Abstract
Oxytocin (OT) has been implicated in reproductive functions, induction of maternal behavior as well as endocrine and neuroendocrine regulation of the cardiovascular system. Here we demonstrate that neonatal manipulation of OT can modulate the mRNAs expression for OT receptor (OTR), atrial natriuretic peptide (ANP), endothelial nitric oxide synthase (eNOS) and estrogen receptor alpha (ERα) in the heart. On the first day of postnatal life, female and male rats were randomly assigned to receive one of following treatments; (a) 50 µl i.p. injection of 7 µg OT, (b) 0.7 µg of OT antagonist (OTA), or (c) isotonic saline (SAL). Hearts were collected either on postnatal day 1 or day 21 (D1 or D21) and the mRNAs expression of OTR, ANP, inducible NOS (iNOS), eNOS, ERα and estrogen receptor beta (ERβ) were compared by age, treatment, and sex utilizing Real Time PCR. OT treatment significantly increased heart OTR, ANP and eNOS mRNAs expression on D1 in both males and females, ERα increased only in females. While there were significant changes in the relative expression of all types of mRNA between D1 and D21 there were no significant treatment effects observed in D21 animals. OTA treatment significantly decreased basal ANP and eNOS mRNAs expression on D1 in both sexes. The results indicate that during the early postnatal period OT can have an immediate effect on the expression OTR, ANP, eNOS, and ERα mRNAs and that these effects are mitigated by D21. Also with the exception of ERα mRNA, the effects are the same in both sexes.
Keywords: Oxytocin, Heart, Oxytocin receptor, NOS, Estrogen receptors
1. Introduction
The neurohypophysial hormone oxytocin (OT) was the first peptide hormone to be chemically synthesized in biologically active form [13]. OT is synthesized primarily in magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus. It is also produced in peripheral tissues [26], including the heart [22]. In addition to OT effects on reproductive functions and induction of maternal behavior, it is involved in endocrine and neuroendocrine regulation of the heart, vasculature, and kidneys [15, 38, 17]. It has been reported that OT acts via neuroendocrine-endocrine-paracrine pathways to regulate blood volume via its natriuretic properties and to modulate blood pressure by stimulating the release of atrial natriuretic peptide (ANP) [15, 17]. In addition, it was demonstrated that in isolated, perfused hearts, an OT antagonist (OTA) blocks basal ANP release [15]. ANP induces vasorelaxation of coronary arteries, inhibition of L-type Ca2+ channels in the myocardium, and suppression of the reninangiotensin system [39]. These effects are recognized as being protective to the cardiovascular system and are also induced by estrogen [27]. Empirical studies suggest that OT may affect or regulate the function of the heart through several different mechanisms.
OT stimulates release of nitric oxide (NO) from human umbilical vein endothelial cells in culture [38]. These findings have led to the suggestion that OT may be an important mediator of vascular function [16]. NO is synthesized from L-arginine in an enzymatic reaction catalyzed by nitric oxide synthase (NOS) [29]. Three different isoforms of NOS catalyze the oxidation of L-arginine to citrulline and NO: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) NOS. Myocardial eNOS appears to be important under physiologic conditions in pregnancy, where it is involved in cardiac hypertrophy [40].
Estrogen (E) stimulates NOS in various organs, including cardiac tissue, in association with an increase of cyclic guanosine monophosphate (cGMP) [42], the second messenger of NO. Recent observations suggest that E’s effects in vascular cells, and possibly in the myocardium, depend on the relative expression of alpha and beta estrogen receptors (ERα and ERβ) [20]. Interactions between OT and E have been reported in several systems. In MCF7 breast cancer cell lines treatment with OT inhibited the ability of E to stimulate mitosis [9]. OT treatment had a number of effects on ERα in the MCF7 cell line, including changes in the production of ERα mRNA, binding affinity, and ERα transcriptional activity [8].
In rats, neonatal treatment with OT produced marked changes in weight and responses to pain [41] and placental and fetal growth during pregnancy as adults [36]. In prairie voles, a single treatment on the day of birth with OT or OTA affected partner preference formation, aggression and reproductive competency in adults [6, 31, 4]. In addition, a single injection of OT or OTA on the day of birth produced a significant increase in OT immunoreactivity in the brain by postnatal day 21 [43]. Based on previous studies from our laboratory and others showing that neonatal exposure to exogenous OT can have long-term effects on the subsequent expression of adult behavior and physiology [6, 7, 31, 36, 41, 43], we used postnatal day 1 (D1) and day 21 (D21) female and male rats in the current study. In an attempt to define the possible role of OTR, ANP, NOS and ERs during the early postnatal effects of OT on the heart, animals were treated with OT or OTA on the day of birth and the mRNAs expression for OTR, ANP, iNOS, eNOS, ERα and ERβ in postnatal day 1 (D1) and day 21 (D21) female and male rats were measured utilizing real time reverse transcription-polymerase chain reaction (RT-PCR).
2. Material and methods
2.1. Animals
Female and male Sprague—Dawley rats (Taconic Farms, Germantown, NY) were used to establish a breeding colony and F1 progeny were used as experimental animals. Due to potential differences between first and subsequent litters, first litters were not used in these studies. Within 24 h of birth, litters were sexed, toe-clipped for identification and culled to 10 pups. Litters were culled to ensure that litter size was uniform, so that feeding demands would be similar between litters. Only mixed-sex litters were used and single-sex litters were not created by culling. Female and male pups were weighed and randomly assigned to one of three treatment groups, with each treatment group being represented at least once per litter. The animals were maintained on a 12:12 h light/dark cycle and provided Purina rat chow and water ad libitum. They were housed in accordance with the USDA and NIH guidelines, and the University of Illinois at Chicago Animal Care and Use Committee approved all procedures described in this study.
2.2. Treatment
On the day of birth (D1), pups (females and males) were given a single intraperitoneal injection (50 µl volume) of isotonic saline (SAL), 7 µg OT/50 µl saline (OT), or 0.7 µg of OT antagonist/50 µl saline (OTA), with an n of 10 per treatment per sex. The dosage of OT was approximately 1 µg/g body weight and for OTA [d(CH2)5, Tyr(Me)², Orn8]-vasotocin, (Peninsula Laboratories, A Division of Bachem, Belmont, CA, USA) was approximately 0.1 µg/g body weight. These doses of OT and OTA were used because there is extensive literature indicating that during the neonatal period these doses can affect a variety of physiological and behavioral responses in both rats and voles [25, 5, 43], as well as affect neuronal activation in neonates [12]. Hearts were collected from half of the treated animals on D1, 2 hours following treatment, while the other half were collected on postnatal day 21 (D21). To collect hearts animals were deeply anesthetized, using a combination of ketamine and xylazine, and then decapitated. The heart was dissected on ice and frozen in dry ice, and then stored at −80°C until RNA extraction.
2.3. RNA extraction
Total RNAs were extracted from each frozen heart tissue according to previous study [2] with slight modification. Briefly, each frozen tissue was homogenized in 5–10 ml LiCl-urea (3M LiCl, 6M urea) per g tissue and incubated overnight at 4°C. Homogenate has then been transferred to Corex tubes and centrifuged at 8000 rpm for 25 min. Supernatant was discarded and the walls of the tubes wiped with cotton swabs. Cold LiCl-urea was added to the tubes at half volume and the process repeated. Then the pellet was dissolved in half volume 10mM Tris pH 7.5, 1mM EDTA, 0.5% SDS. An equal volume of phenol:choloroform:isoamyl alcohol (25:24:1) was added and vortexed. Then the samples were centrifuged at 5000 rpm for 15 min and the upper aqueous layer transferred to the new tubes. Next, 100% EtOH was added to the each sample at 2x the volume plus 10M of NH4OAc at 1/10x the volume and mixed. RNA was precipitated on dry ice for 15 min and then centrifuged at 5000 rpm for 25 min. The pellet has then been rinsed with 70% ETOH and re-suspended in 300µl diethyl pyrocarbonate-treated (DEPC) water. The amount of RNA was estimated by spectrophotometry at 260 nm and the integrity of RNA was verified by agarose gel electrophoresis with ethidium bromide staining.
2.4. Real-time quantitative RT-PCR
cDNA synthesis and polymerase chain reaction (PCR) amplification for OTR, ANP, NOS (iNOS & eNOS) and ERs (ERα & ERβ) was performed using the LightCycler™ (Roche Diagnostic). As we described previously [32], this technique allows amplification and kinetic detection in a single microcapillary tube (Roche Diagnostic). Briefly, cDNA was synthesized using the Superscript-II™ system (Invitrogen Inc.). Oligo (dT) primers were added to 4 µg of total RNA and the samples were denatured at 70°C. MMLV-reverse transcriptase and dNTPs were added to the samples, which were further incubated at 42 °C for 50 min. The reaction was stopped by incubating the samples at 70 °C for 15 min, and the cDNA product was stored at −20 °C. Aliquots (2 µl) of the reverse transcription (RT) products were used for quantification in the LightCycler™ PCR and detection system using the FastStart DNA Master SYBR Green I kit (both from Roche Molecular Biochemicals) as described by the manufacturer.
PCR was set up using 2.5 µl of 10× PCR buffer, 1.25 µl of 5 mM dNTP, supplemented with 1.2 µl of 50 mM MgCl2, 1.0 µl of each forward and reverse 10 µM primers (Table 1), 0.5 µl of 5 U/µl platinum Taq polymerase (Invitrogen Inc.), 0.75 µl of SYBR Green I (Molecular Probes, Eugene, OR, USA), 10.8 µl of H2O, and 1.0 µl of cDNA equivalent to 20 ng total RNA to give a final reaction volume of 20 µl. The sequence of the specific primers for OTR, ANP, iNOS, eNOS, ERα, ERβ and βactin (β-actin was used as a control for the amount of amplified cDNA chosen over at least one intron) was chosen by previous studies [40, 14]. The sequences for the primer pairs are listed in Table 1. As the PCR protocols for the genes were optimized, melting curve analysis (MCA) with fluorescence acquisition was used to determine melting temperature (Tm) of specific amplification products and primer–dimers. For each gene, the specific Tm values were used to create a signal acquisition step (2–3 °C below Tm), which was added onto each elongation period. This phase allows for signal acquisition from specific products without signal interference from primer–dimers and non-specific amplification products. The levels of mRNA were quantified by using the standard curve method. Standard curves for all target genes were constructed by using serially diluted standard template. All PCR reactions were subjected to MCA to determine specificity of amplification. As an additional control of specificity, PCR products were size-fractionated on 2.5% agarose gels and visualized under ultraviolet light. The data were normalized to β-actin to account for differences in reverse transcription efficiencies and amount of template in the reaction mixtures using LightCycler software.
Table 1.
Primer sequences and real-time RT-PCR conditions
| Primers | Product (bp) | PCR parameters |
|
|---|---|---|---|
| Phase | Temperature in °C (time in s) | ||
| Otr | 370 | Denaturation | 95(0) |
| Sense 5′-GTCAATGCGCCCAAGGAAG-3′ | Annealing | 57(5) | |
| Antisense 5′-GTCAATCCTACCCCCGAAGCAGCT-3′ | Extension | 72(10) | |
| Signal | 83(1) | ||
| ANP | 316 | Denaturation | 95(0) |
| Sense 5′-CAGCATGGGCTCCTTCTCCA-3′ | Annealing | 58(5) | |
| Antisense 5′-GTCAATCCTACCCCCGAAGCAGCT-3′ | Extension | 72(10) | |
| Signal | 83(1) | ||
| iNOS | 220 | Denaturation | 95(0) |
| Sense 5′-ATGGAACAGTATAAGCGAAACACC-3′ | Annealing | 57(5) | |
| Antisense 5′-GTTTCCGGTCGATGTCATGAGCAAAGG-3′ | Extension | 72(10) | |
| Signal | 83(1) | ||
| eNOS | 207 | Denaturation | 95(0) |
| Sense 5′-GCAAGACCGATTACACGACA-3′ | Annealing | 57(5) | |
| Antisense 5′-GTCCTCAGGAGGTCTTGCAC-3′ | Extension | 72(10) | |
| Signal | 85(1) | ||
| ERα | 344 | Denaturation | 95(0) |
| Sense 5′-AATTCTGACAATCGACGCCAG-3′ | Annealing | 59(5) | |
| Antisense 5′-GTGCTTCAACATTCTCCCTCCTC-3′ | Extension | 72(10) | |
| Signal | 83(1) | ||
| ERβ | 262 | Denaturation | 95(0) |
| Sense 5′-TTCCCGGCAGCACCAGTAACC-3′ | Annealing | 60(5) | |
| Antisense 5′-TCCCTCTTTGCGTTTGGACTA-3′ | Extension | 72(10) | |
| Signal | 83(1) | ||
| β-Actin | 214 | Denaturation | 95(0) |
| Sense 5′-CCTCTATGCCAACACAGTGC-3′ | Annealing | 58(5) | |
| Antisense 5′-CATCGTACTCCTGCTTGCTG-3′ | Extension | 72(8) | |
| Signal | 83(1) | ||
2.5. Statistical analysis
Data are presented as the mean ± SEM. Statistical comparisons were made using one-way ANOVA followed by Fisher's protected least squares difference test. A level of p<0.05 was accepted as statistically significant.
3. Results
OT receptor and ANP mRNAs expression in the heart of female and male postnatal day 1 and 21 rats following neonatal SAL, OT or OTA administration
Neonatal treatment with OT significantly increased OTR and ANP mRNAs expression on postnatal D1, but not D21, in both sexes compared with SAL groups (Fig. 1A, B, C & D). The OTR mRNA expression was significantly decreased by postnatal D21 in both female and male rats compared with D1 animals. Neonatal treatment with OTA showed no effect on cardiac OTR mRNA expression (Fig. 1A, B). The basal levels of ANP mRNA expression in D21 groups were higher than those of D1 groups. Neonatal OTA treatment decreased basal heart ANP mRNA expression in both sexes compared with SAL groups (Fig. 1C, D).
Figure 1.
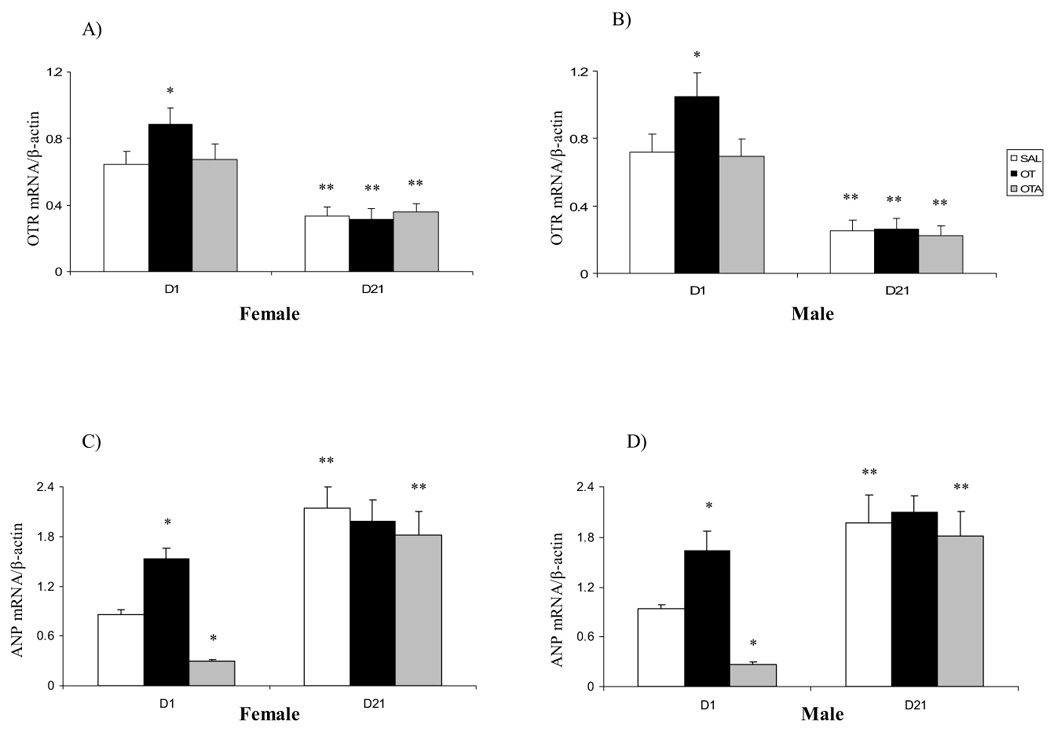
Effect of neonatal treatment with SAL, OT or OTA on heart OTR (A, B) and ANP (C, D) mRNAs expression on postnatal day 1 or 21 in female and male rats. Data represent the mean ± SEM (n=5). * represents significant alteration at p<0.05, when compared to SAL groups within the same age and sex, ** represents significant alteration at p<0.01, when compared to D1 groups within the same treatment and sex.
iNOS and eNOS mRNAs expression in the heart of female and male postnatal day 1 and 21 rats following neonatal SAL, OT or OTA administration
Neonatal treatment with OT or OTA did not affect iNOS mRNA expression of either sex (Fig. 2A, B). We observed a significant increase in eNOS mRNA expression on postnatal D1 in female and male animals following OT treatment compared with SAL groups (Fig. 2C, D). No effect of neonatal treatment with OT was seen on eNOS mRNA expression in postnatal D21 animals. We found that neonatal treatment with OTA decreased basal cardiac eNOS mRNA expression in both sexes compared with SAL groups (Fig. 2C, D).
Figure 2.
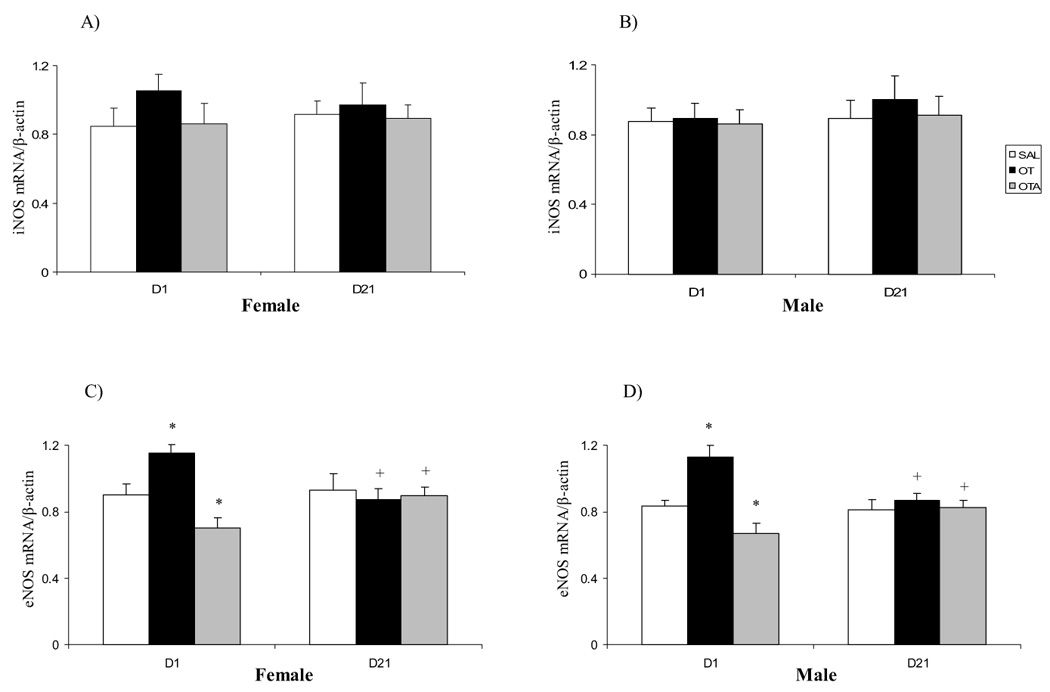
Effect of neonatal treatment with SAL, OT or OTA on heart iNOS (A, B) and eNOS (C, D) mRNAs expression on postnatal day 1 or 21 in female and male rats. Data represent the mean ± SEM (n=5). * represents significant alteration at p<0.05, when compared to SAL groups within the same age and sex, + represents significant alteration at p<0.05, when compared to D1 groups within the same treatment and sex.
ERα and ERβ mRNAs expression in the heart of female and male postnatal day 1 and 21 rats following neonatal SAL, OT or OTA administration
ERα mRNA expression was increased in female (Fig. 3A), but not in male (Fig. 3B), postnatal D1 rats following OT administration compared with SAL groups. ERα mRNA expression in postnatal D21 female and male rats was significantly higher than those in D1 rats (Fig. 3A, B). No effect of OTA treatment on cardiac ERα mRNA expression was seen in either sex (Fig. 3A, B). Similar ERβ mRNA analysis showed age-related changes opposite to those of ERα mRNA. We found that ERβ mRNA expression on postnatal D21 female (Fig. 3C) and male (Fig. 3D) rats was significantly lower than those in D1 rats. No effect of OT or OTA treatment on heart ERβ mRNA expression was seen in either sex (Fig. 3C, D).
Figure 3.
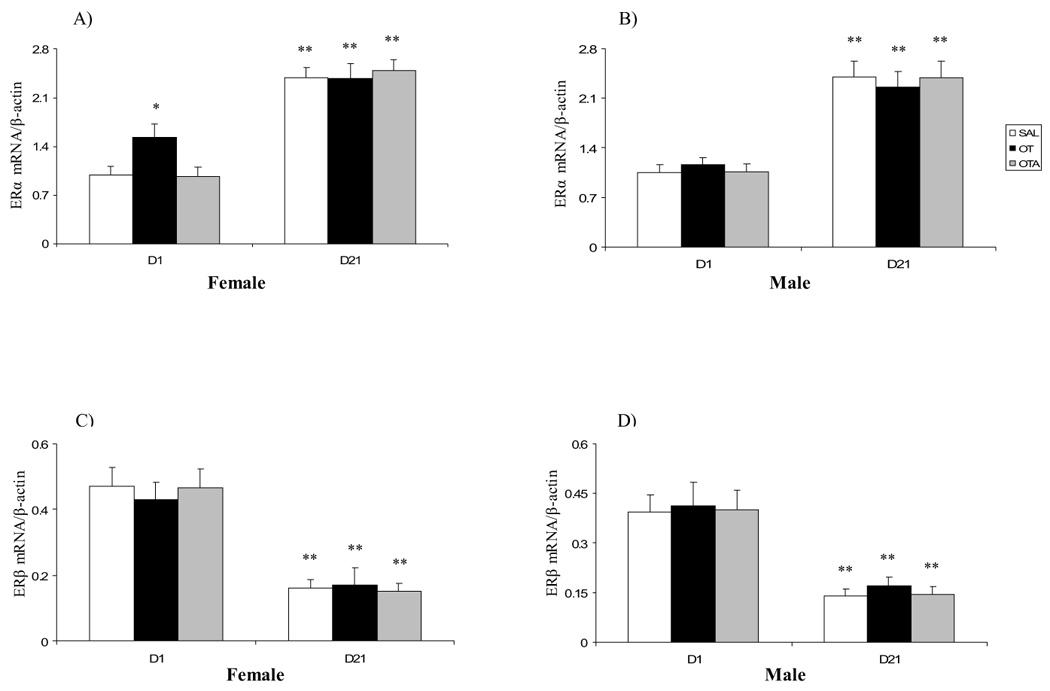
Effect of neonatal treatment with SAL, OT or OTA on heart ERα (A, B) and ERβ (C, D) mRNAs expression on postnatal day 1 or 21 in female and male rats. Data represent the mean ± SEM (n=5). * represents significant alteration at p<0.05, when compared to SAL groups within the same age and sex, ** represents significant alteration at p<0.01, when compared to D1 groups within the same treatment and sex.
4. Discussion
In this study we examined the possible involvement of cardiac OTR, ANP, NOS (iNOS and eNOS) and estrogen receptors (ERα and ERβ) mRNAs expression in the OT actions on the heart in postnatal D1 and D21 female and male rats. The results indicated that OTR, ANP, eNOS and ERα mRNAs expression can be modulated by OT exposure in early postnatal life and this modulation is sexually dimorphic for ERα mRNA expression. In addition to the potential effects of OT on cardiac development we also found that there were significant changes in the expression of mRNAs for OTR, ANP and ERs from the day of birth until weaning on D21.
In the present study we observed an age-dependent decline in cardiac OTR mRNA expression in female and male rats, suggesting that cardiac OTR may be functional in the fetal life. The importance of the OT system during rodents development was validated by a study showing that OT and OTR are expressed in the fetal rat heart and decrease to relatively low levels in adulthood [21]. These findings suggest that cardiac OT may be functional in fetal life, in contrast to low OT synthesis [1] and the low concentration of biologically active OT in the hypothalamus [21]. It might be speculated that OT production in the heart during fetal life and early maturation may supplement low OT production in hypothalamic nuclei. This hypothesis is supported by high plasma OT levels in newborn rats that exceed those of adult rats [19]. We found that early neonatal OT administration caused a significant increase in cardiac OTR mRNA expression of postnatal D1 female and male rats, and OTA treatment elicited no changes in cardiac OT receptors mRNA expression, suggesting that OTR may be one factor that mediates the effects of OT on the cardiovascular system in early life. This is in agreement with a previous study in which P19 embryonic carcinoma cells, a model of mouse embryonic stem cells, express OT receptors (OTRs), and OT stimulates the differentiation of these cells [30]. It was reported that isolated rat heart perfusion with OT induced significant bradycardia [15]. Perfusion of isolated rat hearts with OT results in a dose-dependent negative chronotropic effect while exerting a positive inotropic effect [11]. Further, it was reported that perfusion with OTA alone did not have any effect on heart rate and force of contraction. However, coadministration with OTA completely inhibited the effects of OT on beating rate and force, which suggested that these effects are mediated by OT receptors [28]. Oxytocin receptors in the heart may be localized on intrinsic cholinergic neurons, and upon activation, they release acetylcholine (ACh) to decrease heart rate and force of contraction [28]. Oxytocin may act in an autocrine/paracrine manner to modulate the release of ACh from intrinsic cardiac cholinergic neurons [28], to clarify the OTR localization. It has been shown that stimulation of oxytocin receptors leads to elevation of intracellular Ca2+. Increased Ca2+ stimulate cellular exocytosis [24] and also stimulate ANP secretion by the heart [34].
Because ANP transcription changes during maturation, we have investigated ANP mRNA expression in early postnatal life (D1) and at weaning (D21) in female and male rats. We found that OT administration to female and male rats on the first day of life caused a significant increase in cardiac ANP mRNA expression on postnatal D1 in both sexes, suggesting that ANP may play a role on the effects of OT in rat heart. Haanwinckel and collaborators have shown that OT administration in rats promotes an increase in ANP plasma levels [17]. In the present study neonatal treatment with OTA decreased basal ANP mRNA expression. Several studies have demonstrated that perfusion of isolated rat hearts with OT stimulates ANP release, and an OT receptor antagonist after prolonged perfusion decreases the OT-induced ANP release below that of control hearts [15, 22, 16]. Taken together, our results, and others in which OTA attenuates ANP release, suggest the presence of an intracardiac oxytocinergic system controlling basal ANP release. OT may contribute to the natriuretic action via stimulation of ANP release. Presumably, blood volume expansion via baroreceptor input to the brain causes the release of OT that circulates to the heart. OT-induced ANP release in the heart may be achieved after activation of OT receptors and subsequent elevation of intracellular [Ca2+], which in turn could stimulate exocytosis and ANP secretion [22]. ANP then exerts a negative chrono- and inotropic effect via activation of guanylyl cyclase and release of cGMP [22].
In the present study we have also measured rat heart iNOS and eNOS mRNAs expression in different ages and sexes. We found no changes in cardiac iNOS mRNA expression, but a significant increase in cardiac eNOS mRNA expression following OT treatment and a significant decrease after OTA treatment in female and male postnatal day 1 rats, suggesting that OTR may play a role in OT-induced eNOS mRNA expression in heart. In a study using isolated right atria, it was shown that oxytocin effects on beating rate and force of contraction are totally blocked by the muscarinic blocking drug atropine [19]. Because atropine suppresses transmission from the postganglionic fiber to the cardiac effecter cells, these results imply that oxytocin may primarily act on oxytocin receptors present on parasympathetic postganglionic fibers to stimulate the release of acetylcholine, which consequently acts on muscarinic receptors and results in lower rate and force of contraction. Activation of cardiac muscarinic receptors may influence cardiac functions through regulation of the activity of several ion channels. Muscarinic receptor activation inhibits cardiac L-type calcium channel through interaction with G Proteins [33]. This inhibition is mediated in part via activation of NO synthase and generation of NO, which stimulates soluble guanylyl cyclase to produce cGMP [18], subsequent activation of cGMP-dependent protein kinase G, and dephosphorylation of potassium and calcium channel proteins. We have shown that neonatal OT treatment caused a significant increase in cardiac OTR and eNOS mRNAs expression on postnatal D1. OT-induced activation of OTR would generate NO that would activate guanylyl cyclase, leading to production of cGMP that would dilate the vascular smooth muscle [35], suggesting that the physiological action of OT is vasodilatory and its action is mediated by OT receptors.
It seems that different mechanisms and/or factors may be involved differentially in the effects of OT on the heart. Because of the abundance of estrogen receptors on the heart in rats [37] and the presence of estrogen receptors in myocytes secreting ANP [3], it was hypothesized that estrogen induces ANP synthesis and release from the heart through an ER-dependent mechanism. Since interactions between OT and estrogen have been reported in several systems, we also investigated ERα and ERβ mRNAs expression in early postnatal life and in juvenile female and male rats. Utilizing Real Time PCR analysis, we found that ERs are developmentally regulated in the rat heart. We observed that ERα expression rises from low levels on postnatal D1, whereas ERβ mRNA decreases by postnatal D21 in the rat heart, suggesting a specific role for both receptors in heart maturation. These results are in agreement with previous study in which high ERβ expression was observed in the newborn heart and at 4 days of postnatal life [23], when extensive hyperplasia of the rat heart occurs and the heart grows more rapidly than the body [11]. In adult rats, ERβ expression is low, in contrast to relatively high ERα mRNA levels. Age-dependent increases in ERα suggest that this receptor plays a role in heart maturation. We found no changes in cardiac ERβ mRNA expression, but a significant increase in cardiac ERα mRNA expression following OT treatment only in females on postnatal day 1, suggesting that ERα mRNAs expression can be modulated by OT exposure in early postnatal life, and this modulation is sexually dimorphic. Additional studies will be required to determine the mechanisms of differential modulation of ERs mRNA across age and sex in response to neonatal OT manipulation. Moreover, since maternal OT sharply rises during labor, this OT elevation might modulate expression of OTR, ERs or NOS mRNAs in the neonatal rat heart. Perhaps due to the short time effect of OT on gene expression in the neonatal heart, we could not detect the possible modulation of these mRNAs expression in the current studies. However, additional studies will be required also to evaluate the possible effect of maternal OT on the neonatal cardiovascular system.
In summary, we have investigated the effect of early postnatal OT manipulation on cardiac OTR, ANP, iNOS, eNOS and ERs mRNAs expression in neonatal or juvenile female and male rats. We show that cardiac OTR, ANP, eNOS and ERα mRNAs expression can be modulated by OT exposure in early postnatal life and this modulation is sexually dimorphic for ERα mRNA expression.
Acknowledgements
We thank Dr. Angela J. Grippo, Brain-Body Center, College of Medicine, University of Illinois at Chicago for her valuable comments on the manuscript. This study was supported by National Institute of Child Health and Human Development HD38490 (CSC) and National Institute of Mental Health MH073022 (CSC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almazan G, Lefebvre DL, Zingg HH. Ontogeny of hypothalamic vasopressin, oxytocin and somatostatin gene expression. Dev Brain Res. 1989;45:69–75. doi: 10.1016/0165-3806(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 2.Auffray C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980;107:303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- 3.Back H, Forssmann WG, Stumpf WE. Atrial myoendocrine cells (cardiodilatin/atrial natriuretic polypeptide-containing myocardiocytes) are target cells for estradiol. Cell Tissue Res. 1989;255:673–674. doi: 10.1007/BF00218808. [DOI] [PubMed] [Google Scholar]
- 4.Bales KL, Abdelnabi M, Carter CS. Neonatal injections affect reproductive parameters in male prairie voles. Horm Behav. 2001;39:324. [Google Scholar]
- 5.Bales KL, Abdelnabi M, Cushing BS, Ottinger MA, Carter CS. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles. Physiol Behav. 2004;81:519–526. doi: 10.1016/j.physbeh.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Bales KL, Carter CS. Oxytocin facilitates parental care in female prairie voles (but not in males) Horm Behav. 2002;41:456. [Google Scholar]
- 7.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 8.Cassoni P, Catalano MG, Sapino A, Marrocco T, Fazzari A, Bussolati G, Fortunati N. Oxytocin modulates estrogen receptor alpha expression and function in MCF7 human breast cancer cells. Int J Oncol. 2002;21:375–378. [PubMed] [Google Scholar]
- 9.Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Oncol. 1997;72:340–344. doi: 10.1002/(sici)1097-0215(19970717)72:2<340::aid-ijc23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Clubb FJ, Bishop SP. Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab Invest. 1984;50:571–577. [PubMed] [Google Scholar]
- 11.Coulson CC, Thorp JM, Jr, Mayer DC, Cefalo RC. Central hemodynamic effects of oxytocin and interaction with magnesium and pregnancy in the isolated perfused rat heart. Am J Obstet Gynecol. 1997;177:91–93. doi: 10.1016/s0002-9378(97)70443-5. [DOI] [PubMed] [Google Scholar]
- 12.Cushing BS, Yamamoto Y, Carter CS, Hoffman GE. Central c-Fos expression in neonatal male and female prairie voles in response to treatment with oxytocin. Dev Brain Res. 2003;143:129–136. doi: 10.1016/s0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 13.Du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–957. [PubMed] [Google Scholar]
- 14.Farhangkhoee H, Khan ZA, Mukherjee S, Cukiernik M, Barbin YP, Karmazyn M, Chakrabarti S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J Mol Cel Card. 2003;35:1439–1448. doi: 10.1016/j.yjmcc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM. Oxytocin releases atrial natriuretic peptide: evidence for oxytocin receptors in the heart. Proc Nat Acad Sci U S A. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutkowska J, Jankowski M, Mukaddam-Daher S, McCann SM. Oxytocin is a cardiovascular hormone. Braz J Med Biol Res. 2000;33:625–633. doi: 10.1590/s0100-879x2000000600003. [DOI] [PubMed] [Google Scholar]
- 17.Haanwinckel MA, Elias Lk, Favaretto ALV, Gutkowska J, McCann SM, Antunes-Rodrigues J. Oxytocin mediates atrial natriuretic peptide release and natriuresis after volume expansion in the rat. Proc Nat Acad Sci U S A. 1995;92:7902–7906. doi: 10.1073/pnas.92.17.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Shimoni Y, Giles WR. A cellular mechanism for nitric oxidemediated cholinergic control of mammalian heart rate. J Gen Physiol. 1995;106:45–65. doi: 10.1085/jgp.106.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman RD, Rosella-Dampman LM, Emmert SE, Summy-Long JY. Ontogeny of opioid inhibition of vasopressin and oxytocin release in response to osmotic stimulation. Endocrinology. 1986;119:1–11. doi: 10.1210/endo-119-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101:1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 21.Jankowski M, Danalache B, Wang D, Bhat P, Hajjar F, Marcinkiewicz M, Paquin J, McCann SM, Gutkowska J. Oxytocin in cardiac ontogeny. Proc Nat Acad Sci U S A. 2004;101:13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankowski M, Hajjar F, Al-Kawas S, Mukaddam-Daher S, Hoffman G, McCann S, Gutkowska J. Rat heart: a novel site of oxytocin production and action. Proc Nat Acad Sci U S A. 1998;95:14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowski M, Rachelska G, Wang D, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Nat Acad Sci U S A. 2001;98:11765–11770. doi: 10.1073/pnas.201394198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight DE, von Grafenstein H, Athayde CM. Calcium-dependent and calcium-independent exocytosis. Trends Neurosci. 1989;12:451–458. doi: 10.1016/0166-2236(89)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Kramer KM, Cushing BS, Carter CS. Developmental effects of oxytocin on stress response: single versus repeated exposure. Physiol Behav. 2003;79:775–782. doi: 10.1016/s0031-9384(03)00175-6. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre DL, Giaid A, Bennett H, Lariviere R, Zingg HH. Oxytocin gene expression in rat uterus. Science. 1992;256:1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 28.Mukaddam-Daher S, Yin YL, Roy J, Gutkowska J, Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–296. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- 29.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 30.Paquin J, Danalache BA, Jankowski M, McCann AM, Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Nat Acad Sci U S A. 2002;99:9550–9555. doi: 10.1073/pnas.152302499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeifer L, Bales KL, Carter CS. Neonatal manipulation of oxytocin affects alloparental behavior in male prairie voles. Horm Behav. 2001;39:344. [Google Scholar]
- 32.Pournajafi Nazarloo H, Tanaka Y, Dorobantu M, Hashimoto K. Modulation of corticotropin-releasing hormone receptor type 2 mRNA expression by CRH deficiency or stress in the mouse heart. Regul Pept. 2003;115:131–138. doi: 10.1016/s0167-0115(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 33.Robishaw JD, Hansen CA. Structure and function of G proteins mediating signal transduction pathways in the heart. Alcohol Clin Exp Res. 1994;18:115–120. doi: 10.1111/j.1530-0277.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 34.Ruskoaho H, Toth M, Lang RE. Atrial natriuretic peptide secretion: synergistic effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1985;133:581–588. doi: 10.1016/0006-291x(85)90945-3. [DOI] [PubMed] [Google Scholar]
- 35.Soares TJ, Coimbra TM, Martins AR, Pereira AG, Carnio EC, Branco LG, Albuquerque-Araujo WI, de Nucci G, Favaretto AL, Gutkowska J, McCann SM, Antunes-Rodrigues J. Atrial natriuretic peptide and oxytocin induce natriuresis by release of cGMP. Proc Natl Acad Sci U S A. 1999;96:278–283. doi: 10.1073/pnas.96.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohlström A, Olausson H, Brismar K, Uvnäs-Moberg K. Oxytocin treatment during early life influences reproductive performance in ad libitum fed and food-restricted female rats. Biol Neonate. 2002;81:132–138. doi: 10.1159/000047198. [DOI] [PubMed] [Google Scholar]
- 37.Stumpf WE, Sar M, Aumuller G. The heart: a target organ for estradiol. Science. 1977;196:319–321. doi: 10.1126/science.847474. [DOI] [PubMed] [Google Scholar]
- 38.Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140:1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- 39.Tohse N, Nakaya H, Takeda Y, Kanno M. Cyclic GMP-mediated inhibition of L-type Ca2+ channel activity by human natriuretic peptide in rabbit heart cells. Br J Pharmacol. 1995;114:1076–1082. doi: 10.1111/j.1476-5381.1995.tb13316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ Res. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 41.Uvnä-Moberg K, Alster P, Petersson M, Sohlström A, Bjorkstrand E. Postnatal oxytocin injections cause sustained weight gain and increased nociceptive thresholds in male and female rats. Ped Res. 1998;43:344–349. doi: 10.1203/00006450-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Weiner CP, Knowles RG, Moncada S. Induction of nitric oxide synthases early in pregnancy. Am J Obstet Gynecol. 1994;171:838–843. doi: 10.1016/0002-9378(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Cushing BS, Kramer KM, Epperson P, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]


