Abstract
Alcohol abuse impairs the pulmonary immune response to infection and increases the morbidity and mortality of bacterial pneumonia. Acute alcohol intoxication suppresses lung expression of CXC chemokines bearing the Glu-Leu-Arg motif (ELR+) following lipopolysaccharide (LPS) challenge, but its effect on the structurally-related ELR- CXC chemokines, which attract T cells, is unknown. We therefore investigated the effect of acute alcohol intoxication on the pulmonary response to intratracheal (i.t.) LPS challenge for the ELR- CXC chemokines monokine induced by gamma (MIG or CXCL9), interferon-inducible protein 10 (IP-10 or CXCL10), and interferon-inducible T cell alpha chemoattractant (I-TAC or CXCL11). Male C57BL/6 or C3H/HeN mice were given an intraperitoneal injection of ethanol (3.0g/kg) or PBS 30 min before i.t. LPS challenge. Chemokine mRNA transcripts were measured at 0, 2, 6, and 16 h. Acute alcohol intoxication inhibited the lung’s expression of all three chemokine genes in response to LPS. Lung IFN-γ mRNA was also inhibited by acute intoxication over the same time course. The in vitro effect of ethanol on chemokine secretion was further studied in the MH-S alveolar macrophage cell line. IP-10, MIG, and I-TAC in response to LPS were enhanced by exogenous IFN-γ, and these responses were blunted by exposure to ethanol. Alcohol exposure did not affect MH-S cell NF-kB p65 nuclear localization during challenge, despite dose-dependent inhibition of Erk 1/2 phosphorylation. In addition, phospho-STAT1 was not decreased in the presence of acute ethanol, thereby indicating acute intoxication does not affect IFN-γ signaling in MH-S cells. Recruitment of CD3+ T cells into the alveolar space 4 days after LPS challenge was moderately impaired by acute ethanol intoxication. These results implicate acute ethanol intoxication as a significant inhibitor of lymphocyte chemoattractant expression during pulmonary inflammation.
Keywords: Lung, Lipopolysaccharide, IP-10, MIG, I-TAC, Alcohol Abuse
INTRODUCTION
Effective pulmonary host defense against bacteria requires the coordinated recruitment of effector immune cells into infected tissues to rapidly kill pathogens. Neutrophil recruitment to the alveolar space is the first wave of leukocyte emigration into the lung during bacterial pneumonia and is mediated by the rapid expression of CXC chemokines (Mizgerd, 2002), so named to indicate two conserved cysteine residues near the amino terminus separated by a variable amino acid. The presence of the glutamate-leucine-arginine (ELR) motif immediately preceding the first conserved cysteine in ELR+ CXC chemokines conveys specificity for the CXCR2 receptor, found predominantly on the surface of neutrophils. In contrast, absence of this ELR motif from the sequence of the ELR- CXC chemokines MIG, IP-10, and I-TAC imparts affinity for the CXCR3 receptor. CXCR3 expression is best characterized on the surface of activated T helper 1 (Th1) (Soto et al., 1998) and natural killer (NK) cells, two important sources of IFN-γ (Martin-Fontecha et al., 2004). As these three chemokines are themselves markedly upregulated by IFN-γ (Cole et al., 1998; Farber, 1990; Luster and Ravetch, 1987), it is likely that they play an important role in amplifying the Th1 adaptive response to infection/inflammation.
Since the introduction of effective prophylaxis against opportunistic Pneumocystis jirovecii infection, bacteria pneumonia has emerged as the most common cause of lower respiratory tract infection in HIV infected patients and occurs at nearly six times the rate of that observed in HIV negative controls (Hirschtick et al., 1995). Furthermore, the incidence of gram-negative pneumonia is increased in HIV-infected patients compared to matched seronegative subjects (Afessa and Green, 2000). Taken together, these observations suggest that intact T cell recruitment is critical to the host’s immune response to bacterial pneumonia, particularly that caused by gram-negative organisms.
Alcohol abuse has been identified as an independent risk factor for the development of bacterial pneumonia (Rush B, 1943; Schmidt and De Lint, 1972). As reviewed elsewhere (Happel and Nelson, 2005; Zhang et al., 2002), ethanol intoxication disrupts both innate and adaptive immune responses to pneumonia. Alcohol intoxication has been shown to impair neutrophil influx into the infected/ LPS-challenged lung, and inhibition of the lung’s expression of neutrophil-attracting ELR+ CXC chemokines is a likely mechanism underlying this critical defect (Zhang et al., 1999). Although the lungs express MIG, IP-10, and I-TAC in a murine model of intravenous (i.v.) LPS challenge (Widney et al., 2000a), to our knowledge no published data exists demonstrating the lung’s ELR- chemokine response to intratracheal (i.t.) LPS challenge. We therefore examined the effect of acute alcohol intoxication on the lung’s capacity to express these chemokines following i.t. LPS administration.
Here, we show that the mouse lung robustly expresses IFN-γ and the IFN-γ-inducible ELR- chemokines MIG, IP-10, and I-TAC in response to i.t. LPS, and acute ethanol intoxication suppresses these responses. T cell recruitment into the alveolar space 4 days after challenge is decreased, possibly the result of decreases in these chemokines. In vitro, ethanol inhibits MHS cell expression of these chemokines in response to combined IFN-γ/LPS stimulation, suggesting a direct effect of ethanol.
MATERIALS AND METHODS
Mice
Specific pathogen-free male C3H/HeN (National Cancer Institute; Frederick, MD), wild-type C57BL/6, and C57BL/6 mice homozygous for deletion of IFN-γ (IFN-γ -/-) (Jackson Laboratories; Bar Harbor, ME) were used at 6-8 wk of age. All mice were housed in the LSUHSC vivarium and treated in accordance with institutional guidelines. Mice were provided with water and food ad libitum and received 12 hour light/dark cycles. All procedures were approved by the LSUHSC Animal Care and Use Committee.
Alcohol Administration and Infection
Mice were injected intraperitoneally (i.p.) with ethanol (1.5 or 3.0 g/kg; 20% v/v ethanol in sterile PBS) or an equivalent volume of PBS. Thirty minutes after ethanol administration, animals were anesthetized with isoflurane to expose the trachea for direct intratracheal (i.t.) administration of 10 μg E. coli LPS (List Biological Laboratories; Campbell, CA) in 50μl PBS using a 30 gauge needle. Animals were allowed to recover from anesthesia and returned to their cages.
Lung and BAL Cell Harvest
At designated time points, mice were anesthetized with isoflurane and sacrificed by diaphragmatic interruption. Lungs were removed en bloc and homogenized (Omni GLH, Omni International; Warrenton, VA) in 1 ml buffer RLT (RNeasy system; Qiagen; Valencia, CA) for mRNA analysis. BAL cells were retrieved by lavage of removed lungs with PBS containing 0.1% glucose. A total of 10ml lavage was performed in 1ml aliquots.
Flow Cytometry
BAL cells were analyzed on the FACScalibur (BD Biosciences; Franklin Lakes, NJ) system after surface staining with Pacific Blue conjugated anti-CD3 mAb (eBioscience; San Diego, CA). Lymphocyte populations were identified by forward and side light scatter properties, and T lymphocytes defined as CD3+ cells within this gate. The ratio of CD3+ events to total cellular events was multiplied by the total BAL cell count obtained with a manual hemacytometer to determine total T cells recovered.
Cell Culture
The mouse alveolar macrophage cell line MH-S (ATCC # CRL-2019; Manasas, VA) was used for in vitro studies. Cells were regularly passed in RPMI 1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES and 1.0 mM sodium pyruvate and supplemented with 0.05 mM 2-mercaptoethanol and 10% fetal bovine serum. Cells were seeded at 106 cells/ml for supernatant studies (in 96 well plates) or grown to 80% confluence for STAT1, NF-kB, and MAP kinase studies (in 6 well plates). In designated experiments, culture media was replaced with media containing ethanol at 0, 50, or 100mM concentrations 30 minutes prior to stimulation. These concentrations were maintained by placing plates in dedicated CO2 incubators containing an appropriate amount of ethanol in the water bath, as previously described (Zhang et al., 2001). In vitro stimulation was performed by adding purified E. coli LPS (List Biological Laboratories) at 100 ng/ml final concentration and/or recombinant murine IFN-γ (R&D Systems; Minneapolis, MN) at 100 U/ml final concentration, with or without addition of Erk inhibitors PD98059 or U0126 (Calbiochem; La Jolla, CA).
Chemokine, phospho-Erk 1/2, JNK, and NF-kB p65 ELISA
Concentrations of IP-10, MIG, and I-TAC in cell culture supernatants were determined by colorimetric sandwich ELISA (R&D Systems). Phospho- and total Erk (p44 and p42) and JNK levels in whole MH-S cells were determined using the chemiluminescent version of the fast activated cell-based (FACE™) ELISA kit per manufacturer’s instructions (Acitve Motif; Carlsbad, CA). Nuclear preparations of MH-S cells were made using a commercially available nuclear extraction kit (Active Motif). Detection of immunoreactive nuclear NF-kB p65 was performed using a chemiluminescent ELISA kit (TransAm Chemi; Active Motif) and read on a 96-well plate luminometer (Turner Biosystems; Sunnyvale, CA).
Western Blot
Quantities of phospho- and total p38 and STAT1 were determined by Western blot as described previously (Zhang et al., 2007). Briefly, 20 μg of cell lysates were separated on a 10% acrylamide gel. Following transfer to a nitrocellulose membrane, the presence of phosphorylated STAT1 was detected with a mouse monoclonal antibody specific for phosphotyrosine 701 (Tyr701) at a dilution of 1:1000 while total STAT1 was evaluated with a mouse monoclonal antibody specific for the N-terminus at a dilution of 1:250 (BD Biosciences). For p38, a rabbit polyclonal Ab recognizing phospho-threonine 180 (Thr180) or phospho-tyrosine 182 (Tyr182) and a similar Ab recognizing total p38 were both used at 1:1000 dilution (Cell Signaling; Danvers, MA). Corresponding secondary antibodies conjugated to horseradish peroxidase were used at a concentration of 1:30,000 and the membranes were developed by enhanced chemiluminescence using the ECL Advance Western Blotting Detection Kit (Amersham; Piscataway, NJ) following the manufacturer’s instructions. Densitometry calculations were performed using MI Image software (Kodak Molecular Imaging; New Haven, CT).
Real time RT-PCR
Total RNA from BAL cells and lung homogenates was isolated using the RNeasy mini kit (Qiagen). 10ng of total RNA was subjected to one-step reverse transcription and polymerase chain reaction (RT-PCR) using TaqMan® linear hydrolysis chemistry on the iCycler thermocycler (Bio-Rad; Hercules, CA). Gene-specific primers and dual-labeled FRET probe sequences for MIG, IP-10, I-TAC, IFN-γ, and 18s rRNA were designed using Beacon Designer 2.12 (Premier Biosoft International; Palo Alto, CA), with sequences as follows (primer, primer, probe): MIG 5’- GAG GAA CCC TAG TGA TAA GGA ATG C -3’, 5’- CTG TTT GAG GTC TTT GAG GGA TTT -3’, 5’- 6-FAM/CAG CAC CAG CCG AGG CAC GA/3BHQ1-3’; IP-10 5’- CCA GTG AGA ATG AGG GCC ATA -3’, 5’- ATC GTG GCA ATG ATC TCA ACA -3’, 5’- 6-FAM/AAG CTT GAA ATC ATC CCT GCG /3BHQ-1 -3’ ; I-TAC 5’- TGC GAC AAA GTT GAA GTG ATT GT -3’, 5’- GCG AGC TTG CTT GGA TCT G -3’, 5’- 6-FAM/CTC ATA AAC GAC AAA GGT GCC /3BHQ-1 -3’, IFN-γ 5’- CAT TGA AAG CCT AGA AAG TCT GAA TAA C -3’ 5’- TGG CTC TGC AGG ATT TTC ATG -3’, 5’- 6-FAM/TCA CCA TCC TTT TGC CAG TTC CTC CAG/3BHQ-1 -3’ 18s rRNA 5’-ATT CGA ACG TCT GCC CTA TCA-3’, 5’-GTC ACC CGT GGT CAC CAT G-3’, 5’- 6-FAM/TCG ATG GTA GTC GCC GTG CCT ACC/3BHQ-1-3’. Data are expressed as transcript copy number, using a previously quantified cRNA clone to generate a standard curve for each gene. All values were normalized to 18s ribosomal RNA content.
Statistical analysis
Comparisons of between different treatment groups were made using Student’s t test for simple pairwise comparisons or ANOVA for comparisons of multiple groups. For data not normally distributed, values were log10 transformed prior to analysis. Differences between treatment groups were accepted as significant when p< 0.05.
RESULTS
Pulmonary expression of MIG, IP-10, and I-TAC is inhibited by acute alcohol administration
To determine the kinetics of lung CXC chemokine expression, we challenged male C3H/HeN with i.t. injection of 10 μg purified E. coli LPS. Chemokine mRNA expression in whole lung homogenates was determined by real-time RT-PCR. Lung transcripts for the ELR- chemokines MIG, IP-10, and I-TAC peaked 6 to 16 hours post challenge, and acute ethanol administration (3.0 g/kg) 30 minutes prior to challenge significantly abrogated these responses (Fig. 1). As previously shown for the rat orthologues CINC and MIP-2 (Boe et al., 2001), mRNA encoding the murine ELR+ chemokines KC and MIP-2 were expressed early, peaking 2 hours post challenge, and alcohol also blunted their expression (data not shown).
Figure 1.
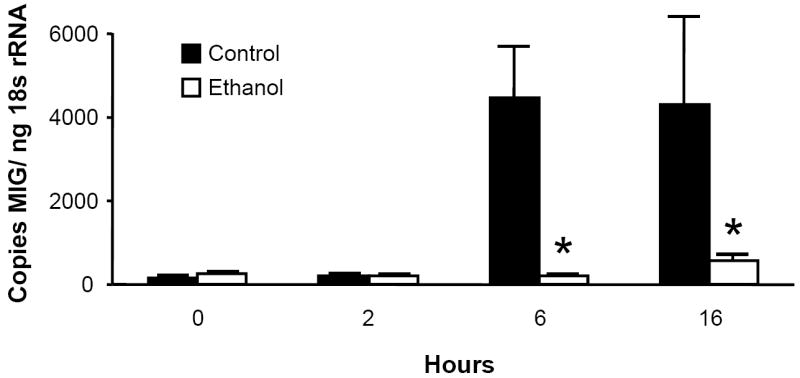
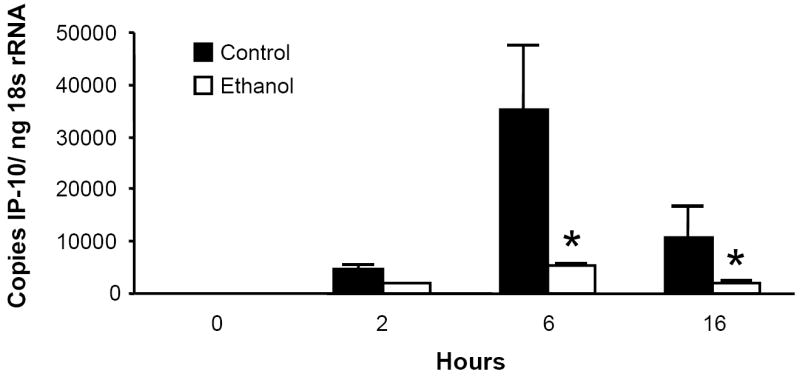
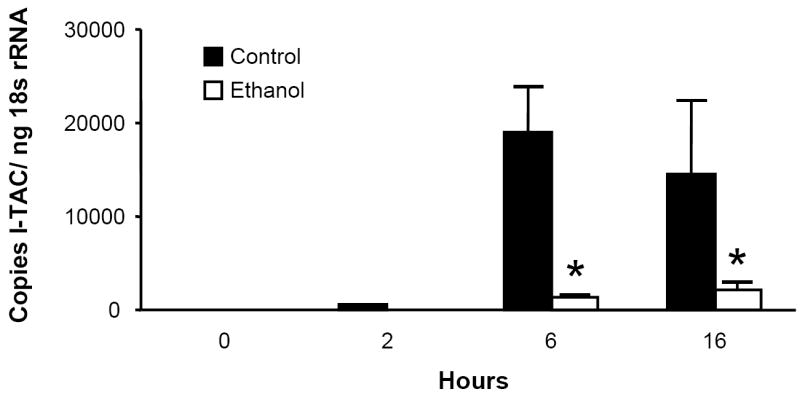
Acute ethanol intoxication inhibits the pulmonary MIG (Fig. 1a), IP-10 (Fig. 1b), and I-TAC (Fig. 1c) mRNA responses to i.t. LPS challenge. Male C3H/HeN mice were administered 3.0g/kg ethanol or PBS i.p. 30 minutes prior to i.t. injection with 10 μg E. coli LPS. Animals were sacrificed at indicated times, and chemokine mRNA expression in whole lung homogenates was determined by real-time RT-PCR. Data are expressed as transcript copy number, normalized for 18s rRNA content. * p <0.05 vs. PBS control at same time point. n=4 to 7 per time point for each treatment.
Pulmonary expression of IFN-γ is inhibited by acute alcohol administration
Prior studies indicate that a 2 week exposure to ethanol inhibits lung IFN-γ expression during K. pneumoniae infection (Widney et al., 2000b; Zisman et al., 1998), but whether a single dose of ethanol prior to i.t. LPS challenge affects the IFN-γ response is unknown. Since MIG, IP-10, and I-TAC are up-regulated by IFN-γ, we next studied the effect of acute intoxication on lung IFN-γ transcripts induced by i.t. LPS. Ethanol exerted a dose-dependent inhibition of lung IFN-γ mRNA (Fig. 2).
Figure 2.
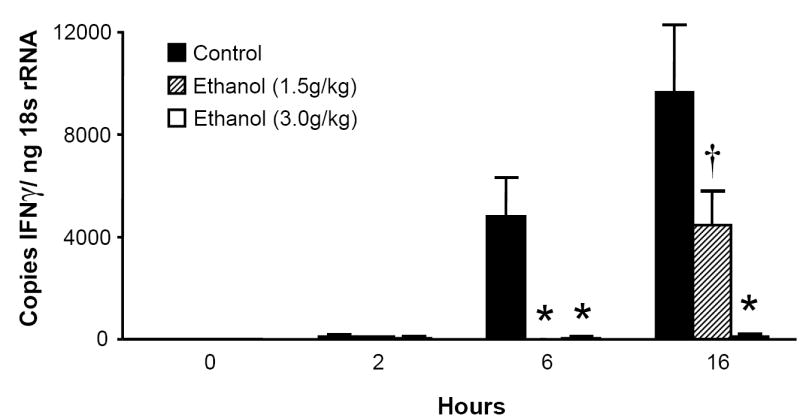
Acute ethanol intoxication exerts a dose-dependent inhibition of IFN- γ mRNA in the lung. Mice were administered 1.5g/kg, 3.0g/kg ethanol or PBS i.p. 30 minutes prior to i.t. injection with 10 μg LPS. Animals were sacrificed at indicated time points and whole lung IFN-γ mRNA measured by real-time RT-PCR. * p <0.05 compared to PBS control group at same time point. † p <0.05 vs. 3.0 g/kg ethanol at same time point. n= 4 to 7 per time point for each treatment group.
IFN-γ is necessary for maximal MIG, IP-10, and I-TAC induction in response to i.t. LPS
Since acute intoxication impairs the lung’s IFN-γ response to LPS, this suppression may explain alcohol’s effect on MIG, IP-10, and I-TAC, as these chemokines are all IFN-γ-inducible. To test this hypothesis, IFN-γ -/- mice and C57BL/6 background control mice received i.t. LPS and were sacrificed at indicated time points. Pulmonary MIG, IP-10, and I-TAC mRNA responses were diminished but not absent in the IFN-γ -/- strain (Fig. 3), indicating a substantial role for IFN-γ in the expression of these ELR- CXC chemokines during airway endotoxin challenge.
Figure 3.
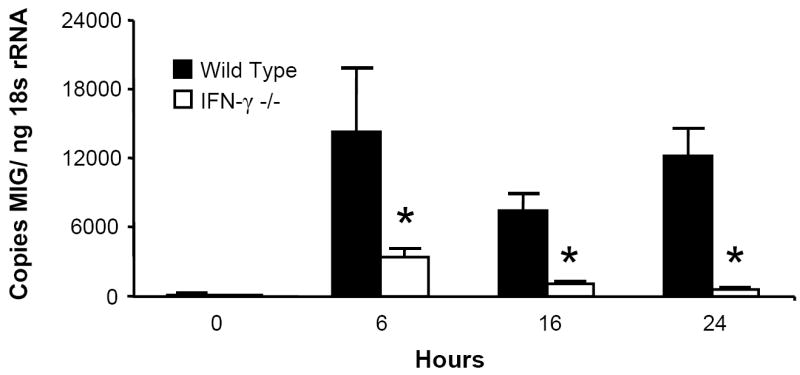
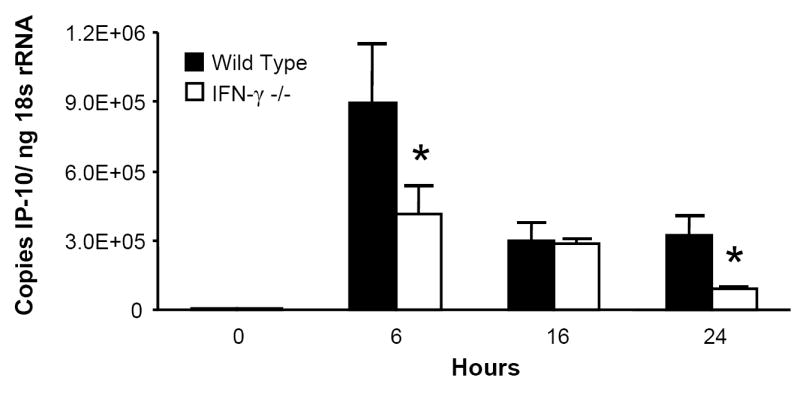
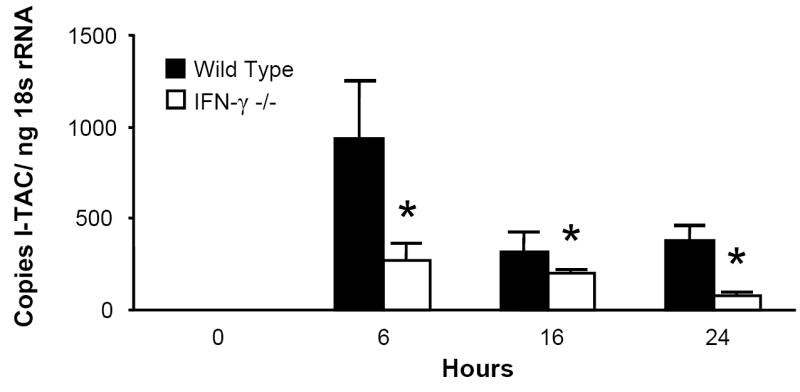
IFN-γ is necessary for maximal MIG, IP-10, and I-TAC induction in response to i.t LPS. IFN-γ -/- and C57BL/6 background control mice received 10 μg i.t. LPS and sacrificed at indicated time points. Whole lung MIG (Fig. 3a), IP-10 (Fig. 3b), and I-TAC (Fig. 3c) mRNA responses were measured by RT-PCR. * p <0.05 vs. background control strain at same time point. n=5 per time point per strain.
MH-S cell production of MIG, IP-10, and I-TAC is suppressed by acute alcohol exposure
Because the macrophage-like cell line RAW 264.7 has been shown to express the ELR- CXC chemokines in response to inflammatory stimuli (Widney et al., 2000c), we examined whether the murine alveolar macrophage cell line MH-S was also capable of expressing these cytokines in response to LPS and whether this response was modulated by in vitro ethanol exposure. Similar to RAW cells, MH-S cells express IP-10 and I-TAC in response to LPS alone but require exogenous IFN-γ for MIG release (Fig. 4). Combined IFN-γ (100 u/ml) and LPS (100ng/ml) exposure induced the most robust levels of all three chemokines, and acute ethanol exposure inhibited these responses.
Figure 4.
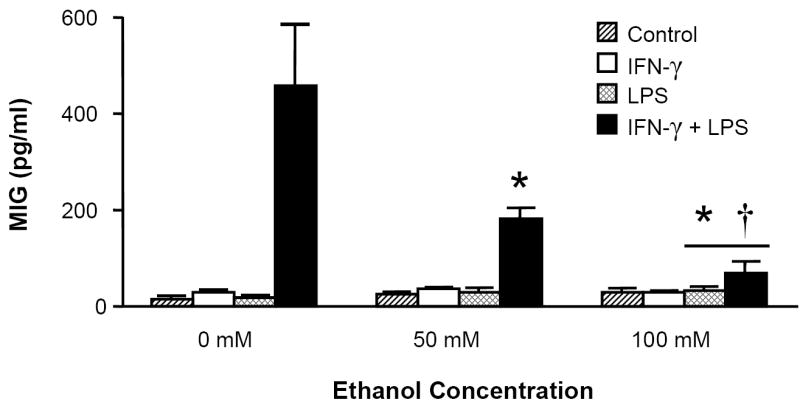
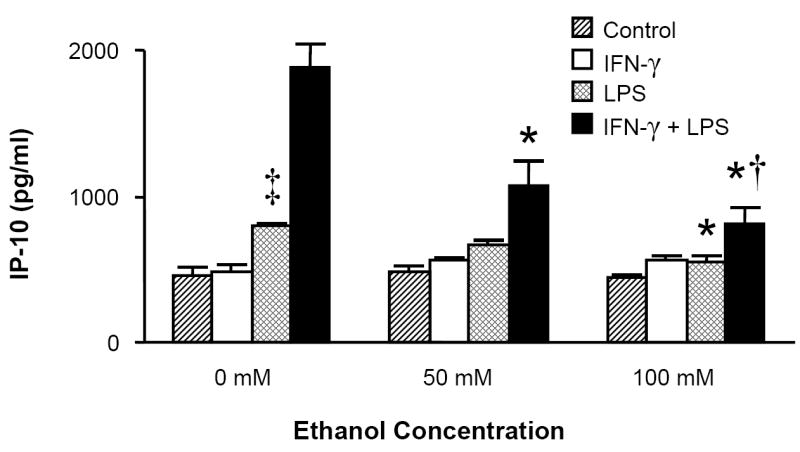
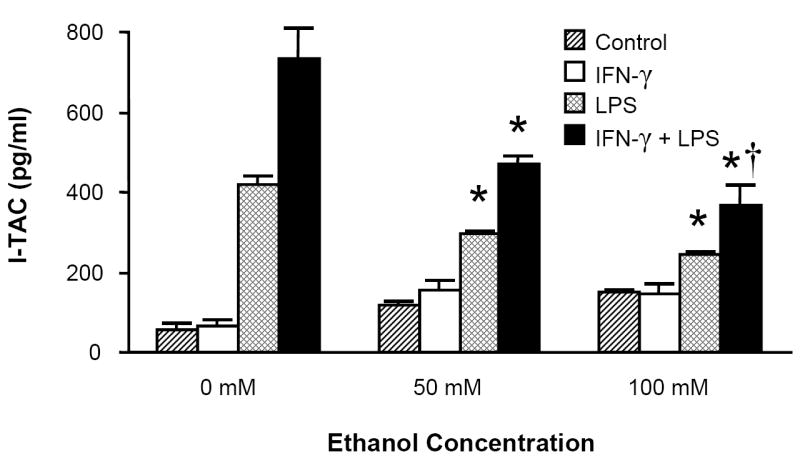
MH-S cell production of MIG, IP-10, and I-TAC is suppressed by acute ethanol exposure. MH-S cells were exposed to 0, 50, or 100mM ethanol for 30 minutes and then stimulated with LPS (100ng/ml), IFN-γ (100 u/ml), LPS+IFN-γ, or media control. Supernatants were collected after 24 hours and assayed for chemokine content. * p <0.05 vs. 0mM ethanol group of identical stimulus. † p <0.05 vs. 50mM ethanol group of identical stimulus. ‡ p <0.05 vs. control (media) stimulus. n=6 per group.
STAT1 signaling is not affected by acute ethanol exposure
Our in vivo results demonstrate that ethanol strongly suppresses pulmonary IFN-γ induction during LPS challenge. The possibility exists that ethanol also interferes with IFN-γ signal transduction in accessory cells, such as alveolar macrophages. Activation (phosphorylation) of STAT1 is critical to the transduction of IFN-γ receptor signaling upon ligation (Darnell, Jr. et al., 1994). Since MH-S cell expression of MIG, IP-10, and I-TAC is dependent on (in the case of MIG) or enhanced by (in the case of IP-10 and I-TAC) the addition of exogenous IFN-γ to LPS stimulation, we next investigated whether acute ethanol intoxication affects MH-S cell STAT1 activation in response to IFN-γ/LPS. Our results demonstrate MH-S cell STAT1 phosphorylation is unaffected by acute ethanol exposure (Fig. 5).
Figure 5.
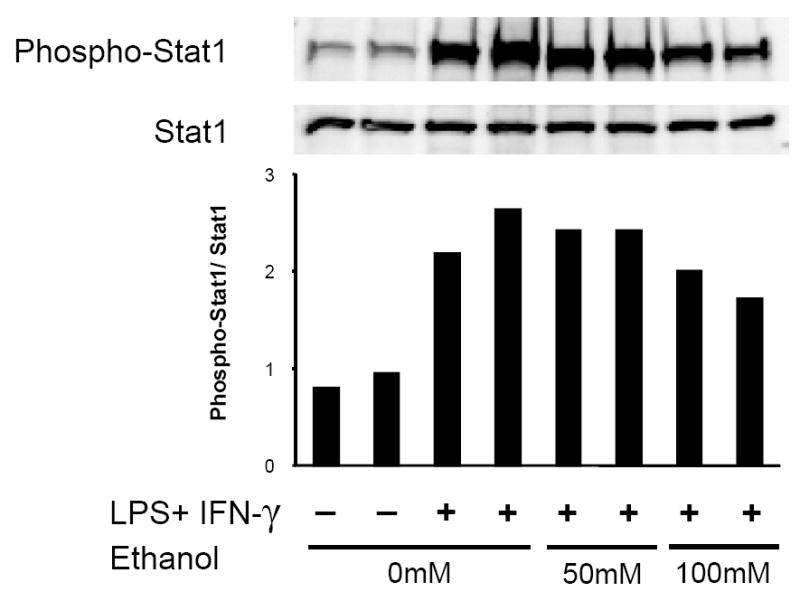
STAT1 signaling is not affected by acute ethanol exposure. MH-S cells were exposed to 0, 50, or 100mM ethanol for 30 minutes and then stimulated with the combination of LPS (100ng/ml) and IFN-γ (100 U/ml). After 20 minutes, cells were lysed and assayed for phospho- and total STAT1 content by quantitative immunoblot. Band intensity was quantified by densitometry, and the data are expressed as the pSTAT1/STAT1 ratio. A representative gel of 6 experiments is shown. n=2 per group.
Acute ethanol exposure does not affect the nuclear localization of NF-kB p65 in LPS-challenged MH-S cells
Acute alcohol intoxication impairs nuclear localization of the critical early inflammatory transcription factor NF-kB in human monocytes (Mandrekar et al., 1999), murine peritoneal cells (Pruett et al., 2004a), as well as the RAW 264.7 cell line (Mandrekar et al., 2006) following LPS challenge. However, the effect of acute intoxication on the alveolar macrophage response to LPS is unknown. We therefore stimulated the MH-S alveolar macrophage cell line with LPS or LPS+IFN-γ and measured the effect of acute ethanol on the nuclear presence of NF-kB p65 (RelA), the major trans-activating domain of the NF-kB family. In contrast to prior studies, acute alcohol did not significantly affect levels of antigenic p65 in nuclear preparations of stimulated MH-S cells (Fig. 6).
Figure 6.
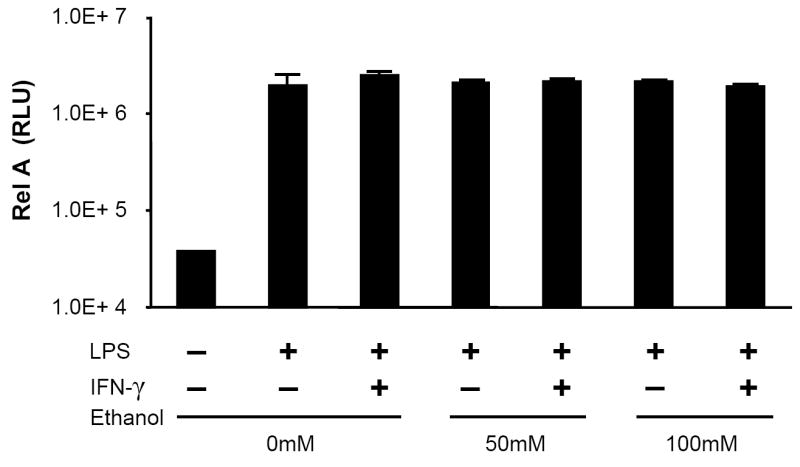
Acute ethanol exposure does not affect the nuclear presence of NF-kB p65 (RelA) in MH-S cells. Cells were exposed to 0, 50, or 100mM ethanol for 30 minutes and then stimulated with LPS (100ng/ml), IFN-γ (100 u/ml), LPS+IFN-γ, or media control. After 30 minutes, cells were lysed, nuclear fractions prepared, and 15 μg of nuclear protein assayed for immunoreactive p65 by chemiluminescent ELISA as described in Methods. Data are expressed as relative light units (RLU). n=3 per group.
Acute ethanol exposure inhibits activation of the mitogen activated protein kinase (MAPK) Erk 1/2 but not p38 or JNK in MH-S cells
MAPK activity is a critical component of the early inflammatory response to LPS in many cell types, including macrophages (Dong et al., 2002; Guha and Mackman, 2001). Acute ethanol treatment has been associated with a decrease in MAPK phosphorylation in response to LPS following both in vivo (Goral et al., 2004; Kato et al., 2005) and in vitro intoxication (Oak et al., 2006). We therefore examined the effect of acute ethanol exposure on p38 (Figs. 7a and 7b), Erk 1/2 (Fig. 7c), and JNK (data not shown) phosphorylation in MH-S cells following LPS or combined LPS + IFN-γ stimulation and found an effect only on Erk activation.
Figure 7.

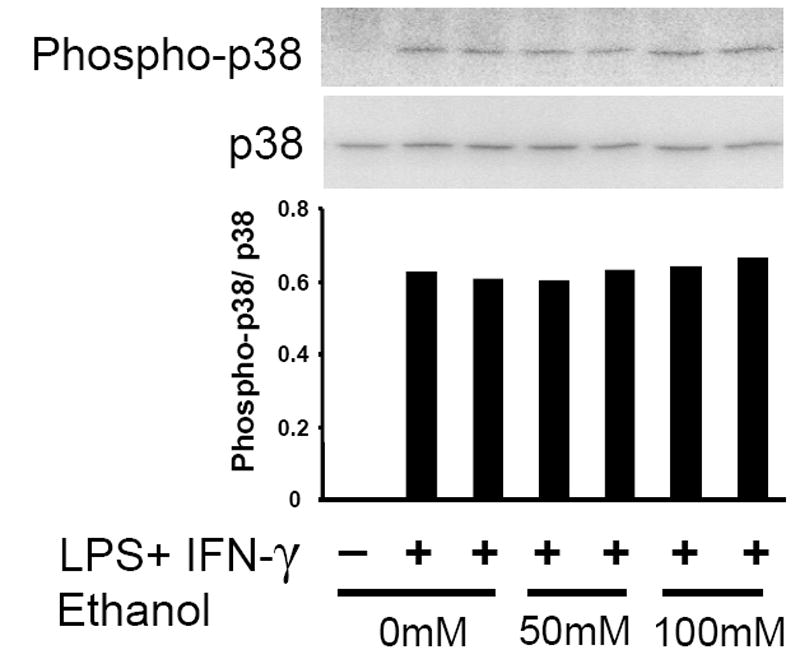
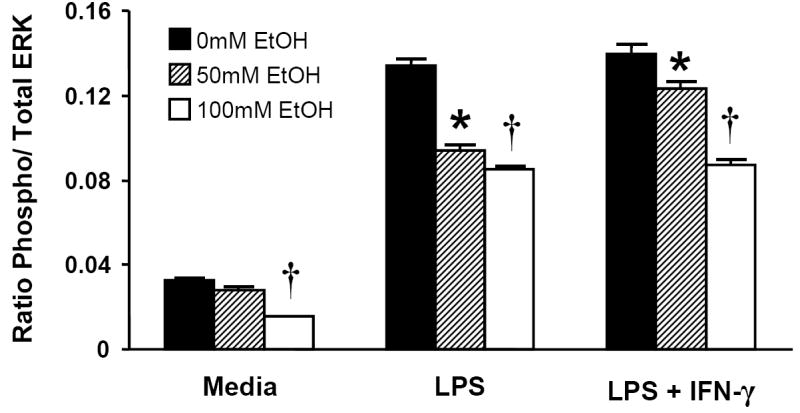
Acute ethanol exposure inhibits activation of Erk 1/2 but not p38 in MH-S cells. Cells were exposed to 0, 50, or 100mM ethanol for 30 minutes and then stimulated with LPS (100ng/ml), IFN-γ (100 u/ml), LPS+IFN-γ, or media control. After 30 minutes, cells were lysed, and 20 μg of total lysate protein was assayed for phospho-p38 and total p38 by quantitative immunoblot (Figs. 7a and 7b). Densitometry data indicate phospo-p38/ p38 ratio. n=2 per group. For phospho-Erk 1/2, chemiluminescent ELISA was performed on fixed MH-S cells as described in Methods (Fig. 7c). Data are expressed as relative light units and are normalized for cellular content as quantified by crystal violet staining. * p < 0.05 vs. no ethanol; † p < 0.05 vs. 50mM EtOH. n=12 per group.
Erk is necessary for MIG, IP-10, and I-TAC secretion in LPS-stimulated MH-S cells
To investigate whether ethanol’s inhibition of Erk might underlie the defect in MIG, IP-10, and I-TAC expression, we pre-incubated MH-S cells with specific inhibitors of MEK 1/2, kinases responsible for Erk 1/2 phosphorylation. Each compound strongly suppressed the secretion of these chemokines in response to LPS (Fig. 8).
Figure 8.
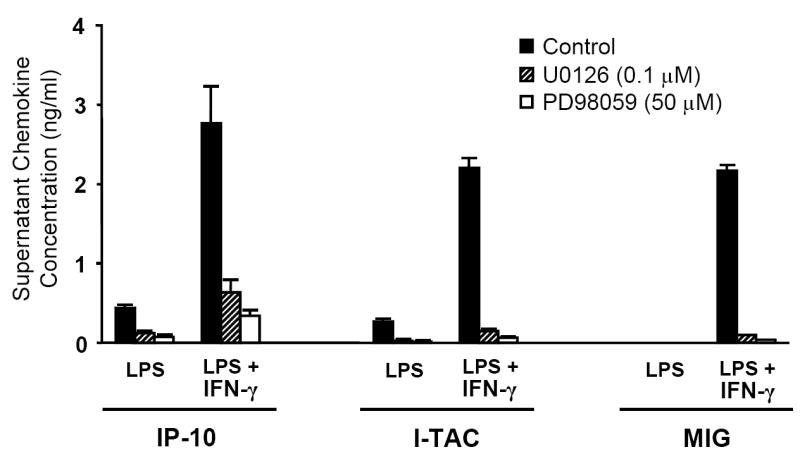
Inhibition of Erk activation impairs MH-S cell expression of MIG, IP-10, and I-TAC. MH-S cells were pre-treated with the MEK 1/2 specific inhibitors U0126 (0.1 μM), PD98059 (50 μM), or solvent control for 90 min prior to stimulation. Supernatants were collected after 9 hours of stimulation and assayed for chemokine content. p <0.001 for all groups exposed to an inhibitor vs. solvent control. n=12 per group.
Acute intoxication impairs the alveolar recruitment of T cells following i.t. LPS challenge
The initial wave of pulmonary neutrophil recruitment following i.t LPS treatment is followed by the influx of lymphocytes, which peaks between 3 and 5 days (Morris et al., 1997). Because ethanol impairs the ELR- CXC chemokine response to i.t. LPS, we hypothesized that intoxication would also decrease the alveolar recruitment of T lymphocytes, which bear the cognate receptor (CXCR3) for these ligands. To test this hypothesis, animals were injected i.p. with ethanol (3.0g/kg) or PBS and administered i.t. LPS 30 minutes later. 4 days after i.t. LPS challenge, animals which had been acutely intoxicated demonstrated a small but significant attenuation in alveolar CD3+ T lymphocyte recruitment as recovered by bronchoalveolar lavage (Fig. 9).
Figure 9.
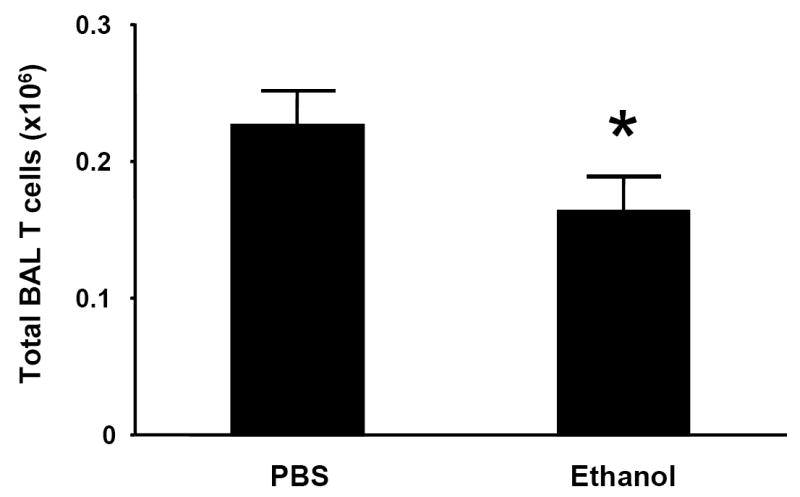
Acute alcohol intoxication impairs the alveolar recruitment of T cells following i.t. LPS challenge. C3H/HeN mice were injected i.p with ethanol (3.0 g/kg) or PBS and administered 10 μg LPS via i.t. injection 30 minutes later. 4 days after i.t. LPS challenge, animals were sacrificed and alveolar cells recovered by bronchoalveolar lavage (BAL). Total BAL T cell counts were calculated as described in Methods. * p <0.05 vs. PBS control group. n=6 per group.
DISCUSSION
Our current study indicates that acute alcohol intoxication suppresses the pulmonary MIG, IP-10, and I-TAC response to airway endotoxin challenge. This inhibition occurs, at least, at the level of transcriptional regulation as evidenced by decreased chemokine mRNA expression in vivo. In agreement with prior studies showing inhibition of accessory cell function as a result of acute ethanol exposure (Szabo et al., 2001; Szabo et al., 2004), the in vitro data demonstrate that ethanol directly and dose-dependently inhibits the MHS cell response to LPS or LPS + IFN-γ. Furthermore, to our knowledge, these data are the first to show that the early pulmonary IFN-γ response to LPS is attenuated by a single dose of ethanol. Hence, these data support both direct (decreased MH-S cell chemokine mRNA) and indirect (decreased lung IFN-γ transcripts) mechanisms underlying inhibition of ELR- CXC chemokine expression in the lung.
The association of alcohol abuse and poor outcome from pneumonia has been well documented and is associated with substantial cost (Saitz et al., 1997). Recruitment of immune effector cells is critical to optimal response to respiratory infection and host survival. Alcohol impairs the neutrophil response to pulmonary infection (Astry et al., 1983; Zhang et al., 1999), and this effect is at least partially due to the attenuated expression of chemokines (Boe et al., 2001). While it is clear that alcohol intoxication alters the early neutrophil response, it is plausible that no such effect would be seen on later cellular recruitment events since ethanol is rapidly metabolized in the mouse, with little to no detectable blood alcohol content 4 to 6 hours following intoxication. It is therefore interesting that we found a measurable defect in lymphocyte recruitment 4 days after challenge as a result of a single ethanol injection. While products of ethanol metabolism may contribute to the in vivo finding, we speculate that the in vitro finding of impaired chemokine release in LPS-stimulated intoxicated MHS cells supports a direct effect of ethanol, as other studies have shown that defects in inflammatory responses by alveolar macrophages are observed despite blockade of ethanol metabolism (Greenberg et al., 1999).
Two weeks of ethanol feeding in CD-1 mice can impair the IFN-γ response to pulmonary K. pneumoniae challenge, a response which occurs 3 days after challenge (Zisman et al., 1998). Perhaps as a result of our using a relatively high dose of LPS, we were able to readily demonstrate pulmonary IFN-γ transcripts as early as 6 hours after challenge. We speculate that gamma delta-T cells and NK cells are important early sources of IFN-γ in our model, based on prior reports identifying these cells as producing (NK cells) or critical for the expression of (gamma delta-T cells) IFN-γ during bacterial pneumonia (Deng et al., 2001; Moore et al., 2000).
Toll-like receptors are well-characterized pattern recognition receptors which recognize a diverse group of conserved microbial products (Medzhitov, 2001). Significant progress has been made in our understanding of ethanol’s modulation of Toll-signaling, with varying effects of alcohol exposure depending on the Toll ligand of interest (Oak et al., 2006; Pruett et al., 2004b; Pruett et al., 2004a). CD14/TLR4 activation by LPS is accompanied by rapid nuclear translocation of NF-kB, and prior studies have shown inhibition of LPS-induced NF-kB induction by acute ethanol exposure both in vivo (human monocytes) and in vitro (murine Raw 264.7 cells). It is interesting that our current studies did not show a decrease in the nuclear presence of NF-kB RelA (p65) in stimulated MH-S cells acutely cultured in ethanol. Although it is possible that other NF-kB family members responsible for induction of MIG, IP-10, and I-TAC were downregulated by ethanol, we find this an unlikely explanation of our results since RelA is the most abundant trans-activating member of this transcription factor family involved in monocye/macrophage responses to LPS (Guha and Mackman, 2001). Furthermore, others have shown that increases in nuclear p65 as a result of TLR3 (Pruett et al., 2004a) or TLR4 (Mandrekar et al., 1999) activation are abrogated by ethanol. Discrepancies between these and our observations may be related to differences in the cell type studied, dosing of alcohol, or amount of LPS exposure. Nonetheless, we conclude that acute alcohol inhibits MH-S cell ELR- chemokine elaboration in a manner not reliant on NF-kB suppression. This finding suggests alternative intracellular events downstream of TLR4 activation are affected by acute alcohol.
TLR4 activation by LPS has been shown to result in activation of several MAP kinases, including p38 (Lu et al., 1999), Erk 1/2 (Weinstein et al., 1992), and JNK (Hambleton et al., 1996). Because no effects on NF-kB were observed in MH-S cells, we then sought to determine if MAPK activity was affected by acute intoxication of this cell line. We observed a dose-dependent inhibition of Erk 1/2 phosphorylation as a result of acute intoxication. These results are similar to prior studies which indicate defects in Erk activation after acute in vivo ethanol treatment followed by ex vivo LPS stimulation of macrophages (Goral et al., 2004; Kato et al., 2005).
In contrast, we found no ethanol effect on LPS-induced phosphorylation of p38 or JNK (JNK data not shown). Others have shown acute ethanol (25mM) actually increases human monocyte p38 activation following in vitro LPS stimulation (Drechsler et al., 2006). Our finding of no effect on LPS-induced JNK activation by acute intoxication is consistent with previous work (Oak et al., 2006). To support our hypothesis that ethanol’s inhibition of Erk may underlie the defect in chemokine secretion during intoxication, we observed that two specific MEK inhibitors also strongly down-regulate ELR- chemokine expression during LPS challenge. These results demonstrate a strong dependence on Erk signaling for expression of these chemokines, and they support our conclusion that Erk inhibition is a critical mechanism underlying the effect of acute intoxication on these cytokines.
Ethanol-induced suppression of lung neutrophil recruitment has been extensively studied (as reviewed by Zhang et al., 2002), but the effect on pulmonary lymphocyte influx has not been described. Because CXCR3 is expressed on activated T cells, we hypothesized that suppression of MIG, IP-10, and I-TAC by acute ethanol administration would be accompanied by an attenuation of LPS-induced pulmonary T cell recruitment. Following acute intoxication, we observed a modest yet statistically significant decrease in T cells recruited into the alveolar space 4 days after LPS instillation. We find these results interesting since this T cell recruitment defect occurs 4 days after the acute intoxication event. Although we suggest that this defect is at least partially a result of the defective ELR- chemokine expression currently observed, several caveats must be addressed. First, T cells express other chemokine receptors such as CCR2 and CCR5, and inhibition of the ligands for these receptors, such as the monocyte chemotactic protein (MCP) family for CCR2 and the macrophage inflammatory protein (MIP) family for CCR5, may contribute to decreased T cell presence in BAL following LPS challenge. Furthermore, direct effects of alcohol on the T cells themselves may impair their ability to traffic into inflamed tissues, as has been described in neutrophils (MacGregor et al., 1974; Zhang et al., 1998). It is also possible that acute alcohol’s induction of IL-10, either in the lung (Happel et al., 2006) or the systemic compartment (Szabo et al., 2001) may contribute to the blunted recruitment of T cells into inflamed lung tissue.
In summary, our work shows that acute alcohol intoxication impairs the lung’s expression of the ELR- CXC chemokines MIG, IP-10, and I-TAC, and this suppression may be the result of ethanol’s inhibition of Erk MAPK activation. Acute ethanol decrease the alveolar presence of T cells 4 days after intoxication, indicating that even a single dose of ethanol can affect cellular recruitment days after the disappearance of ethanol from the circulation. These data further our understanding of the specific mechanisms by which acute alcohol intoxication interferes with the pulmonary inflammatory response to bacterial challenge.
Acknowledgments
This work was supported by NIH NIAAA grants P60AA009803 (PZ, GB, SN), P50HL076100 (JS), and K08AA15163 (KH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117(4):1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- Astry CL, Warr GA, Jakab GJ. Impairment of polymorphonuclear leukocyte immigration as a mechanism of alcohol-induced suppression of pulmonary antibacterial defenses. Am Rev Respir Dis. 1983;128(1):113–117. doi: 10.1164/arrd.1983.128.1.113. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184(9):1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187(12):2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Deng JC, Tateda K, Zeng X, Standiford TJ. Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun. 2001;69(10):6382–6390. doi: 10.1128/IAI.69.10.6382-6390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol. 2006;177(4):2592–2600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75(3):553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Xie J, Ouyang J, Zhao X. Ethanol metabolism is not required for inhibition of LPS-stimulated transcription of inducible nitric oxide synthase. Alcohol. 1999;17(3):203–213. doi: 10.1016/s0741-8329(98)00048-2. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 1996;93(7):2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Happel KI, Odden AR, Zhang P, Shellito JE, Bagby GJ, Nelson S. Acute alcohol intoxication suppresses the interleukin 23 response to Klebsiella pneumoniae infection. Alcohol Clin Exp Res. 2006;30(7):1200–1207. doi: 10.1111/j.1530-0277.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- Kato H, Negoro M, Wakabayashi I. Effects of acute ethanol administration on LPS-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase in rat alveolar macrophages. Alcohol Clin Exp Res. 2005a;29(12 Suppl):285S–293S. doi: 10.1097/01.alc.0000191809.29775.41. [DOI] [PubMed] [Google Scholar]
- Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18(7):1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166(4):1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor RR, Spagnuolo PJ, Lentnek AL. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11(11):1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30(1):135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14(2):123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165(5):2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- Morris PE, Glass J, Cross R, Cohen DA. Role of T-lymphocytes in the resolution of endotoxin-induced lung injury. Inflammation. 1997;21(3):269–278. doi: 10.1023/a:1027393715300. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. J Immunol. 2006b;176(12):7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004a;173(4):2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004b;33(2):147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rush B. An inquiry into the effects of ardent spirits upon the human body and mind. Q J Stud Alcohol. 1943;4:321–341. [Google Scholar]
- Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157(13):1446–1452. [PubMed] [Google Scholar]
- Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. 1972;33(1):171–185. [PubMed] [Google Scholar]
- Soto H, Wang W, Strieter RM, Copeland NG, Gilbert DJ, Jenkins NA, Hedrick J, Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci U S A. 1998;95(14):8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol. 2004;33(3):241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin Exp Res. 2001;25(12):1766–1772. [PubMed] [Google Scholar]
- Weinstein SL, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267(21):14955–14962. [PubMed] [Google Scholar]
- Widney DP, Xia YR, Lusis AJ, Smith JB. The murine chemokine CXCL11 (IFN-inducible T cell alpha chemoattractant) is an IFN-gamma- and lipopolysaccharide-inducible glucocorticoid-attenuated response gene expressed in lung and other tissues during endotoxemia. J Immunol. 2000c;164(12):6322–6331. doi: 10.4049/jimmunol.164.12.6322. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Front Biosci. 2002;7:d1314–d1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Stoltz DA, Summer WR, Nelson S. Granulocyte colony-stimulating factor modulates the pulmonary host response to endotoxin in the absence and presence of acute ethanol intoxication. J Infect Dis. 1999;179(6):1441–1448. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Xie M, Stoltz DA, Summer WR, Nelson S. Acute ethanol intoxication inhibits neutrophil beta2-integrin expression in rats during endotoxemia. Alcohol Clin Exp Res. 1998;22(1):135–141. [PubMed] [Google Scholar]
- Zhang P, Zhong Q, Bagby GJ, Nelson S. Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide. Alcohol Clin Exp Res. 2007;31(1):113–121. doi: 10.1111/j.1530-0277.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol Clin Exp Res. 2001;25(3):444–449. [PubMed] [Google Scholar]
- Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, Standiford TJ. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcohol Clin Exp Res. 1998;22(3):621–627. doi: 10.1111/j.1530-0277.1998.tb04303.x. [DOI] [PubMed] [Google Scholar]


