MMP inhibitor of the kind that blocks IA memory is ∼1200 μm. Taken together, these data suggest that IA training induces a slowly emerging, but subsequently protracted period of MMP-mediated proteolysis critical for enabling long-lasting synaptic modification that underlies long-term memory consolidation.
New information is learned and remembered through functional and structural modifications of synaptic connections (Bliss and Collingridge 1993; Rogan et al. 1997; Rioult-Pedotti et al. 1998, 2000; Jorntell and Hansel 2006; Pastalkova et al. 2006; Whitlock et al. 2006). Several kinds of learning and memory tasks have been shown to drive changes in neurotransmitter receptor function and/or localization (Cammarota et al. 1995; Bevilaqua et al. 2005; Rumpel et al. 2005; Whitlock et al. 2006), as well as induce over time changes in synapse number or morphology (O'Malley et al. 2000; Geinisman et al. 2001; Eyre et al. 2003; Leuner et al. 2003; Maviel et al. 2004; Lamprecht et al. 2006; Rekart et al. 2007). These observations suggest that there must be learning-induced cellular mechanisms for coordinating functional and structural remodeling of synaptic connectivity to enable long-term memory, but there is little known about the molecules that could fulfill such a role.
Learning-related, regulated extracellular proteolysis could be one mechanism for coordinating functional and structural synaptic plasticity, thereby enabling memory (Huang et al. 1996; Madani et al. 1999; Calabresi et al. 2000; Pawlak et al. 2002; Tamura et al. 2006). It is well-recognized that extracellular matrix (ECM) proteins as well synaptic cell adhesion molecules contribute to both functional and structural aspects of synaptic plasticity, are modified by learning or plasticity-inducing stimuli, and in some cases are essential for certain types of memory (Lüthi et al. 1994; Tang et al. 1998; Bozdagi et al. 2000; Chun et al. 2001; Chan et al. 2003, 2006; Dityatev and Schachner 2003; Okamura et al. 2004; Bernard-Trifilo et al. 2005; Huang et al. 2006; Kramar et al. 2006; Bukalo et al. 2007; Lopez-Fernandez et al. 2007). Generally, in other tissues of the body, ECM and the cell-surface proteins through which they interact are maintained and dynamically modified by tightly regulated, extracellular proteolysis mediated by matrix metalloproteinases (MMPs), a family of mostly secreted, very potent proteolytic enzymes (Sternlicht and Werb 2001; Stamenkovic 2003). MMPs in brain are synthesized and secreted by both neurons and neuroglia in an inactive (pro) form (Szklarczyk et al. 2002; Arai et al. 2003; Nagy et al. 2006; Bozdagi et al. 2007). Upon removal of the pro-peptide sequence and other regulatory steps, they become proteolytically active, where they participate in physical remodeling of the pericellular microenvironment and cell signaling by liberating latent growth factors or other ligands harbored in the matrix, or by exposing cryptic bioactive fragments (Nagase and Woessner 1999; Sternlicht and Werb 2001).
In brain, MMPs have well-documented roles both in early development, during axonal pathfinding and circuit formation (McFarlane 2003), and in maturity under injurious or neuropathological contexts, where they contribute to protracted and often deleterious remodeling that accompanies inflammation, stroke, or degeneration (Zhang et al. 1998; Lo et al. 2002; Szklarczyk et al. 2002; Reeves et al. 2003). Standing views of MMP function are expanding, however, because several lines of recent evidence reveal previously unrecognized roles for MMPs in long-lasting synaptic plasticity of the kind associated with normal, nonpathological brain function. In hippocampus, MMP-9 is up-regulated and becomes proteolytically active selectively during the maintenance phase of long-term potentiation (LTP) at CA3–CA1 synapses (Nagy et al. 2006; Bozdagi et al. 2007). Once active, MMP-9 induces a slowly emerging potentiation of synaptic signal strength that is mediated by integrins. When MMP-9 activity is blocked pharmacologically or with specific neutralizing antibodies, the stable maintenance of LTP is significantly impaired, with no effects on LTP induction or early expression. Similar findings have been recently reported in rat prefrontal cortex (Okulski et al. 2007). This suggests that MMPs may function in cellular processes that contribute to learning and memory.
Several recent studies support the idea of a role for MMPs in learning and memory (Meighan et al. 2006; Nagy et al. 2006; Brown et al. 2007). However, no data have yet been provided showing that MMP proteolytic activity is actually enhanced in response to learning. Here, we address this and related questions using an inhibitory avoidance (IA) task. This is an ideal task for the question: it is learned in a single trial and creates a strong and long-lasting memory (Milekic and Alberini 2002).
Results
Elevation of MMP-9 protein levels and proteolytic activity induced by inhibitory avoidance learning
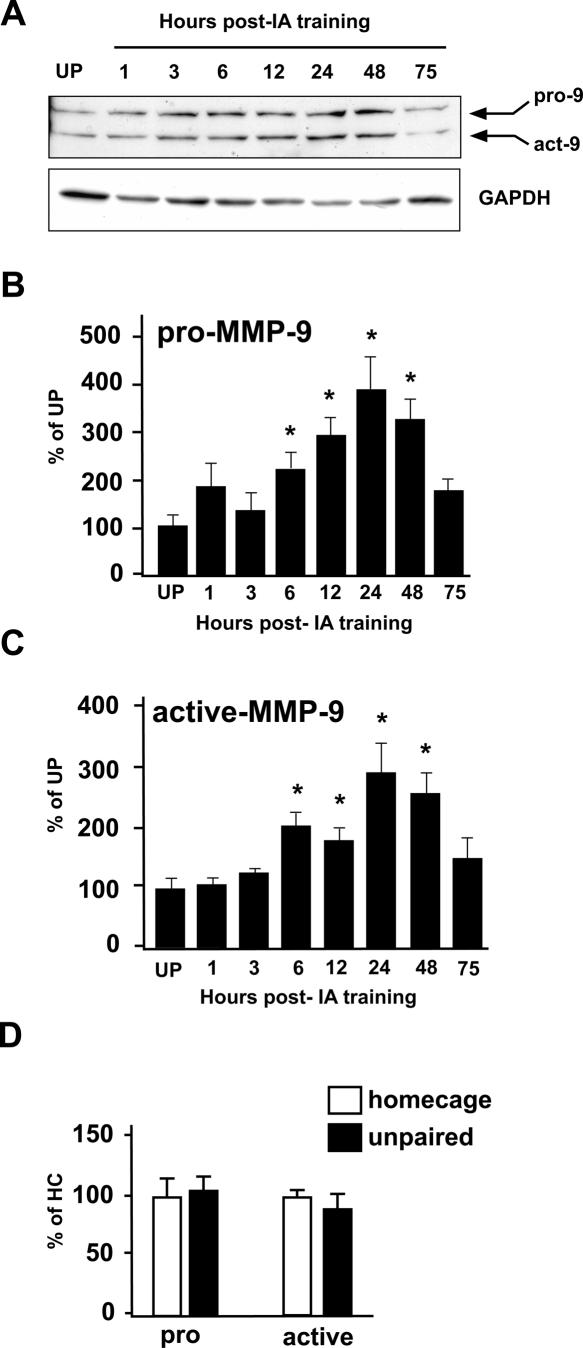
We first tested effects of IA learning on MMP-9 protein levels in hippocampus. An experimental group of rats was subjected to IA training and subsequently killed at times ranging from 1 to 75 h post-training (Fig. 1A). An unpaired (UP) control group was exposed to the IA context and received the footshock, but these two experiences were separated by 1 h and were therefore unpaired, preventing IA learning (Garcia-Osta et al. 2006). Immunoblot analysis using MMP-9-specific antisera that recognize both pro- and active-forms shows that IA training induces a gradual and transient increase in levels of both forms of MMP-9 (Fig. 1B,C). Protein levels of pro-MMP-9 (Fig. 1B) and active-MMP-9 (Fig. 1C) become significantly elevated between 3 and 6 h post-training in comparison with those of unpaired control animals, reach a peak elevation 24–48 h post-training, then decline to baseline values by 75 h post-training. We confirmed that MMP-9 protein levels in the unpaired control group were unchanged by exposure to either the IA context or footshock, because there were no differences between unpaired control rats and home cage control rats in MMP-9 levels (Fig. 1D).
Figure 1.
MMP-9 protein levels increase in response to IA training. (A) Representative immunoblot of hippocampal homogenates prepared from unpaired (UP) control rats or from rats killed post-IA training at the times indicated (in hours). Membranes were probed with antibodies that recognize both pro- and active-forms of MMP-9 (pro-9, act-9, respectively), or GAPDH, which was used as a loading control. (B,C) Graphs showing quantification of immunoblots from trained and unpaired animals (n = 4 animals per UP group or per post-training time point, *P < 0.05 in comparison with levels in UP controls). Levels of both pro- (B) and active- (C) forms of MMP-9 rise significantly between 3 and 6 h post-training, peak at 24–48 h, then decline to control levels by 75 h post-training. (D) Graph showing quantification of immunoblots from UP and HC control animals (n = 6 UP, n = 3 HC). There were no differences between these two control groups in levels of pro- or active-MMP-9.
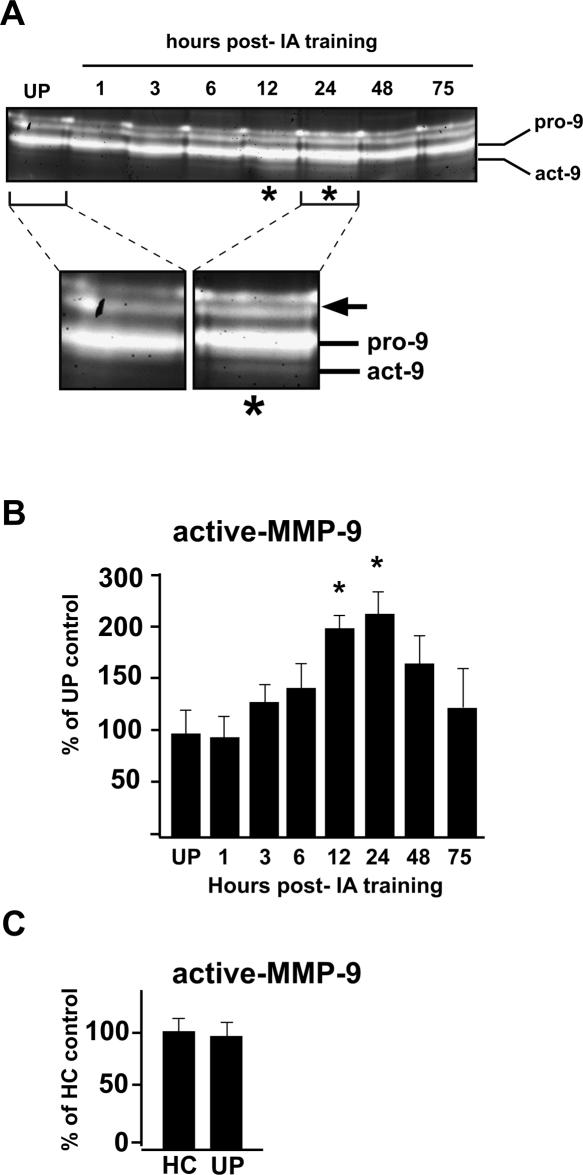
The significant increase in MMP-9 protein levels suggests that MMP-9 becomes proteolytically active following IA learning. To test this, hippocampal lysates from the experimental and control groups of rats described above were subjected to gelatin substrate zymography. In comparison with unpaired control rats, proteolytic activity of the active form of MMP-9 becomes significantly elevated by 12 h following IA learning which is sustained through 24 h, then returns to baseline values by 48–75 h post-training (Fig. 2A,B). There were no differences in levels of proteolytic activity between the unpaired control animals and home cage control animals (Fig. 2C), consistent with the lack of any differences in protein levels between these two control groups. Taken together, these data indicate that MMP-9 protein levels and proteolytic activity are increased specifically in response to associative learning.
Figure 2.
MMP-9 proteolytic activity increases in response to IA training. (A) Representative in vitro gelatin zymograph showing time course in elevation of MMP-9 proteolytic activity post-IA training in comparison with an unpaired (UP) control rat. The zymograph lanes of the UP control and the 24-h post-training rats are shown at higher magnification in the insets, below. The pro-form of MMP-9 is the middle, thick band (pro-9); the proteolytically active-form of MMP-9 (act-9) is the thin, fainter band immediately subjacent to the pro-band. The pro-form appears in zymographs because of some auto-activation following the partially denaturing conditions of SDS gel electrophoresis; the higher molecular weight activity bands (arrow) may represent unprocessed forms that also undergo some auto-activation (Snoek-van Beurden and Von den Hoff 2005). Asterisks indicate time points (12 and 24 h) of significant elevation in proteolytic activity in comparison with UP control levels. (B) Graph showing quantification of MMP-9 proteolysis (active-MMP-9) following training. MMP-9 activity reaches significantly elevated levels by 12–24 h post-IA training, then declines to baseline values by 48–75 h (*P < 0.05; n = 4 animals per group (UP) or per post-training time point). (C) Graph showing quantification of MMP-9 proteolysis (active-MMP-9) in lysates from home cage (HC) and UP controls. There are no differences between these two control groups in levels of MMP-9 proteolysis.
MMP proteolytic activity is required for IA memory
The increase in MMP-9 protein levels and the enhancement of MMP-9 proteolytic activity by IA learning suggests that MMP-9 contributes to cellular processes underlying IA memory. We tested this in a series of experiments in which we infused a potent, MMP-2/9-specific inhibitor (Inhibitor II) or vehicle into hippocampus bilaterally at certain times post-IA training and tested effects of neutralizing MMP activity on memory retention at 24 h post-training, a time when animals normally exhibit robust IA memory (Taubenfeld et al. 1999; Bevilaqua et al. 2005). We used Inhibitor II because its potency in inhibiting MMP-2 and MMP-9 proteolytic activity has been established previously using a fluorometric enzymatic assay (Nagy et al. 2006), and, by blocking MMP-9 activity, it impairs maintenance of hippocampal area CA1 long-term potentiation (LTP) in both acute slices (Nagy et al. 2006) and in adult rats in vivo (Bozdagi et al. 2007).
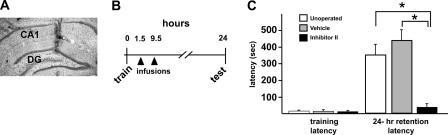
In a first series of experiments, we infused the MMP inhibitor into the hippocampus at 1.5 and 9.5 h after IA training (Fig. 3A,B). The goal here was to neutralize MMP activity prior to and extending into the protracted period over which MMP-9 activity rises in response to IA training. During IA training, there were no differences in mean entrance latencies between animals in which bilateral cannulae were implanted and unoperated control rats (Fig. 3C, P > 0.4), indicating that the surgical procedures or the presence of indwelling cannulae per se had no adverse effects on behavioral performance. At the 24-h retention test, rats receiving bilateral infusions of vehicle exhibited robust retention of the IA response, with entrance latencies that were significantly longer (442 ± 60 sec) in comparison with their own group’s mean training latency (20 ± 4 sec; Fig. 3C, P < 0.0025). Such retention latencies displayed by the vehicle-infused rats were not significantly different than those of unoperated control rats (352 ± 75 sec; P > 0.4), indicating that the diluent had no effect on IA memory. In contrast, animals receiving intrahippocampal infusions of MMP inhibitor following IA training exhibited severely impaired IA memory when tested at 24 h (Fig. 3C). Their group mean 24-h retention latency (44 ± 17 sec) was significantly shorter than those of the unoperated or diluent groups (Fig. 3C, P < 0.004). The mean 24-h retention latency of the MMP inhibitor group was not significantly different than their own group’s mean training latency (15 ± 3 sec; P > 0.1), indicating that these animals were amnesic for IA memory.
Figure 3.
Intrahippocampal infusion of MMP-9 inhibitor blocks IA memory. (A) Representative Nissl-stained section through dorsal hippocampus showing the track of the infusion needle used to deliver MMP inhibitor or vehicle. DG, dentate gyrus; CA1, area CA1. (B) Timeline of blocking experiments. Animals were trained at time 0 h, then received bilateral intrahippocampal infusions of Inhibitor II or vehicle at 1.5 and 9.5 h post-training (arrowheads). Retention latencies were then tested at 24 h (test). (C) Graph showing mean IA training and retention latencies of three groups of rats (n = 9 unoperated; n = 5 vehicle-infused; n = 6 MMP inhibitor-infused). Retention latency was assessed at 24 h. The MMP inhibitor-infused rats displayed significantly shorter 24-h retention latencies in comparison with those of the other two groups (*P < 0.004). Within the MMP inhibitor group, there was no significant difference between their training and retention latencies, indicating that they were amnesic when tested for IA memory at 24 h. Data are means + SEM.
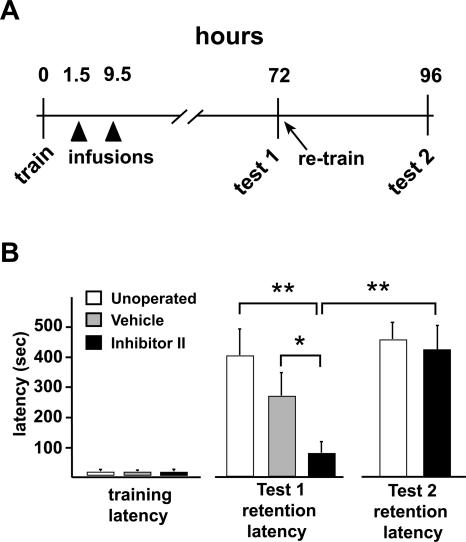
To determine if the significant memory impairment at 24 h post-training was persistent, we repeated these experiments on a second series of animals but extended the duration between IA training and the memory retention test to 72 h (Fig. 4A). As with the first series of experiments, there were no differences in entrance latencies across the unoperated and the cannulated groups of animals during IA training (Fig. 4B, P > 0.1). At 1.5 and 9.5 h after training, one cannulated group received bilateral infusions of vehicle; the other cannulated group received bilateral infusions of Inhibitor II (Fig. 4A). At 72 h, the unoperated animals displayed robust IA memory as expected (Fig. 4B; Milekic and Alberini 2002). The vehicle-infused animals displayed a group mean retention latency that was slightly, but not significantly, lower than that of the unoperated animals (Fig. 4B, P > 0.2), indicating that this group also had strong IA memory at 72 h. In contrast, the MMP inhibitor-infused animals displayed significantly impaired IA memory when tested at 72 h (Fig. 4B), with a group mean 72-h retention latency (104 ± 36 sec) that was significantly shorter in comparison with the 72-h retention latencies of unoperated (410 ± 84 sec, P < 0.002) and vehicle-infused rats (274 ± 76 sec, P < 0.05). To rule out the possibility of permanent cytotoxic effects of Inhibitor II as a basis for long-lasting memory impairment, we tested the ability of the MMP inhibitor-infused animals to relearn the task immediately following the 72-h retention test. We reasoned that, if hippocampal integrity was sound and uncompromised by the earlier inhibitor infusions, then the animals displaying profound memory impairment at 72 h should be capable of relearning the IA task and subsequently displaying robust IA memory. At the 72-h retention test, the inhibitor-infused animals were given a 0.65-mA reminder shock upon entering the dark chamber and were retested for IA memory 24 h later (Fig. 4A). As predicted, these animals now displayed robust IA memory (Fig. 4B), with a group mean retention latency (482 ± 58 msec) that is comparable to unoperated animals tested for IA memory at 24 h post-training (432 ± 84 msec, P > 0.1). Thus, these data indicate that pharmacological blockade of MMP-9-mediated proteolysis within the first 10 h following IA learning produces a persistent memory impairment that is not likely attributable to permanent, deleterious effects on hippocampal function.
Figure 4.
IA memory impairment following MMP-9 inhibition is persistent. (A) Timeline of experiments. The initial training and infusion schedule (arrowheads) was identical to that shown in Fig. 3, but, in these experiments, retention latancies were first assessed at 72 h (test 1). The MMP inhibitor-infused rats were then retrained immediately following the 72-h retention test and retested for IA memory 24 h later (test 2). (B) Graph showing mean IA training and retention latencies of three groups of rats (n = 10 unoperated; n = 9 vehicle-infused; n = 8 MMP inhibitor-infused). The MMP inhibitor-infused rats had significantly shorter 72-h retention latencies in comparison with those of the other two groups (*P < 0.05, **P < 0.01), indicating significantly impaired IA memory. There was no significant difference in Test 1 retention latencies between the unoperated and vehicle-infused control groups (P > 0.2). The MMP inhibitor-infused rats were immediately retrained and tested 24 h later (Test 2). Now, they exhibited retention latencies that were significantly longer than their own group’s Test 1 latency (**P < 0.01), and similar in magnitude to that of unoperated control animals tested at 24 h (P > 0.1). These experiments demonstrate that IA memory impairment at 72 h is not a reflection of toxic or other deleterious effects that permanently disrupt hippocampal function. Data are means + SEM.
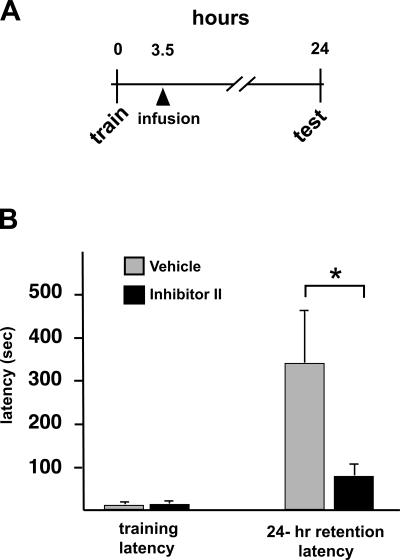
We next investigated effects on IA memory retention of lowering the levels of the MMP inhibitor delivered to the hippocampus. For this, a third series of experiments was undertaken that was similar in design to the others except that only a single injection of MMP inhibitor or diluent was administered intrahippocampally at an intermediate (3.5 h post-training) time point (Fig. 5A). As expected from the previous series, there were no differences between groups in mean entrance latencies during training (Fig. 5B; P > 0.4). At the 24-h retention test, the mean entrance latency of the vehicle-infused rats was significantly longer (339 ± 96 sec) in comparison with their own group’s mean training latency (13 ± 5 sec; P < 0.008), indicating, as expected, a robust IA response. In contrast, rats receiving a single intrahippocampal infusion of MMP inhibitor had significantly impaired IA memory at the 24-h retention test (Fig. 5B), with a group mean retention latency (80.1 ± 26 sec) that was significantly shorter than the mean 24-h retention latency of the vehicle-group (P < 0.01). Taken together, these data suggest that enhanced MMP-9 proteolytic activity induced by IA learning contributes to the cellular processes necessary for IA memory that commence between 3 and 6 h post-training.
Figure 5.
A single, post-training infusion of MMP-9 inhibitor blocks IA memory. (A) Timeline of experiment. The design is identical to that shown in Fig. 1, except that only a single infusion (arrowhead) of vehicle or MMP inhibitor was made at 3.5 h post-training. (B) Graph showing IA training and retention latencies of vehicle-infused rats (n = 7) or MMP inhibitor-infused rats (n = 9). Retention latencies were assessed at 24 h. MMP inhibitor-infused rats displayed significantly shorter entrance latencies in comparison with those of the vehicle-infused group (*P < 0.01). Data are means + SEM.
Spatial limits of LTP impairment following MMP inhibitor infusion
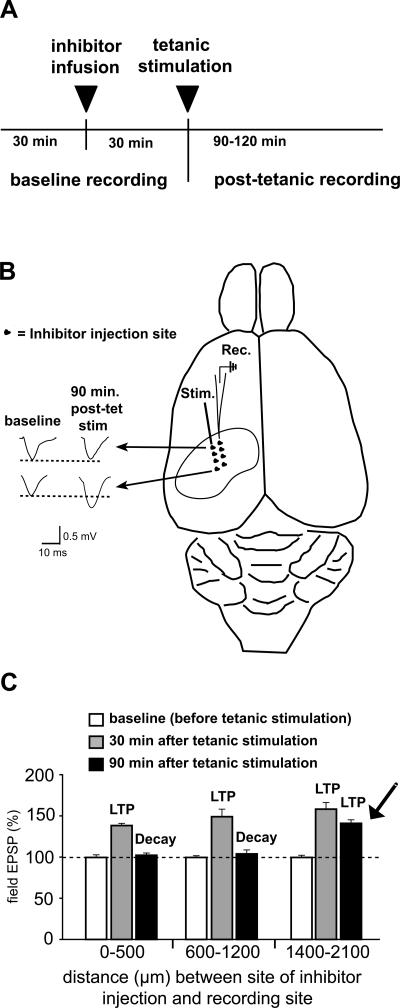
Our goal for the next series of experiments was to determine the effective spread of the inhibitor throughout the dorsal hippocampus in order to better understand a potential cellular basis for the effects on IA memory that we observed following infusion of the MMP inhibitor. The experiments were based on the prior demonstration that intrahippocampal administration of Inhibitor II blocks maintenance of LTP at CA3–CA1 synapses in adult rats in vivo, with no effects on induction of LTP or baseline properties of synaptic neurotransmission (Bozdagi et al. 2007). Here, in urethane-anesthetized adult rats, stimulating and recording electrodes were positioned in the dorsal hippocampus to elicit LTP in area CA1 stratum radiatum following tetanic stimulation of the Schaffer-collaterals as described previously (Bozdagi et al. 2007). After establishing a baseline of synaptic responses, a single injection of Inhibitor II was placed into the dorsal hippocampus at one of several different sites that spanned 0–2100 μm away from the recording electrode (Fig. 6A,B) using injection parameters, volumes, and concentrations identical to those used in the behavioral experiments. Each animal received only a single inhibitor injection at only one site; the position of the one injection site in relationship to the position of the recording electrode varied across animals (Fig. 6B). After allowing time for diffusion (Fig. 6A), tetanic stimulation of Schaffer-collaterals was used to elicit LTP of synaptic responses recorded from area CA1 stratum radiatum; synaptic responses were then monitored for 120 min following tetanic stimulation (Fig. 6B,C). Distances between infusion and recording sites were grouped into three ranges (in μm: 0–500, 600–1200, 1400–2100). Robust LTP was evident at 30 min post-tetanic stimulation in all three groups (gray “LTP” bars, Fig. 6C), consistent with the lack of effects of the MMP inhibitor on induction of LTP in vivo (Bozdagi et al. 2007). In animals where distances between the inhibitor infusion site and recording site were ≤1200 μm, synaptic responses gradually decayed back to baseline values by 90 min post-stimulation (black “Decay” bars, Fig. 6C), consistent with impairment of LTP maintenance. However, at distances ≥1400 μm separation, the inhibitor failed to block LTP maintenance, as LTP persisted throughout the 120 min recording period (arrow, Fig. 6C). Thus, these data indicate that a single injection of MMP inhibitor, sufficient for impairing IA memory, affects synaptic plasticity across a relatively spatially limited extent (∼1200 μm) of dorsal hippocampus.
Figure 6.
Range of effective blockade of synaptic plasticity across dorsal hippocampus following infusion of MMP-9 inhibitor. A combination of pharmacological and electrophysiological methods was applied to urethane-anesthetized adult rats in order to determine the effective distance over which a single intrahippocampal infusion of MMP-9 inhibitor blocks maintenance of LTP of CA3–CA1 synapses. Parameters of infusion were identical to those used to block IA memory. (A) Schematic showing timeline and experimental design. (B) Surface view schematic of rat brain showing position of stimulating (Stim) and recording (Rec) electrodes used to elicit LTP in area CA1, and the positions of single inhibitor infusions into the dorsal hippocampus (black circles). Each animal received only a single infusion. (Left) Representative EPSP traces shown at two such positions: potentiation decayed back to baseline by 90 min post-tetanic stimulation (post-tet stim) when infusion site distances were ≤1200 μm away from the recording site (upper trace), while no effects on LTP were observed when infusion site distances were ≥1400 μm away from the recording site (lower trace). (C) Distances between recording and infusion were grouped into three ranges. LTP was elicited reliably in all experiments (gray “LTP” bar, taken at 30 min post-tetanic stimulation), but decayed back to baseline at sites ≤1200 μm separation distance (black “Decay” bars). Only at sites ≥1400 μm separation was LTP maintained (arrow).
Discussion
Recent studies have revealed previously unrecognized roles for MMP-mediated extracellular proteolysis in long-lasting hippocampal synaptic physiology and plasticity. In an extension of these observations, we demonstrate here that MMP-9 protein levels and proteolytic activity are significantly elevated within hippocampus following inhibitory avoidance training. The elevation in levels and proteolytic activity commences between 3 and 6 h post-training, peaks by 12–24 h, then declines to baseline values by ∼72 h. When MMP function is abrogated by intrahippocampal infusion of a potent gelatinase (MMP-2 and MMP-9) inhibitor 3.5 h following IA training, a time point prior to the onset of up-regulation, IA memory retention is significantly diminished when tested 1–3 d later. The effect on IA memory is not likely attributed to toxic or other deleterious effects that permanently impair hippocampal function, since animals exhibit strong IA memory when retrained. In anesthetized adult rats, the effective distance over which synaptic plasticity is impaired by intrahippocampal infusion of the MMP inhibitor of the kind that blocks IA memory is ∼1200 μm. Since LTP is a form of long-lasting synaptic plasticity that is induced by IA training (Whitlock et al. 2006) and thought to represent a cellular basis for memory consolidation (Bliss and Collingridge 1993; Silva 2003), these data overall suggest that IA training induces a slowly emerging, but subsequently protracted, period of MMP-mediated proteolysis critical for enabling long-lasting synaptic modification that underlies long-term memory consolidation.
The delayed onset of MMP-9 up-regulation and activation following training suggests that MMP-9-mediated proteolysis is not involved in early cellular mechanisms enabling short-term IA memory that can be recalled within the first few hours, but instead is related selectively to later-occurring cellular plasticity mechanisms that stabilize longer-term memory. Such mechanisms very likely involve the maintenance of long-lasting changes in synaptic strength, such as LTP. Tetanically evoked LTP of CA3–CA1 synapses exhibits an early phase that reflects fast post-translational modifications of certain synaptic proteins including AMPA-type glutamate receptors (Bredt and Nicoll 2003) and a maintenance phase that requires additional mechanisms including mRNA transcription and protein synthesis (Reymann and Frey 2007). Previous studies of the role of MMP-9 in LTP at CA3–CA1 synapses using acute hippocampal slices (Nagy et al. 2006) or in urethane-anesthetized adult rats in vivo (Bozdagi et al. 2007) have shown that MMP-9 becomes proteolytically active within area CA1 stratum radiatum during the maintenance phase of LTP, and, when its proteolytic activity is neutralized with either broad-spectrum MMP inhibitors or MMP-9-specific blocking reagents, the maintenance of LTP is abolished without any effects on the induction or early expression of LTP, or on baseline properties of synaptic neurotransmission. This time course of activation and role in stable expression, but not induction, of LTP under experimentally induced conditions (tetanic stimulation) is consistent with how the regulation and function of MMP-9 might contribute to later-occurring processes of long-term memory consolidation triggered by behavioral experience. Inhibitory avoidance training induces LTP at CA3–CA1 synapses that exhibits many of the molecular and temporal features of tetanically evoked LTP, including rapid changes in AMPA receptor phosphorylation, localization, and binding (Cammarota et al. 1995; Whitlock et al. 2006), as well as later, stable expression of synaptic potentiation lasting at least 4 h post-IA training (when experiments were terminated) (Whitlock et al. 2006). Thus, infusion of the gelatinase inhibitor at 3.5 h post-IA training, prior to the period of onset of MMP-9 up-regulation, is likely to have impaired the stable maintenance of IA training-induced LTP. Such IA-associated LTP within area CA1 is widely distributed (Whitlock et al. 2006), consistent with theoretical and previous experimental mapping studies suggesting that IA and other forms of learning engage widely distributed neural circuits throughout hippocampus (Buzsaki et al. 1990; Treves and Rolls 1994; Guzowski et al. 1999; Taubenfeld et al. 2001; de Hoz et al. 2005; Fletcher et al. 2006). Our experiments here showing that the maintenance of LTP was impaired up to ∼1200 μm from the site of the inhibitor infusion suggest that acute interference with synaptic plasticity mechanisms over a spatially limited extent within dorsal hippocampus has significant effects on behavioral performance.
The functional involvement of MMPs in IA memory described here is based on the use of an MMP inhibitor (Inhibitor II) known to be a potent blocker of MMP-2 and MMP-9 proteolytic activity (Nagy et al. 2006). Although Inhibitor II was originally reported as being highly selective for MMP-2 and MMP-9, with little or no inhibitory effects on other MMPs (Tamura et al. 1998), more recent studies suggest that Inhibitor II may also affect the activity of members of the related ADAMs (a disintegrin and metalloproteinase) family (Mifune et al. 2005), which, along with MMPs, are members of the metzincin superfamily (Sternlicht and Werb 2001). Thus, we cannot rule out that some of the effects we observed on IA memory retention with Inhibitor II could be attributed to involvement of ADAMs or possibly even other metalloproteases. Nevertheless, our data show clearly that MMP-9 proteolytic activity is up-regulated by IA learning. Additionally, the effects on hippocampal LTP elicited in vivo in the presence of an MMP-9-specific function-blocking antibody are identical to those observed using Inhibitor II or other broad-spectrum MMP inhibitors (Bozdagi et al. 2007), indicating that the major effects of the pharmacological inhibitors, including Inhibitor II, on long-lasting synaptic plasticity are largely attributable to blockade of MMP-9 activity without major “off-target” effects on other types of proteases. Finally, in preliminary experiments, we have injected the function-blocking MMP-9 antibody intrahippocampally and observed IA memory deficits when tested at 24 h similar to those observed with Inhibitor II (V. Nagy and G.W. Huntley, unpubl.), consistent with the idea that the effects of Inhibitor II on IA memory are largely attributable to blockade of MMP activity.
Once proteolytically active within hippocampus, MMP-9 causes a slowly emerging synaptic potentiation that is mutually occluded by tetanically evoked LTP (Bozdagi et al. 2007) and is mediated by integrin receptors (Nagy et al. 2006). It is known that integrin receptors are required both for the consolidation of LTP (Staubli et al. 1990; Bahr et al. 1997; Chun et al. 2001; Kramar et al. 2002; Huang et al. 2006), as well as for some types of hippocampal-dependent learning and memory tasks (Chan et al. 2003, 2006). In the context of the present study, it is possible that MMP-9 contributes to long-term IA memory via cleavage and/or exposure of latent ligands that then bind to and activate integrin receptors, although the role of integrins in IA memory specifically has not been tested. It is not clear at present whether MMP-9 participates in other forms of hippocampal-dependent or independent memory, for example, as part of a general mechanism of synaptic plasticity, or whether its role is limited to IA memory. Like MMP-9, integrins, as discussed above, are also required for LTP maintenance generally, but targeted deletion of the β1 subunit produces deficits in working memory selectively, without affecting spatial or contextual memories (Chan et al. 2006). It also remains unknown what the endogenous target substrates of MMP-9 proteolysis are during LTP or memory. Generally, MMPs cleave a potentially large number of extracellular matrix proteins and other cell-surface or cell-adhesion molecules (Sternlicht and Werb 2001), many of which are expressed in hippocampus and are involved in synaptic plasticity and/or memory (Strekalova et al. 2002; Dityatev and Schachner 2003). A recent study (Michaluk et al. 2007) has identified β-dystroglycan (β-dg) as an activity-dependent target of MMP-9 cleavage in cultured cortical neurons. β-dg is a transmembrane protein that links, via association with α-dystroglycan, proteins of the extracellular matrix with the actin cytoskeleton (Higginson and Winder 2005). Whether and under what natural conditions such cleavage would occur in vivo, and the functional consequences of such cleavage, remain unknown.
Another likely contribution of MMP-9 to mechanisms enabling long-term memory is to facilitate synaptic structural remodeling, since one canonical function of MMPs throughout all tissues is in remodeling of the cellular microenvironment (Stamenkovic 2003). Synaptic remodeling in hippocampus both accompanies long-lasting LTP (Hosokawa et al. 1995; Engert and Bonhoeffer 1999; Toni et al. 1999; Weeks et al. 2001; Lang et al. 2004; Matsuzaki et al. 2004; Nägerl et al. 2004) and occurs with several hippocampal-dependent associative learning and memory tasks (Geinisman et al. 2001; Eyre et al. 2003; Leuner et al. 2003; Rekart et al. 2007). Stereological analyses of synapse density in the dentate gyrus molecular layer have shown that spine number begins to increase ∼3 h post-IA training, peaks by 6 h, then returns to baseline values by 72 h (O'Malley et al. 2000). This time course of synaptic remodeling parallels that over which MMP-9 is regulated by IA training. MMPs can cleave structural molecules of the synapse, including, for example, cadherins (Steinhusen et al. 2001; Monea et al. 2006), and MMP-7 has been shown to influence spine morphology in cultured hippocampal neurons (Bilousova et al. 2006). Further, following LTP of CA3–CA1 synapses in vivo, focal “hotspots” of MMP-9-immunopositive gelatinolytic puncta are distributed throughout the neuropil of area CA1 stratum radiatum that codistributes with synaptic molecular markers, thus indicating that MMP-mediated proteolysis is ideally positioned to modify locally synaptic structure and function (Bozdagi et al. 2007).
The cellular pathways and mechanisms coupling IA training with MMP-9 up-regulation and activation remain unknown. NMDA receptor activity during IA training may be involved, as NMDA receptors are required both for IA learning (Izquierdo and Medina 1997), and for the up-regulation of MMP-9 protein levels and onset of MMP-9 proteolytic activity during hippocampal LTP (Nagy et al. 2006; Bozdagi et al. 2007). The up-regulation in MMP-9 protein levels likely reflects increased transcriptional and translational activity, as these represent major mechanisms regulating MMP levels in other tissues (Sternlicht and Werb 2001). Several temporally discrete waves of protein synthesis are triggered by IA training, including one that spans 3–6 h post-training (Quevedo et al. 1999), which corresponds to the time period when MMP-9 levels begin to rise. Further, the promoter region of MMP-9 contains binding sites for the transcription factor nuclear factor-kappa B (NF-κB) (Sato and Seiki 1993); transcriptional activity of NF-κB is increased following IA training with a time course consistent with driving the increase in MMP-9 levels as we have shown here (Freudenthal et al. 2005). Additionally, a recent microarray analysis of gene regulation following IA training revealed an overrepresentation of genes regulated at ∼3 h post-IA training that contained within their promoter regions NF-κB binding sites (O'Sullivan et al. 2007). In addition to MMP-9, it is likely that IA training induces changes in MMP regulatory proteins and possibly other MMPs. For example, TIMPs (tissue inhibitors of MMPs), which are endogenous protein inhibitors of active MMPs, are regulated by certain learning and memory tasks and, when eliminated genetically, display learning and memory deficits (Jaworski et al. 2005; Jourquin et al. 2005; Chaillan et al. 2006). It is possible that the narrower temporal window of significantly elevated proteolytic activity revealed by zymography (12–24 h) in comparison with the broader temporal window of significantly elevated levels of the active form of the protein revealed by immunoblot (6–48 h) reflects training-induced functional regulation of the active form of the protein by TIMPs.
In conclusion, the present observation that MMP proteolytic activity is both regulated during, and contributes to, an IA learning and memory task is important because a predominant view of MMP function in mature brain is that MMPs are activated under pathophysiological contexts (see the introduction). The present study contributes to a growing body of evidence that MMPs also play crucial roles in normal, nonpathological synaptic and behavioral plasticity.
Materials and Methods
Animals
This study was carried out on 104 adult male Long-Evans rats and 12 adult male Sprague-Dawley rats, all weighing 200–250 g at the time of behavioral testing or electrophysiology. The Sprague-Dawley rats were used only for in vivo electrophysiology as described below; all other experiments were conducted on the Long-Evans rats. All procedures involving live animals were approved by Mount Sinai’s Institutional Animal Care and Use Committee, and adhered strictly to Institutional and NIH guidelines for the care and treatment of animals. Rats were housed individually in a temperature- and light-controlled vivarium, and allowed free access to food and water. They were allowed minimally 5 d to habituate to the vivarium prior to experimentation.
Surgery
Rats were anesthetized with a mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg, i.m.) and placed in a stereotaxic instrument. Guide cannulae (22 gauge, Plastics One) were implanted bilaterally using the coordinates (from bregma): 4.0 mm posterior, ±2.6 mm lateral, and 2 mm ventral from the skull surface. Two anchoring screws fastened into the skull and dental cement were used to immobilize the cannulae, which were capped with stylets to prevent clogging. Animals were allowed to recover for 3 d after surgery, then were handled daily for an additional 7 d prior to IA training to habituate them to the infusion procedure. Twice prior to IA training (at 24 h before, and immediately before), an infusion needle extending 1.5 mm longer than the guide cannulae was briefly inserted into and withdrawn from the guide cannulae.
Inhibitory avoidance task
All behavioral training and testing were performed during the morning hours (9:00 am–12:00 pm) in a sound- and light-attenuated room. The IA apparatus consisted of a rectangular box divided into two compartments separated by a sliding door. The safe compartment was white and illuminated by a light attached to the lid; the shock compartment was dark. For IA training, animals were placed into the lit safe compartment facing away from the door, which was in the closed position. After 10 sec of exploration, the sliding door was raised automatically, allowing the rat to enter the dark compartment. At 1 sec after entering with all four paws, the door closed automatically, and a brief footshock (2 sec, 0.65 mA) was delivered via the floor grid. Animals were left in the dark compartment for 15 sec following the shock, then returned to their home cages for variable periods depending on the experiments as described in the Results. Rats were subsequently tested for IA memory at 24 or 72 h post-training by placing them back into the safe lit compartment as described. Retention was assessed by measuring their latency to reenter the dark compartment. The experiment was terminated either when the rat entered the dark compartment (in which case the door would close) or if 540 sec elapsed while they remained in the lit safe compartment. In the experiments in which animals were tested for retention at 72 h, those that reentered the dark chamber were given a second footshock (2 sec, 0.65 mA), returned to their home cages, then retested for IA memory 24 h later as described above. For biochemical experiments, controls consisted of home cage (HC) rats that were neither exposed to the training apparatus nor received a footshock, and unpaired (UP) control rats that were allowed to explore the compartments and received a footshock, but these two experiences were unpaired in time. This was accomplished by placing rats in the lit safe compartment and allowing them to enter and explore the dark compartment for 15 sec, but without receiving a footshock. Animals were returned to their home cages for 1 h, then brought back and footshocked directly (2 sec, 0.65 mA), without further exploration. Previous studies have shown that such UP controls fail to form IA memory or display IA training-induced changes in expression of certain genes (Garcia-Osta et al. 2006). After footshock, UP control rats were returned to their home cages and were sacrificed at 24 or 48 h post-shock.
Drug delivery
Rats received bilateral intrahippocampal infusions of MMP inhibitor or diluent at two time points (1.5 and 9.5 h) or one time point (3.5 h) post-IA training, depending on the experiment as described in the Results. Both the inhibitor and the diluent were delivered through an infusion needle inserted into, and extending 1.5 mm below, the guide cannulae, placing the tip into the hippocampus. The needle was attached, via polyethylene tubing, to a Hamilton syringe fitted into an infusion pump. A total of 2 μL of MMP inhibitor or diluent was delivered, per side, at a rate of 0.3 μL/min. The needle was left in place for 1–2 min to allow for diffusion into the parenchyma. Animals were subsequently returned to their home cages until tested for memory retention. To block MMP-9 activity, a potent gelatinase (MMP-2 and MMP-9) inhibitor was used (250 μM Inhibitor II [Calbiochem] diluted in 0.6% dimethylsulfoxide [DMSO]). This MMP inhibitor was chosen because its potency in blocking MMP-2 and MMP-9 proteolytic activity over a range of concentrations has been established previously using a fluorometric enzymatic assay (Nagy et al. 2006). Functionally, at the concentration used, Inhibitor II blocks the maintenance phase of LTP at CA3–CA1 synapses in acute slices and in adult animals in vivo, as well as blocks a slowly emerging synaptic potentiation induced by exogenously applied recombinant-active MMP-9 (Nagy et al. 2006; Bozdagi et al. 2007). Control animals received injections of the diluent (0.6% DMSO) alone. At the termination of the experiments, rats were killed by intracardiac perfusion (4% paraformaldehyde, 10 min). Brain sections were Nissl-stained and used to verify placement of the infusion needle.
Immunoblotting
IA training-induced changes in levels of MMP-9 were assessed by immunoblotting according to procedures described in detail previously (Bozdagi et al. 2007) with some modifications. None of the animals used for immunoblotting had received cannulae or any other kind of surgery. Hippocampi from IA-trained (n = 4 per time point), unpaired (n = 6) and home cage (n = 3) control animals were removed bilaterally at various times post-training, as indicated in the text, and snap frozen. Tissue was homogenized using a hand-held Teflon homogenizer in the following solution: 50 mM Tris-HC (pH 7.6), 150 mM NaCl, 5 mM CaCl2, 0.05% NaN3, and 1% Triton X-100. Homogenates were centrifuged at 4°C for 15 min at 12,000g. Pellets were discarded and supernatants were subjected to either SDS-polyacrylamide gel electrophoresis and Western blotting or in vitro gelatin-substrate zymography as described below. Fifty micrograms of tissue homogenate (determined by Bio-Rad Protein Assay) were mixed with 2× reducing sample buffer (0.125 M Tris-HCl, at pH 6.8, 20% glycerol, 4% SDS, 0.003% Bromophenol blue, and 5% 2-mercaptoethanol), boiled for 2 min, then loaded onto a 10% SDS-polyacrylamide gel and electrophoresed. Gels were then transferred onto membranes which were probed with antisera to MMP-9 (1:500; Torrey Pines Biolabs). The specificity of the anti-MMP-9 antisera has been verified previously (Nagy et al. 2006). The antisera recognize recombinant pro- and active-forms of MMP-9 in Western blots of recombinant proteins and hippocampal lysates, but does not cross-react with either form (pro- or active-) of the structurally and functionally related MMP-2. Following incubation in HRP-conjugated secondary antibodies, antibody binding was visualized using SuperSignal West Pico Lumino/Enhancer Solution (Pierce) and developed on X-Omat LS Imaging Film (Eastman Kodak). Blots were then stripped and reprobed with antisera to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:5000; Trevigen), which was used as a loading control. Blots were scanned and analyzed by Metamorph software using densitometry. Within each lane, pro- and active-MMP-9 band intensities were normalized against the appropriate GAPDH band intensities; data were expressed as a ratio of MMP-9 levels in trained animals versus UP animals, or UP animals versus HC animals.
In vitro gelatin substrate zymography
MMP-9 proteolytic activity was assessed by gelatin substrate zymography on the hippocampal protein extracts described in the preceding section. Zymographic methods were according to previous descriptions (Zhang and Gottschall 1997). Briefly, 6 mg of each hippocampal lysate were incubated with gelatin-coated sepharose beads (Amersham Biosciences) for 1 h at 4°C with constant agitation in order to extract MMP-9. Beads were briefly centrifuged, washed once with extraction buffer, then resuspended in extraction buffer with 10% DMSO. Samples (10 μL) were then electrophoresed after loading onto 5-mm thick, 10% SDS-polyacrylamide gels that had been copolymerized with 0.1% gelatin. Each gel contained a complete set of lysates from each post-training time point plus an UP control. Recombinant pro- and active-MMP-9 were also loaded to verify identity of MMP-9 bands. Gels were washed twice for 30 min with 2.5% Triton X-100 and incubated for 48 h at 37°C in assay buffer (21 mM Tris-HCl at pH 7.6, 10 mM CaCl2, and 0.04% NaN3). Clear bands of gelatinolysis were visualized against a dark background after gels were incubated in Coomasie blue and subsequently destained. Gels were then scanned with a flat-bed scanner at high resolution. For quantification, images were inverted in Adobe Photoshop and subjected to densitometric analysis of the active form of MMP-9 using Metamorph software. Within each gel, for each time point, the intensity of the active band was normalized to that of the UP control that was run on that same gel. The ratios at each time point were then averaged across gels (n = 4 rats per UP group or time point). Comparisons between HC and UP controls were conducted identically, except that levels of the active band from UP animals were normalized to those from HC animals.
In vivo electrophysiology
These experiments were carried out on anesthetized (urethane, 1.5 g/kg; i.p.) adult male rats. Details of the in vivo stimulation and recording methods used here for inducing LTP have been described previously (Bozdagi et al. 2007). Briefly, recordings of field excitatory postsynaptic potentials (fEPSPs) were made from the stratum radiatum in area CA1 in response to stimulation of the ipsilateral Schaffer collateral-commissural projection. After a baseline of synaptic responses was established, a single intrahippocampal infusion of Inhibitor II (250 μM, 2 μL) was given at one of several different sites that were grouped into three ranges: 0–500 μm, 600–1200 μm, and 1400–2100 μm away from the recording site (n = 4 rats per each grouped range). The infusion was delivered via an infusion cannula connected to a Hamilton syringe that was attached to the infusion pump, using identical delivery parameters to those used for the behavioral experiments described above. After Inhibitor II injection, baseline was recorded for an additional 30 min. Previous studies have shown that Inhibitor II has no effects on baseline properties of synaptic neurotransmission (Bozdagi et al. 2007). At the end of this period, LTP at CA3–CA1 synapses was induced by delivering four trains of 100 Hz, 1-sec tetanic stimulation, each separated by 5 min (Bozdagi et al. 2007). Synaptic responses were then collected for an additional 120 min post-tetanus, after which rats were killed by intracardiac perfusion (4% paraformaldehyde, 10 min). Brain sections (50 μm) were Nissl-stained and used to verify placement of stimulating/recording electrodes.
Statistical analyses
All data are expressed as mean values plus standard errors of the mean where P < 0.05 is considered significant. Western blots and zymographs were compared with unpaired Student’s t-tests using Prism 4 software. Behavioral data were analyzed using two-way analysis of variance (ANOVA; with entrance latency as a within-group variable, and drug treatment as a between-group variable) followed where relevant by Scheffe’s post-hoc tests (JMP version 5, SAS Institute, Inc.). In some cases, paired Student’s t-tests (within a group) or unpaired Student’s t-tests (between two groups) were also used to compare group mean training and retention entrance latencies.
Acknowledgments
This work was supported by The Mount Sinai School of Medicine and grant no. MH075783-01 from the National Institute of Mental Health (NIMH), United States Public Health Service. We thank Cristina Alberini, Sophie Tronel, Ana Garcia-Osta, and Stephen Taubenfeld for help in designing the behavior experiments, and Cristina Alberini and Peter Rapp for critically reading the manuscript.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.678307
References
- Arai K., Lee S.R., Lo E.H. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- Bahr B.A., Staubli U., Xiao P., Chun D., Ji Z.-X., Esteban E.T., Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: Pharmacological studies and the characterization of a candidate matrix receptor. J. Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Trifilo J.A., Kramar E.A., Torp R., Lin C.Y., Pineda E.A., Lynch G., Gall C.M. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J. Neurochem. 2005;93:834–849. doi: 10.1111/j.1471-4159.2005.03062.x. [DOI] [PubMed] [Google Scholar]
- Bevilaqua L.R., Medina J.H., Izquierdo I., Cammarota M. Memory consolidation induces N-methyl-D-aspartic acid-receptor- and Ca2+/calmodulin-dependent protein kinase II-dependent modifications in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor properties. Neuroscience. 2005;136:397–403. doi: 10.1016/j.neuroscience.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bilousova T.V., Rusakov D.A., Ethell D.W., Ethell I.M. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J. Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V.P., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bozdagi O., Shan W., Tanaka H., Benson D.L., Huntley G.W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Bozdagi O., Nagy V., Kwei K.T., Huntley G.W. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brown T.E., Forquer M.R., Cocking D.L., Jansen H.T., Harding J.W., Sorg B.A. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn. Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O., Schachner M., Dityatev A. Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J. Neurosci. 2007;27:6019–6028. doi: 10.1523/JNEUROSCI.1022-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Chen L.S., Gage F.H. Spatial organization of physiological activity in the hippocampal region: Relevance to memory formation. Prog. Brain Res. 1990;83:257–268. doi: 10.1016/s0079-6123(08)61255-8. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Napolitano M., Centonze D., Marfia G.A., Gubellini P., Teule M.A., Berretta N., Bernardi G., Frati L., Tolu M., et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur. J. Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- Cammarota M., Izquierdo I., Wolfman C., de Levi Stein M., Bernabeu R., Jerusalinsky D., Medina J.H. Inhibitory avoidance training induces rapid and selective changes in [3H]AMPA receptor binding in the rat hippocampal formation. Neurobiol. Learn. Mem. 1995;64:257–264. doi: 10.1006/nlme.1995.0008. [DOI] [PubMed] [Google Scholar]
- Chaillan F.A., Rivera S., Marchetti E., Jourquin J., Werb Z., Soloway P.D., Khrestchatisky M., Roman F.S. Involvement of tissue inhibition of metalloproteinases-1 in learning and memory in mice. Behav. Brain Res. 2006;173:191–198. doi: 10.1016/j.bbr.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.S., Weeber E.J., Kurup S., Sweatt J.D., Davis R.L. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J. Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.S., Weeber E.J., Zong L., Fuchs E., Sweatt J.D., Davis R.L. β1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J. Neurosci. 2006;26:223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D., Gall C.M., Bi X., Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience. 2001;105:815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- de Hoz L., Moser E.I., Morris R.G. Spatial learning with unilateral and bilateral hippocampal networks. Eur. J. Neurosci. 2005;22:745–754. doi: 10.1111/j.1460-9568.2005.04255.x. [DOI] [PubMed] [Google Scholar]
- Dityatev A., Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Engert F., Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Eyre M.D., Richter-Levin G., Avital A., Stewart M.G. Morphological changes in hippocampal dentate gyrus synapses following spatial learning in rats are transient. Eur. J. Neurosci. 2003;17:1973–1980. doi: 10.1046/j.1460-9568.2003.02624.x. [DOI] [PubMed] [Google Scholar]
- Fletcher B.R., Calhoun M.E., Rapp P.R., Shapiro M.L. Fornix lesions decouple the induction of hippocampal arc transcription from behavior but not plasticity. J. Neurosci. 2006;26:1507–1515. doi: 10.1523/JNEUROSCI.4441-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal R., Boccia M.M., Acosta G.B., Blake M.G., Merlo E., Baratti C.M., Romano A. NF-κB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur. J. Neurosci. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A., Tsokas P., Pollonini G., Landau E.M., Blitzer R., Alberini C.M. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. J. Neurosci. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y., Berry R.W., Disterhoft J.F., Power J.M., Van der Zee E.A. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J.F., McNaughton B.L., Barnes C.A., Worley P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Higginson J.R., Winder S.J. Dystroglycan: A multifunctional adaptor protein. Biochem. Soc. Trans. 2005;33:1254–1255. doi: 10.1042/BST0331254. [DOI] [PubMed] [Google Scholar]
- Hosokawa T., Rusakov D.A., Bliss T.V., Fine A. Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: Evidence for changes in length and orientation associated with chemically induced LTP. J. Neurosci. 1995;15:5560–5573. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Y., Bach M.E., Lipp H.P., Zhuo M., Wolfer D.P., Hawkins R.D., Schoonjans L., Kandel E.R., Godfraind J.M., Mulligan R., et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc. Natl. Acad. Sci. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Shimazu K., Woo N.H., Zang K., Muller U., Lu B., Reichardt L.F. Distinct roles of the β1-class integrins at the developing and the mature hippocampal excitatory synapse. J. Neurosci. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I., Medina J.H. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Jaworski D.M., Boone J., Caterina J., Soloway P., Falls W.A. Prepulse inhibition and fear-potentiated startle are altered in tissue inhibitor of metalloproteinase-2 (TIMP-2) knockout mice. Brain Res. 2005;1051:81–89. doi: 10.1016/j.brainres.2005.05.057. [DOI] [PubMed] [Google Scholar]
- Jorntell H., Hansel C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Jourquin J., Tremblay E., Bernard A., Charton G., Chaillan F.A., Marchetti E., Roman F.S., Soloway P.D., Dive V., Yiotakis A., et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) modulates neuronal death, axonal plasticity, and learning and memory. Eur. J. Neurosci. 2005;22:2569–2578. doi: 10.1111/j.1460-9568.2005.04426.x. [DOI] [PubMed] [Google Scholar]
- Kramar E.A., Bernard J.A., Gall C.M., Lynch G. α3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience. 2002;110:29–39. doi: 10.1016/s0306-4522(01)00540-1. [DOI] [PubMed] [Google Scholar]
- Kramar E.A., Lin B., Rex C.S., Gall C.M., Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc. Natl. Acad. Sci. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R., Farb C.R., Rodrigues S.M., LeDoux J.E. Fear conditioning drives profilin into amygdala dendritic spines. Nat. Neurosci. 2006;9:481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Lang C., Barco A., Zablow L., Kandel E.R., Siegelbaum S.A., Zakharenko S.S. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc. Natl. Acad. Sci. 2004;101:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B., Falduto J., Shors T.J. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo E.H., Wang X., Cuzner M.L. Extracellular proteolysis in brain injury and inflammation: Role for plasminogen activators and matrix metalloproteinases. J. Neurosci. Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Lopez-Fernandez M.A., Montaron M.F., Varea E., Rougon G., Venero C., Abrous D.N., Sandi C. Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J. Neurosci. 2007;27:4552–4561. doi: 10.1523/JNEUROSCI.0396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A., Parent J.-P., Figurov D., Muller D., Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Madani R., Hulo S., Toni N., Madani H., Steimer T., Muller D., Vassalli J.D. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Honkura N., Ellis-Davies G.C., Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T., Durkin T.P., Menzaghi F., Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McFarlane S. Metalloproteases: Carving out a role in axon guidance. Neuron. 2003;37:559–562. doi: 10.1016/s0896-6273(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Meighan S.E., Meighan P.C., Choudhury P., Davis C.J., Olson M.L., Zornes P.A., Wright J.W., Harding J.W. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Michaluk P., Kolodziej L., Mioduszewska B., Wilczynski G.M., Dzwonek J., Jaworski J., Gorecki D.C., Ottersen O.P., Kaczmarek L. β-Dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Mifune M., Ohtsu H., Suzuki H., Nakashima H., Brailoiu E., Dun N.J., Frank G.D., Inagami T., Higashiyama S., Thomas W.G., et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- Milekic M.H., Alberini C.M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Monea S., Jordan B.A., Srivastava S., DeSouza S., Ziff E.B. Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J. Neurosci. 2006;26:2300–2312. doi: 10.1523/JNEUROSCI.3521-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Woessner J.F. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nägerl U.V., Eberhorn N., Cambridge S.B., Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nagy V., Bozdagi O., Matynia A., Balcerzyk M., Okulski P., Dzwonek J., Costa R.M., Silva A.J., Kaczmarek L., Huntley G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K., Tanaka H., Yagita Y., Saeki Y., Taguchi A., Hiraoka Y., Zeng L.H., Colman D.R., Miki N. Cadherin activity is required for activity-induced spine remodeling. J. Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okulski P., Jay T.M., Jaworski J., Duniec K., Dzwonek J., Konopacki F.A., Wilczynski G.M., Sanchez-Capelo A., Mallet J., Kaczmarek L. TIMP-1 abolishes MMP-9-dependentlong-lasting long-term potentiation in the prefrontal cortex. Biol. Psychiatry. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- O'Malley A., O'Connell C., Murphy K.J., Regan C.M. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- O'Sullivan N.C., McGettigan P.A., Sheridan G.K., Pickering M., Conboy L., O'Connor J.J., Moynagh P.N., Higgins D.G., Regan C.M., Murphy K.J. Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning. J. Neurochem. 2007;101:1085–1098. doi: 10.1111/j.1471-4159.2006.04418.x. [DOI] [PubMed] [Google Scholar]
- Pastalkova E., Serrano P., Pinkhasova D., Wallace E., Fenton A.A., Sacktor T.C. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pawlak R., Nagai N., Urano T., Napiorkowska-Pawlak D., Ihara H., Takada Y., Collen D., Takada A. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001. doi: 10.1016/s0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Quevedo J., Vianna M.R., Roesler R., de-Paris F., Izquierdo I., Rose S.P. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: Protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn. Mem. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves T.M., Prins M.L., Zhu J., Povlishock J.T., Phillips L.L. Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J. Neurosci. 2003;23:10182–10189. doi: 10.1523/JNEUROSCI.23-32-10182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekart J.L., Sandoval C.J., Bermudez-Rattoni F., Routtenberg A. Remodeling of hippocampal mossy fibers is selectively induced seven days after the acquisition of a spatial but not a cued reference memory task. Learn. Mem. 2007;14:416–421. doi: 10.1101/lm.516507. [DOI] [PubMed] [Google Scholar]
- Reymann K.G., Frey J.U. The late maintenance of hippocampal LTP: Requirements, phases, 'synaptic tagging', 'late-associativity' and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Hess G., Donoghue J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Donoghue J.P. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rogan M.T., Staubli U.V., LeDoux J.E. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rumpel S., LeDoux J., Zador A., Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sato H., Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Silva A.J. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J. Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Snoek-van Beurden P.A.M., Von den Hoff J.W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Staubli U., Vanderklish P., Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behav. Neural Biol. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- Steinhusen U., Weiske J., Badock V., Tauber R., Bommert K., Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J. Biol. Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T., Sun M., Sibbe M., Evers M., Dityatev A., Gass P., Schachner M. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol. Cell. Neurosci. 2002;21:173–187. doi: 10.1006/mcne.2002.1172. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A., Lapinska J., Rylski M., McKay R.D., Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Watanabe F., Nakatani T., Yasui K., Fuji M., Komurasaki T., Tsuzuki H., Maekawa R., Yoshioka T., Kawada K., et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-Sulfonylamino acid derivatives. J. Med. Chem. 1998;41:640–649. doi: 10.1021/jm9707582. [DOI] [PubMed] [Google Scholar]
- Tamura H., Ishikawa Y., Hino N., Maeda M., Yoshida S., Kaku S., Shiosaka S. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J. Physiol. 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Hung C.P., Schuman E.M. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S.M., Wiig K.A., Bear M.F., Alberini C.M. A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S.M., Wiig K.A., Monti B., Dolan B., Pollonini G., Alberini C.M. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δ co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J. Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N., Buchs P.A., Nikonenko I., Bron C.R., Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Weeks A.C., Ivanco T.L., Leboutillier J.C., Racine R.J., Petit T.L. Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: III. Long-term maintenance phase. Synapse. 2001;40:74–84. doi: 10.1002/1098-2396(200104)40:1<74::AID-SYN1028>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Whitlock J.R., Heynen A.J., Shuler M.G., Bear M.F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Gottschall P.E. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. J. Neurosci. Methods. 1997;76:15–20. doi: 10.1016/s0165-0270(97)00065-4. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Deb S., Gottschall P.E. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur. J. Neurosci. 1998;10:3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]