Abstract
The c-Jun N-terminal kinase (JNK) branch of the mitogen-activated protein kinase (MAPK) signaling pathway regulates cellular differentiation, stress responsiveness and apoptosis in multicellular eukaryotic organisms. Here we investigated the functional importance of JNK signaling in regulating differentiated cellular growth in the post-mitotic myocardium. JNK1/2 gene-targeted mice and transgenic mice expressing dominant negative JNK1/2 were determined to have enhanced myocardial growth following stress stimulation or with normal aging. A mechanism underlying this effect was suggested by the observation that JNK directly regulated nuclear factor of activated T-cell (NFAT) activation in culture and in transgenic mice containing an NFAT-dependent luciferase reporter. Moreover, calcineurin Aβ gene targeting abrogated the pro-growth effects associated with JNK inhibition in the heart, while expression of an MKK7–JNK1 fusion protein in the heart partially reduced calcineurin-mediated cardiac hypertrophy. Collectively, these results indicate that JNK signaling antagonizes the differentiated growth response of the myocardium through direct cross-talk with the calcineurin–NFAT pathway. These results also suggest that myocardial JNK activation is primarily dedicated to modulating calcineurin–NFAT signaling in the regulation of differentiated heart growth.
Keywords: calcineurin/cardiac growth/cross-talk/JNK signaling/NFAT
Introduction
The mitogen-activated protein kinases (MAPKs) represent an essential signal transduction cascade that regulates cell growth, differentiation, apoptosis and transformation (reviewed by Garrington and Johnson, 1999). In its broadest sense, MAPK signaling is comprised of at least three branches of successively acting phosphorylation cascades: the extracellular signal-regulated protein kinases (ERKs), the c-Jun N-terminal kinases (JNKs) and the p38 MAP kinases (Garrington and Johnson, 1999). While each of the MAPK branches can mediate specific signaling events, their ability to elicit a biological response is often coordinated with the activity of other parallel signaling pathways. Deciphering these coordinated cross-talking signaling pathways will permit the elucidation of integrated networks that ultimately mediate diverse biological responses (Downward and Goff, 2002).
JNKs are activated in response to environmental stress or membrane-bound receptor signaling through GTPases of the Rho family, which can initiate MAPK kinase kinase (MAPKKK) signaling (reviewed by Davis, 2000). These MAPKKKs then promote activation of the MAPKKs such as MKK4 and MKK7, which function as dual-specificity protein kinases to directly phosphorylate JNK1, JNK2 and JNK3 (Davis, 2000). Once activated, JNK proteins phosphorylate diverse transcriptional and apoptotic regulatory factors to initiate predetermined biological responses. For example, gene targeting in the mouse has revealed a critical role for JNK signaling in regulating cellular viability, induction of apoptosis and cellular proliferation through phosphorylation of AP-1, p53, c-Myc and Bcl-2 family members (reviewed by Weston and Davis, 2002). However, the role that JNK signaling plays in regulating the growth of differentiated cells has not been well characterized.
One potential mechanism whereby JNKs might influence the growth response of differentiated cells is through cross-talk with other intracellular signaling pathways. For example, JNKs directly phosphorylate nuclear factor of activated T-cell (NFAT) transcription factors, thus antagonizing the effects of calcium-regulated signaling through the protein phosphatase calcineurin (PP2B) (Chow et al., 1997; Porter et al., 2000; Yang et al., 2002). In response to elevations in intracellular calcium concentration, calcineurin becomes activated resulting in the direct dephosphorylation of NFATc1, NFATc2, NFATc3 and NFATc4 transcription factors within the cytoplasm, facilitating their nuclear translocation and the activation of gene expression (reviewed by Crabtree, 1999). Calcineurin–NFAT signaling plays a pivotal role in regulating lymphocyte maturation, the growth response of both cardiac and skeletal muscle myocytes, differentiation of keratinocytes, chondrogenesis and the proper formation of the vascular network during development (Graef et al., 2001; Crabtree and Olson, 2002). Thus intracellular cross-talk between the JNK and calcineurin–NFAT signaling pathways represents a potential mechanism to coordinate cellular proliferation and differentiation.
Cardiac myocytes represent an ideal model system for investigating the molecular determinants that underlie the differentiated growth response. Cardiac myocytes within the rodent myocardium irreversibly exit the cell cycle ∼1 week after birth, yet the myocardium continues to grow well into adulthood through the hypertrophy of individual myocytes (Claycomb, 1977). The adult myocardium can also undergo physiologic and pathologic hypertrophy in response to increased workloads or certain disease associated stimuli (reviewed by Lorell and Carabello, 2000). To investigate the function of JNK signaling in regulating the differentiated growth response of the heart, we analyzed JNK1 and JNK2 gene-targeted mice, as well as cardiac-specific transgenic mice expressing dominant negative mutants of JNK1/2 and mice expressing an activated JNK1 protein by fusion with MKK7. Our results indicate that JNK signaling coordinates the differentiated cardiac growth response through direct cross-talk with the calcineurin–NFAT signaling pathway.
Results
Characterization of genetically altered mice with reduced JNK signaling
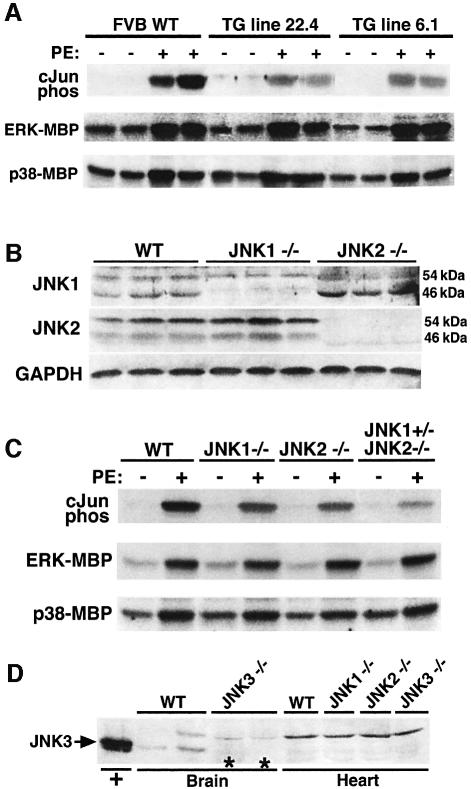
To assess the importance of JNK signaling as a regulator of the cardiac growth response, transgenic mice were generated that express dominant negative mutants of JNK1 and JNK2 under the control of the cardiac-specific α-myosin heavy chain 5.5 kb promoter. Both constructs were mixed during the oocyte injection procedure to generate transgenic mice that coexpress each dominant negative protein. This strategy produced three independent transgenic lines that coexpressed equal amounts of both dominant negative proteins within the heart (data not shown). To verify the effectiveness of this strategy, two of the lines were subjected to acute phenylephrine (PE) injection (15 mg/kg) for 30 min to induce JNK activation in the heart. JNK activity was assessed by c-Jun pull-down kinase assays, which demonstrated a significant reduction in cardiac JNK activity in line 22.4 and line 6.1 transgenic mice (heterozygotes) compared with wild-type controls (Figure 1A). No inhibitory effect on ERK1/2 or p38 activation was observed as assessed by immune kinase assay with myelin basic protein (MBP) as a substrate (Figure 1A).
Fig. 1. Characterization of mice with diminished JNK activity. (A) Non-transgenic littermates and dnJNK1/2 transgenic mice from lines 22.4 and 6.1 were injected with either PBS or PE (15 mg/kg), and cardiac JNK activity was measured 30 min later using GST-cJun (1–79) as a substrate. ERK and p38 kinase activities were also measured using myelin basic protein (MBP) as the substrate after immunoprecipitation. (B) Western blot analysis with antibodies partially specific for JNK1 and JNK2 from the hearts of JNK1 null and JNK2 null mice. (C) JNK activity assay showing diminished PE-induced JNK activity in the hearts of JNK1–/–, JNK2–/– and 3-allele JNK deleted mice, yet ERK and p38 activities were unaffected. (D) Western blot analysis with a JNK3-specific antibody showed no detectable JNK3 protein in the wild-type mouse heart and no upregulation of JNK3 in either JNK1–/– or JNK2–/– hearts. Controls consisted of HEK293 cell lysate after JNK3 plasmid transfection or brain protein lysate from wild-type and JNK3 targeted mice.
To support the transgenic approach described above, JNK1 and JNK2 gene-targeted mice were also analyzed. While combinatorial deletion of JNK1 and JNK2 results in embryonic lethality, individually JNK1 and JNK2 targeted mice are viable and overtly normal (Kuan et al., 1999; Sabapathy et al., 1999). JNK1 and JNK2 genes each encode a 54 and 46 kDa isoform, although JNK1 primarily encodes the 46 kDa isoform while JNK2 primarily encodes the 54 kDa isoform in the heart (Figure 1B). Western blotting from cardiac protein extracts confirmed the absence of either JNK1 or JNK2 protein from each null animal, although the JNK1-specific antibody was determined to cross-react weakly with endogenous JNK2 of the 54 kDa isoform (Figure 1B). Interestingly, in the absence of JNK2, the 46 kDa JNK1 isoform was significantly increased. To assess the degree of reduction in total cardiac JNK activity a c-Jun pull-down kinase assay was performed from cardiac protein extracts at baseline or from mice subjected to acute PE injection (Figure 1C). Both JNK1 and JNK2 null mice showed a partial reduction in c-Jun kinase activity after PE injection compared with wild-type mice. An even greater reduction in c-Jun kinase activity was observed in combinatorial gene-targeted mice in which three of the four JNK1/2 alleles were missing (JNK1+/– JNK2–/–) (Figure 1C). No alterations were observed in ERK or p38 kinase activity in the hearts of PE-injected JNK gene-targeted mice (Figure 1C). Similar results were observed in three independent experiments. Finally, JNK3 expression was also evaluated to rule out additional compensatory effects in JNK1/2 null mice (Figure 1D). Western blotting with a JNK3-specific antibody demonstrated expression in the brain, but not in the heart at baseline or in the absence of JNK1 or JNK2 (Figure 1D). These results indicate that only JNK1 and JNK2 genes contribute in a significant way to JNK activity in the heart.
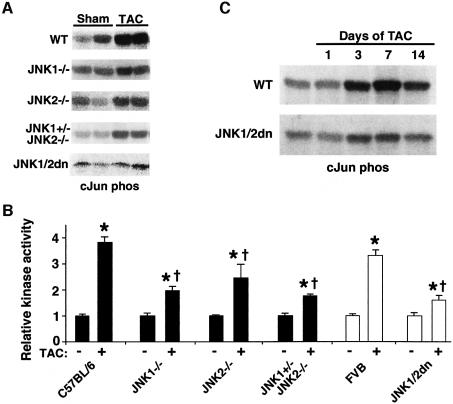
To further evaluate the effectiveness of the dominant negative JNK1/2 transgene and JNK1 or JNK2 gene disruption, a series of c-Jun pull-down kinase assays was performed from the hearts of mice subjected to transverse aortic constriction (TAC) (Figure 2). Pressure overload is a physiologically relevant stimulus for the heart that was previously shown to induce JNK signaling (Choukroun et al., 1999). The dominant negative transgenic mice and each of the gene-targeted mice showed reduced JNK activity in the heart following 7 days of pressure overload stimulation compared with strain-matched controls (N = 6) (Figure 2A and B). The three-allele JNK null mice and the dominant negative JNK1/2 transgenic mice showed the greatest degree of JNK inhibition in the heart (Figure 2B). A time-course analysis was also performed, which revealed significant JNK activation 3, 7 and 14 days after TAC stimulation in wild-type mice, but consistent inhibition in JNK1/2 dominant negative mice (Figure 2C).
Fig. 2. TAC-induced cardiac JNK activation is reduced in dominant negative mice and gene-targeted mice. (A) Autoradiograph of 32P-labeled GST-cJun (1–79) phosphorylation from heart protein extracts of the indicated mice at baseline or 7 days after TAC stimulation (some lanes were spliced together for simplicity). (B) Quantitative analysis of JNK activity in each of the indicated groups (N = 6 each) (*P < 0.05 versus sham; †P < 0.05 versus C57BL/6 or FVB WT TAC). (C) Time course of cJun kinase activity in wild-type (WT) or JNK1/2 dn mice following TAC stimulation.
Reduction of JNK activity enhances the cardiac hypertrophic growth response
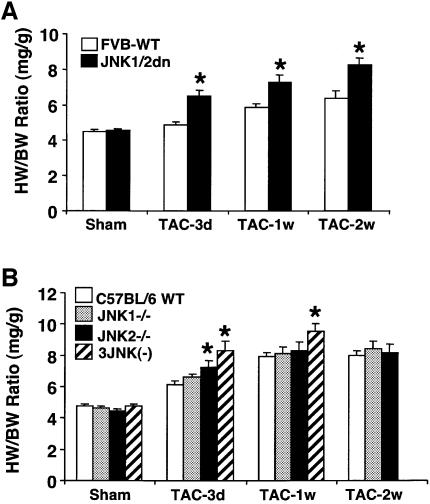
JNK1/2dn transgenic mice were originally generated to test the hypothesis that JNK signaling positively regulates the cardiac hypertrophic growth response. This initial hypothesis was based on the ability of MAP kinase kinase 7 (MKK7) to induce hypertrophic growth in cultured cardiac myocytes (Wang et al., 1998), and on the ability of dominant negative MKK4 to attenuate hypertrophic growth in vitro and in vivo (Choukroun et al., 1998; 1999). However, given the potential for cross-talk amongst MAPKKs (such as MKK4), we sought a more proximal inhibitory approach. Accordingly, JNK1/2dn transgenic mice (FVB strain) and non-transgenic littermate controls were subjected to pressure-overload stimulation by TAC. JNK1/2dn mice showed a 43%, 60% and 81% increase in the heart-to-body weight ratio at 3, 7 and 14 days after TAC compared with only an 8%, 30% and 42% increase, respectively, in wild-type mice at the same time points (P < 0.05) (Figure 3A). A nearly identical profile of enhanced hypertrophic growth was also observed in desiccated hearts normalized to body weight, indicating that cardiac edema was not skewing the results (data not shown).
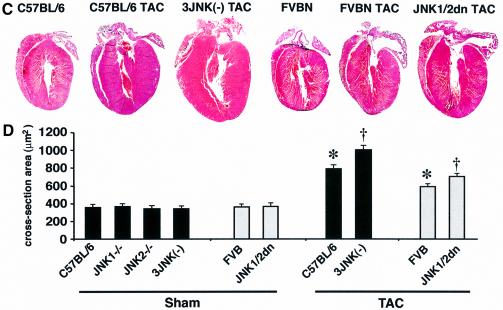
Fig. 3. JNK-inhibited mice show enhanced cardiac hypertrophy following TAC. (A) Two-month-old JNK1/2dn transgenic mice showed enhanced cardiac hypertrophy following 3, 7 and 14 days of TAC compared with FVB wild-type (FVB-WT) control mice (as assessed by wet heart-to-body weight ratio HW/BW) (N = 10–15 mice in each group; *P < 0.05 versus FVB-WT TAC). (B) TAC-induced increase in HW/BW ratio was significantly higher in JNK2 null and JNK1+/– JNK2–/– three-allele null mice compared with strain-matched wild-type controls (C57BL/6) at 3 days and/or 1 week (N = 6–15 for each group; *P < 0.05 versus C57BL/6 TAC). (C) Histological analysis of HE-stained hearts in frontal section from 1-week TAC- stimulated mice. (D) Assessment of cardiomyocyte cross-sectional areas at baseline or 7 days after TAC stimulation (N ≥ 100 cells in each group; *P < 0.05 versus respective sham; †P < 0.05 versus WT TAC).
Since overexpression approaches are not without limitations, JNK1 and JNK2 gene-targeted mice were also analyzed to verify the potential specificity of this prohypertrophic effect. JNK2 null mice showed enhanced growth during the initiation phase of the hypertrophic response at 3 days post-TAC, while the three-allele JNK null mice (JNK1+/– JNK2–/–) had a 77% and 103% increase in the heart-to-body weight ratio compared with a 28% and 65% increase in wild-type strain-matched controls at 3 days and 1 week after TAC, respectively (P < 0.05) (Figure 3B). Unfortunately, three-allele JNK null mice were somewhat ‘fragile’ and did not survive 14 days of TAC. However, the results obtained at 3 and 7 days verify that diminished JNK activity renders the heart more susceptible to growth following pressure-overload stimulation.
The cellular and molecular determinants of the hypertrophic response were also evaluated in JNK1/2dn transgenic mice and JNK gene-targeted mice following 7 days of TAC. Histological analysis demonstrated no overt abnormalities in any of the JNK-inhibited mice after TAC, although a profile of greater eccentric growth was observed (Figure 3C). These histological results were further verified by echocardiography (data not shown). Finally, histological sections were stained with wheat germ agglutinin–TRITC to demarcate cellular membranes for measurement of cross-sectional areas (Figure 3D). Consistent with the heart weight analyses described above, TAC stimulation produced a significant increase in cellular hypertrophy in wild-type mice, which was enhanced in JNK-inhibited mice (93% in dnJNK1/2 versus 62% in FVB, and 193% in JNK1+/– JNK2–/– versus 120% in C57BL/6, P < 0.05) (Figure 3D). Collectively, these data implicate JNK signaling as antagonistic to adaptive cardiac growth.
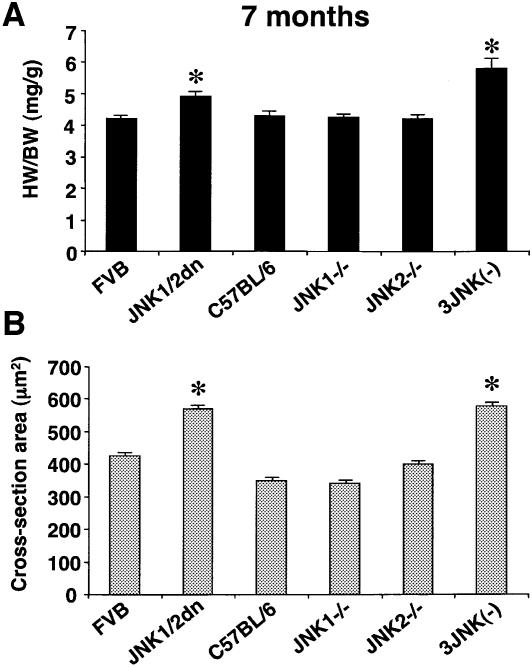
Given that JNK-inhibited mice showed an enhanced growth response following pressure overload, we further reasoned that JNK-inhibited mice might gradually show increased heart growth as they age, without stimulation. While 2- and 3-month-old JNK-inhibited mice showed normal ratios of heart to body weight and fiber cross-sectional areas, 7-month-old double allele JNK1/2dn transgenic mice and three of four-allele JNK gene-targeted mice each showed enhanced growth of the whole organ and individual myocytes (16% and 28% increase in heart-to-body weight ratio, respectively; P < 0.05) (Figure 4A and B). For this experiment double-allele JNK1/2 transgenic mice (two copies of the transgene integration) were employed as a means of further inhibiting JNK activity in the heart. The spontaneous hypertrophy of the heart over time in JNK-inhibited mice is consistent with the hypothesis that JNK and calcineurin constitute a reciprocal regulatory circuit whereby NFAT activity is controlled around a set equilibrium point.
Fig. 4. JNK-inhibited mice show spontaneous cardiac hypertrophy with aging. (A) Wet heart-to-body-weight ratios HW/BW were determined for 7-month-old mice C57BL/6 WT or three-allele JNK-targeted mice (N = 6 in each group; *P < 0.05) or in FVB WT and double-allele JNK1/2dn transgenic mice (N = 10–13; *P < 0.05). (B) Cross-sectional area measurements from wheat germ agglutinin–TRITC stained histological sections for each cohort. At least 100 muscle fibers on sections from two separate mice were measured for each group (*P < 0.05).
JNK signaling regulates NFAT nuclear translocation in cardiomyocytes
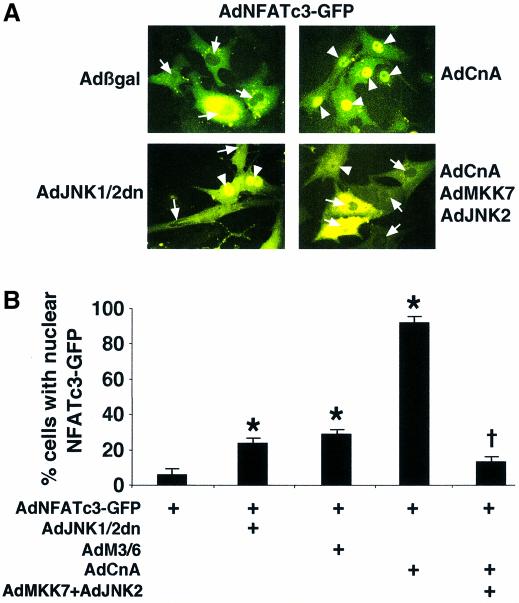
The observation that JNK-inhibited mice displayed enhanced hypertrophic growth following pressure-overload stimulation suggested an antihypertrophic role for this signaling pathway in the heart. Indeed, JNK was originally shown to phosphorylate NFATc3 directly (Chow et al., 1997), while subsequent studies showed that JNK could also associate with and phosphorylate NFATc2 (Porter et al., 2000) but not NFATc4 (Yang et al., 2002). Since calcineurin–NFAT signaling plays a critical role in regulating cardiac hypertrophic growth (Molkentin et al., 1998), it was hypothesized that JNK might attenuate this response by antagonizing the ability of select NFAT isoforms to translocate to the nucleus. To examine this hypothesis, cultured cardiomyocytes were infected with an adenovirus encoding NFATc3–Green Fluorescent Protein (GFP) in combination with JNK activating or inhibitory adenoviruses. NFATc3–GFP resided almost exclusively within the cytoplasm of unstimulated cardiomyocytes [Figure 5A (arrows) and B]. However, coinfection with an activated calcineurin-expressing adenovirus (AdCnA) induced greater than 92% nuclear occupancy of NFATc3–GFP [Figure 5A (arrowheads) and B]. Remarkably, coinfection of dominant negative JNK1/2 encoding adenoviruses significantly enhanced basal NFATc3 nuclear occupancy (25%; P < 0.05) (Figure 5A and B). As an alternative inhibitory approach, cardiomyocytes were also infected with an adenovirus encoding the JNK-specific dual-specificity phosphatase M3/6, which similarly enhanced NFATc3–GFP nuclear occupancy (28%; P < 0.05) (Figure 5B). Conversely, adenoviral-mediated overexpression of wild-type JNK1 or JNK2 in the presence of MKK7 antagonized calcineurin-induced NFATc3 nuclear translocation (13%; P < 0.05) (Figure 5A and B). Nearly identical results were observed with an adenovirus expressing an NFATc1–GFP fusion protein, suggesting that JNK can also regulate NFATc1 (data not shown). All results were quantified in three independent experiments.
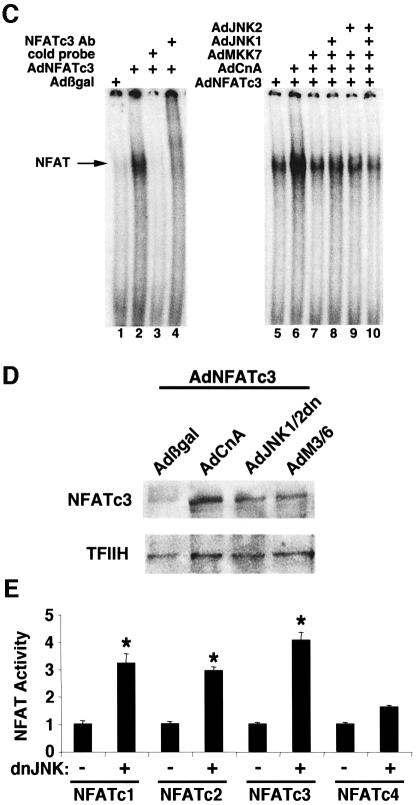
Fig. 5. MKK7/JNK pathway negatively regulates NFATc3 activation. (A) Representative fluorescent images showing the nuclear-cytoplasmic shuttling of NFATc3–GFP in neonatal rat cardiomyocytes infected with AdNFATc3–GFP, Adβgal, AdJNK1/2dn, AdCnA, AdMKK7 and/or AdJNK1/2. Arrows show cytoplasmic localization and arrowheads show nuclear localization of NFATc3–GFP. (B) Quantitation from three independent experiments of the percentage of cells with nuclear NFATc3–GFP by each of the indicated recombinant adenoviruses (*P < 0.05 versus AdNFATc3–GFP alone). (C) EMSA showing that MKK7/JNK signaling inhibits calcineurin-induced NFATc3 DNA binding activity in nuclear protein extracts from recombinant adenoviral infected neonatal rat cardiomyocytes. Nuclear protein extracts were generated 24 h after viral infection. (D) NFATc3 western blot from nuclear protein extracts derived from adenoviral-infected cardiomyocytes. All cells were infected with wild-type AdNFATc3 and Adβgal, AdCnA, AdJNK1/2dn or AdM3/6. TFIIH was used as a nuclear protein loading control. (E) Transient transfection experiment with an NFAT- dependent luciferase reporter in Cos-7 cells shows that dominant negative JNK1/2 augments the activity of NFATc1, NFATc2 and NFATc3 (*P < 0.05 versus no dnJNK1/2 cotransfection). The results represent the average of triplicate assays.
To evaluate this paradigm of altered NFAT nuclear translocation more carefully, electrophoretic mobility shift assays (EMSAs) and transient transfection reporter assays were performed. An adenovirus encoding full-length NFATc3 was generated and used to infect cultured neonatal cardiomyocytes for subsequent analysis of DNA binding activity present in the nuclear protein fraction (24 h after infection). AdNFATc3-infected myocytes showed a mobility shift that was competed with cold oligonucleotide and NFATc3-specific antibody (Figure 5C). Coinfection of AdNFATc3 and AdCnA dramatically enhanced the intensity of the NFATc3-specific mobility shift from cardiomyocyte nuclear extracts (Figure 5C). In contrast, coinfection of AdNFATc3 and AdCnA with wild-type AdMKK7, AdJNK1 or AdJNK2 significantly reduced the calcineurin-induced NFATc3 mobility shift, indicating partial blockade of nuclear translocation (Figure 5C).
The observations described above were extended by performing western blots from nuclear protein extracts derived from unstimulated cardiomyocytes infected with AdNFATc3 in combination with Adβgal control or a JNK-inhibitory virus. At baseline, very little NFATc3 protein was identified in the nuclear extracts of Adβgal coinfected myocytes. However, inhibition of endogenous JNK signaling with adenoviruses encoding dominant negative JNK1/2 or the phosphatase M3/6 each augmented NFATc3 nuclear content, while a control protein (TFIIH) showed equivalent protein loading (Figure 5D). To evaluate which NFAT isoform is subject to JNK regulation, a series of transient transfection assays were performed with an NFAT-dependent luciferase reporter. Dominant negative JNK1/2 significantly enhanced NFAT-dependent reporter activity from cotransfections of NFATc1, NFATc2 and NFATc3, but not NFATc4 (P < 0.05) (Figure 5E).
Dominant negative JNK1/2 transgenic mice show enhanced NFAT activity in vivo
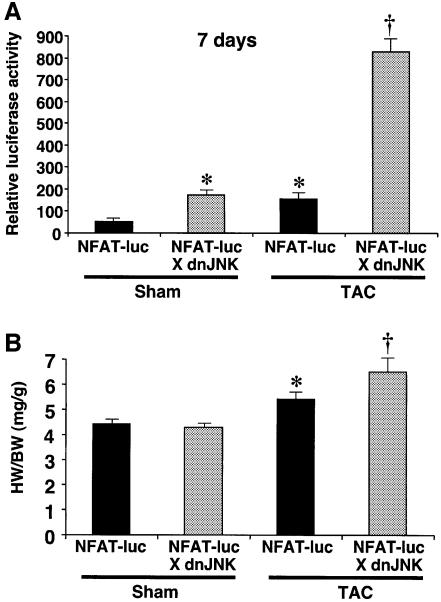
While inhibition of endogenous JNK signaling enhanced NFAT nuclear occupancy in cultured cardiomyocytes, it was uncertain whether this mechanism of regulation was responsible for the enhanced growth response observed in JNK-inhibited mice described above. To evaluate the activity of NFAT factors more directly in vivo, NFAT reporter transgenic mice were generated (nine NFAT DNA binding elements upstream of a basal promoter and a luciferase cDNA). After screening a large number of founders, two transgenic lines were identified that demonstrated robust calcineurin transgene-induced expression in the heart that was blocked with cyclosporine A (B.J.Wilkins and J.D.Molkentin, in preparation). Mice containing this NFAT-dependent reporter transgene were crossed with mice containing the dominant negative JNK1/2 transgene to evaluate cross-talk between these two signaling pathways in vivo. At baseline, the dominant negative JNK1/2 transgene induced a 2.4-fold activation of the NFAT reporter transgene in the hearts of 2-month-old mice (Figure 6A). By comparison, 7 days of TAC stimulation induced a >2-fold activation of the NFAT–luciferase reporter transgene in wild-type hearts. Strikingly, TAC stimulation of JNK1/2dn mice showed a synergistic increase in NFAT–luciferase activity (15-fold; P < 0.05) (Figure 6A). Heart weights were also assessed from these intercrossed cohorts, and consistently showed greater hypertrophy in the presence of the JNK1/2dn transgene after TAC stimulation (P < 0.05) (Figure 6B). These data further extend the proposed mechanism whereby JNK1/2 signaling directly cross-talks with the calcineurin–NFAT pathway in the heart.
Fig. 6. JNK inhibition enhances NFAT activation in vivo. (A) dnJNK1/2 mice were crossed with the transgenic NFAT indicator line and activity in the heart was assessed in 2-month-old mice at baseline or after 7 days of TAC stimulation (N = 4–6 mice in each group). (B) The increased NFAT activity correlated with the enhanced hypertrophic response to TAC in the same dnJNK1/2 transgenic mice as assessed by heart-to-body weight ratio HW/BW. (*P < 0.05 versus sham NFAT-Luc; †P < 0.05 versus NFAT indicator TG alone after TAC).
Calcineurin Aβ gene targeting blocks JNK1/2dn-augmented hypertrophic growth
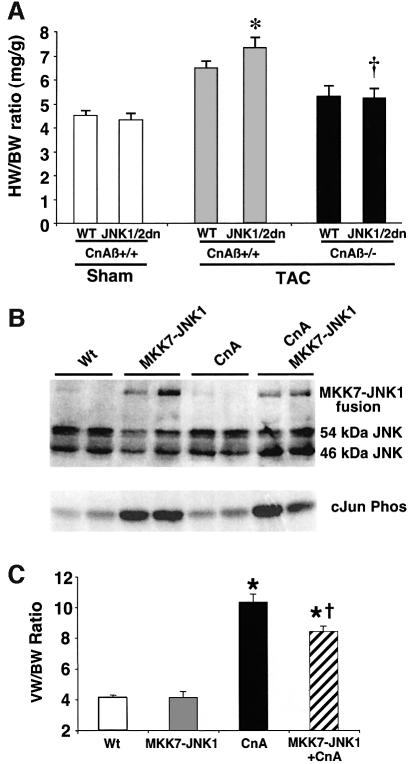
Since calcineurin signaling normally activates NFAT while JNK signaling attenuates it, we reasoned that reducing calcineurin signaling by gene targeting should diminish the effectiveness of the growth response associated with JNK inhibition. In other words, basal calcineurin signaling and JNK inhibition each tend to augment NFAT nuclear translocation, so that calcineurin Aβ gene targeting could somewhat diminish the positive effect of diminished JNK signaling. Calcineurin Aβ gene-targeted mice were recently shown to be partially defective in NFAT translocation and in mounting a cardiac growth response (Bueno et al., 2002a,b). JNK1/2dn transgenic mice were crossed with calcineurin Aβ disrupted mice as a means of reducing NFAT activation. Control littermates from the intercross that were transgene negative and wild type for the calcineurin Aβ locus showed a significant increase in cardiac growth following TAC stimulation (41% increase, wet heart weights; P < 0.05) (Figure 7A). Once again, the presence of the JNK1/2dn transgene significantly enhanced the growth response in wild-type mice from the intercross (70% increase, wet heart weights; P < 0.05). However, in the absence of the calcineurin Aβ gene, this enhanced TAC-induced growth response associated with the JNK1/2dn transgene was abrogated (P < 0.05) (Figure 7A). This result indicates that the enhanced growth response associated with JNK inhibition is dependent, in part, on calcineurin–NFAT signaling.
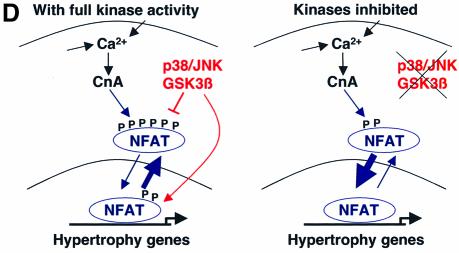
Fig. 7. Calcineurin–NFAT signaling is interconnected with JNK signaling in vivo. (A) The dnJNK1/2 transgene was crossed with calcineurin Aβ gene-targeted mice to generate null mice (CnAβ–/–) or wild-type littermate controls (CnAβ+/+) for gravimetric analysis 1 week after TAC or sham operation. (N = 4–9 mice in each group; *P < 0.05 versus wild-type mice subjected to TAC; †P < 0.05 versus JNK1/2dn subjected to TAC). (B) Western for JNK1/2 protein from the hearts of MKK7–JNK1 fusion transgenic mice (upper band), wild-type mice, activated calcineurin transgenic mice or mice containing both the activated calcineurin and MKK7–JNK1 transgenes. The lower panel shows cJun kinase activity. (C) Analysis of wet ventricle-to-body weight ratios VW/BW from MKK7–JNK1 transgenic mice crossed with activated calcineurin transgenic mice (N = 4 mice in each group; *P < 0.05 versus WT; †P < 0.05 versus CnA transgenic. (D) Model whereby NFAT nuclear translocation and occupancy are controlled by the interplay between calcineurin-mediated dephosphorylation and kinase-mediated phosphorylation (left). Inhibition of a key NFAT kinase, such as JNK, shifts the equilibrium so that the same activation signal by calcineurin now results in greater NFAT translocation and nuclear occupancy.
MKK7–JNK1 transgenic mice show reduced calcineurin-mediated hypertrophy
The data described in Figure 7A indirectly suggests that JNK inhibition regulates the cardiac growth response through a calcineurin-dependent mechanism. However, to establish this hypothesis more directly, a JNK gain-of-function mouse model was generated and crossed with transgenic mice expressing the activated calcineurin cDNA in the heart (Molkentin et al., 1998). To produce specific activation of JNK signaling in the heart, an activated MKK7–JNK1 fusion construct was used which produces highly specific JNK activation and limits the potential for MKK7 cross-talk (Lei et al., 2002). The cardiac-specific α-MHC promoter was again employed to drive expression of the MKK7–JNK1 fusion in transgenic mice. Using a JNK-specific antibody, hearts from these transgenic mice showed reasonable MKK7–JNK1 protein expression in the heart by western blotting that correlated with increased c-Jun kinase activity (Figure 7B). MKK7–JNK1 transgenic mice were crossed with transgenic mice expressing activated calcineurin in the heart, a model previously shown to undergo massive hypertrophy (Molkentin et al., 1998). Remarkably, the MKK7–JNK1 transgene significantly reduced the hypertrophy response driven by activated calcineurin at 4 weeks of age (18.3% inhibition). While this reduction was rather mild, it is roughly similar to the reduction in calcineurin-transgene-mediated heart growth observed in NFATc3 gene-targeted mice (Wilkins et al., 2002) or due to GSK3β overexpression in the heart (Antos et al., 2002). Collectively, these results indicate that upregulation of JNK signaling in the heart antagonizes calcineurin-mediated hypertrophy, most likely by partially reducing the degree of NFAT nuclear translocation.
Discussion
JNK signaling negatively regulates cardiac growth in multiple models
Two distinct approaches were utilized to inhibit JNK signaling in vivo: gene targeting and transgenesis. A transgenic approach circumvents two significant concerns sometimes levied against gene-targeting approaches in mice. First, traditional gene targeting disrupts function within all tissues so that non-myocytes might influence the cardiac growth process through secondary mechanisms. Secondly, gene-targeting experiments can lead to developmental compensation in the expression of related family members in an attempt to restore homeostasis. Indeed, JNK2 null mice showed an upregulation of JNK1 protein in the heart (Figure 1B). However, a transgenic approach can also be criticized for potential non-specific effects due to overexpression itself. Here, a simultaneous analysis employing both models was performed.
The dominant negative JNK1/2 mutant proteins (TPY to APF) employed in this study were previously shown to potently and specifically block endogenous JNK signaling in mammalian cells (Gupta et al., 1995, 1996). The JNK1/2 dominant negative transgenic mice showed a significant reduction in JNK activation following both acute (PE injection) and chronic (TAC) stimulation, without downregulating ERK1/2 or p38 activation. These observations further reduce the likelihood that overexpression of the JNK1/2-APF mutant proteins within the heart elicits a non-specific effect. As discussed above, the independent analysis of JNK1 and JNK2 gene-targeted mice further validated the specificity of the JNK1/2 dominant negative approach. Another consideration is that single disruptions of JNK1 or JNK2 either had no effect on the pressure-overload-induced growth response or mildly increased it early on. However, mice missing three of the four JNK1/2 alleles, which had a more significant reduction in total cardiac JNK activity, showed a more prominent increase in the hypertrophic response at two different time points. Taken together, these data indicate that JNK signaling has a specific effect on the hypertrophic response whereby reduced signaling is permissive to greater cardiac growth.
Cross-talk between calcineurin–NFAT and JNK signaling pathways
The observation that inhibition of JNK signaling enhanced stimulus-induced hypertrophy suggests that JNK negatively regulates the activity of another growth-inducing pathway in the heart. Indeed, JNK phosphorylates a diverse array of transcription factors which directly influence cellular proliferation, differentiation and apoptosis (reviewed by Davis 2000). One potential mechanism whereby JNK signaling could influence cellular growth is through direct co-regulation of the calcium-sensitive calcineurin–NFAT signaling pathway (Chow et al., 1997; Porter et al., 2000; Yang et al., 2002). Here, we have shown that inhibition of JNK signaling enhances NFATc1, c2 and c3 nuclear localization, suggesting that endogenous JNK signaling normally antagonizes the growth response partially attributed to NFAT factors.
In the heart, calcineurin–NFAT signaling functions as an important regulator of the hypertrophic growth response (reviewed by Wilkins and Molkentin, 2002). In fact, a convincing case can now be made that calcineurin–NFAT signaling represents a central control point of the cardiac growth response. For example, transgene-mediated inhibition of calcineurin in the heart using four independent inhibitory factors significantly reduced stimulus-induced hypertrophy (Wilkins and Molkentin, 2002). More recently, gene targeting of calcineurin Aβ in mice rendered the heart largely unable to hypertrophy in response to pressure overload or agonist stimulation (Bueno et al., 2002b). Here we show that reduction of NFAT activation by calcineurin Aβ gene targeting blocked the enhanced hypertrophic response associated with JNK inhibition in vivo (Figure 7A). In other words, the tendency of NFAT to translocate and/or reside within the nucleus is regulated by the equilibrium between calcineurin-mediated dephosphorylation and kinase- mediated phosphorylation. Inhibition of JNK signaling would shift the equilibrium, thus making endogenous calcineurin more effective in activating NFAT (Figure 7D). In contrast, reduction of calcineurin signaling would shift the equilibrium so that JNK-mediated phosphorylation is no longer necessary to fully restrain NFAT nuclear occupancy (Figure 7D). Lastly, increased JNK activity in the heart was observed to partially antagonize calcineurin-induced cardiac hypertrophy, although the absolute magnitude of inhibition was small given the robust nature of activated calcineurin cDNA (Figure 7C).
Additional evidence that NFAT factors function as critical hypertrophic mediators was suggested by overexpression of activated glycogen synthase kinase-3β (GSK-3β) in the mouse heart, which similarly antagonizes NFAT nuclear translocation and reduces the hypertrophic growth response in vivo (Antos et al., 2002). More recently, we have determined that p38 signaling can also partially regulate NFAT nuclear translocation and the ensuing hypertrophic response in transgenic mice (Braz et al., 2003), which is consistent with the ability of p38 MAPKs also to directly phosphorylate NFAT factors (Gomez del Arco et al., 2000; Porter et al., 2000; Yang et al., 2002). Finally, we have determined that NFATc3 gene-targeted mice show a minimal, albeit significant, reduction in calcineurin transgene-induced hypertrophy as early as 3 weeks of age up to 10 weeks (Wilkins et al., 2002). These observations indicate that NFAT factors are central mediators of the cardiac hypertrophic response and that alterations in the activity of NFAT effector kinases can dramatically influence hypertrophic growth.
Previous studies implicating a role for JNK in cardiac hypertrophic growth
JNK is rapidly activated in cultured cardiomyocytes or in the adult heart following agonist treatment or stress stimulation (Ramirez et al., 1997; Choukroun et al., 1999; He et al., 1999; Esposito et al., 2001). However, data implicating a causative role for JNK signaling in mediating the cardiac growth response has been controversial. While adenoviral-mediated overexpression of MKK7 in cultured cardiac myocytes induced the characteristic features of hypertrophy (Wang et al., 1998; Liang et al., 2001), it is difficult to rule out the possibility that MKK7 might regulate the activity of kinases other than JNK (Fleming et al., 2000). In addition, the growth response of cultured neonatal cardiomyocytes is not identical to the growth response of the adult heart, since neonatal myocytes in culture lack the highly organized signaling complexes associated with the Z-disk and T-tubule network found in adult myocytes. Indeed, MKK7-overexpressing transgenic mice were recently described and determined not to have overt cardiac hypertrophy (Petrich et al., 2002). A similar discordance between cultured neonatal myocytes and the adult heart was observed with overexpression of activated MKK3 (stimulates p38), MKK6 (stimulates p38) and MKK5 (stimulates ERK5) (Liao et al., 2001; Nicol et al., 2001). Taken together, these data suggest that MKK7 signaling, presumably through JNKs, does not initiate a significant cardiac growth response in vivo. However, this assertion does not disregard a potential role for JNK signaling in regulating developmental growth of the heart, cardiac remodeling or cell death.
Previous studies have also employed a loss-of-function approach toward investigating the necessary function of JNKs as mediators of the growth response. Specifically, adenoviral gene transfer of a dominant negative MKK4 protein into either cultured cardiomyocytes or the adult rat heart blocked JNK activation and reduced hypertrophic growth induced by agonist stimulation or pressure overload (Choukroun et al., 1998,1999; De Windt et al., 2000). However, the specificity of MKK4 has been shown to include other kinases in different cell types (Kyriakis and Avruch, 1996; Fleming et al., 2000). We observed that neither JNK1dn, JNK2dn nor the JNK-specific M3/6 phosphatase inhibited agonist-induced growth of cultured neonatal cardiomyocytes, in contrast with the effects of dnMKK4 (see Supplementary data available at The EMBO Journal Online.). In vivo, two independent groups have recently reported the cardiac growth phenotype of MEKK1 gene-targeted mice (MEKK1 can regulate JNK activation through MKK4 and MKK7). Schneider and colleagues crossed the MEKK1 null mutation with the Gαq model of hypertrophic cardiomyopathy, resulting in rescue of both Gαq-mediated hypertrophic growth and diminished functional performance (Minamino et al., 2002). In contrast, Sadoshima and colleagues evaluated pressure overload in MEKK1 null mice demonstrating normal growth at 7 days after aortic constriction, but an enhanced growth response at 14 days (Sadoshima et al., 2002). The enhanced hypertrophic response of MEKK1 null mice following 14 days of aortic constriction is consistent with the enhanced growth response observed in dominant negative JNK1/2 transgenic mice and the three-allele JNK1/2 gene-targeted mice described here. However, the assertion that MEKK1 is an exclusive upstream regulator of JNK signaling only is unlikely given its ability to act as an E3 ubiquitin ligase and to regulate MEK1-ERK1/2 signaling in non-myocytes (Karandikar et al., 2000; Lu et al., 2002). In conclusion, our analysis of JNK-targeted mice suggests that this kinase effector functions to antagonize the growth response of the adult heart through cross-talk with the calcineurin–NFAT signaling pathway.
Materials and methods
Animals
Dominant negative (dn) JNK1 and JNK2 cDNAs, in which the dual phosphorylation motif TPY was changed to APF, and the MKK7–JNK1 chimeric cDNA were each fused downstream of the α-MHC promoter and used to generate transgenic mice (FVB/N strain). Heterozygous JNK1, JNK2 and JNK3 knockout mice in C57BL/6 background were obtained from Dr Michael Karin and/or Dr Richard Flavell (Yang et al., 1997; Kuan et al., 1999; Sabapathy et al., 1999). JNK1 and JNK2 gene-targeted mice were intercrossed to generate 3-allele JNK deleted mice (JNK1+/– JNK2–/–), which generated wild-type mice in the same mixed background as controls. To generate a model of cardiac pressure overload, TAC was performed in 8-week-old mice using a 7–0 silk suture that was tied to a 27-gauge constriction at the aortic arch.
Western blot analysis
Protein samples were prepared as described previously (De Windt et al., 2000). Blots were incubated overnight in 3% milk with primary antibodies against JNK1 (Santa Cruz), JNK2 (Cell Signaling) and JNK3 (StressGen Bio Corporation, Canada), which was followed by incubation of alkaline phosphatase-conjugated secondary antibodies for 1 h at room temperature in 3% milk. Chemiluminescent detection was performed with Vistra ECF (Amersham Pharmacia Biotech).
Immune complex kinase assays
Cardiac ERK, JNK and p38 kinase activities were determined using a published method with minor modifications (Whitmarsh and Davis, 2001). Mouse ventricular tissue was minced in lysis buffer and sonicated briefly. Soluble protein extracts (1 mg) were incubated with agarose-conjugated antibodies to ERK1 and ERK2 or p38 for 3 h. Complexes were washed twice in lysis buffer and twice in kinase buffer. MBP was used as substrate for ERK and p38, while GST-c-Jun (amino acids 1–79) was used to isolate JNK kinases and as a substrate. The reaction was started by the addition of 100 µM cold ATP spiked with 10 µCi [γ-32P]ATP and incubated at 30°C for 20 min followed by SDS–PAGE electrophoresis.
Morphological analyses
For microscopic analysis, hearts were harvested, rinsed in phosphate-buffered saline (PBS), blotted dry, weighed and fixed in 10% phosphate-buffered formalin. Serial 7 µm heart sections were cut and stained with hematoxylin and eosin (HE) or wheat germ agglutinin–TRITC conjugate to identify sarcolemmal membranes for measuring myocyte cross-sectional area (at least 100 cells from two independent hearts each).
Replication-deficient adenovirus
Murine JNK1 and JNK2 plasmids were obtained from Dr K.Yoshioka (Ito et al., 1999), and the JNK-specific phosphatase M3/6 was obtained from Dr S.Arkinstall (Muda et al., 1996). The cDNAs encoding JNK1, JNK2, MKK7, M3/6, NFATc3, dnJNK1 and dnJNK2 were each subcloned into the shuttle vector pACCMVpLpA to generate replication-deficient adenoviruses as previously described (De Windt et al., 2000). The adenovirus expressing a constitutively active form of calcineurin A (AdCnA) was reported before (De Windt et al., 2000), and the adenovirus expressing NFATc3–GFP was provided by Dr R.B.Marchase (Hunton et al., 2002).
Cell culture, viral infection and transfection
Primary cultures of neonatal rat cardiomyocytes were prepared as described previously (De Windt et al., 2000). Cardiomyocytes were maintained in serum-free M199 media for 48 h and then infected with AdNFATc3–GFP in combination with Adβgal or AdJNK1/2dn or AdCnA in the absence or presence of AdMKK7 plus AdJNK1/2 (same for Cos-7 cells). Transfections in Cos-7 cells utilized an NFAT-dependent luciferase reporter plasmid and FuGENE 6 reagent (Roche).
Electrophoretic mobility shift assays (EMSAs)
Nuclear extracts were prepared from cultures of primary neonatal rat cardiomyocytes as described previously (Schreiber et al., 1989), while EMSAs were performed as described previously (Liang et al., 2001). 32P-labeled double-stranded oligonucleotide containing the NFAT motif from the IL-4 promoter was used as the probe in EMSAs. Supershift experiments utilized 0.6 µg of antibody to the EMSA reaction.
Statistical analysis
Data were expressed as mean ± SE. Differences between experimental groups were evaluated for statistical significance using Student’s t-test for unpaired data or one-way ANOVA for multiple groups followed by Bonferroni’s post-test. P values <0.05 were considered to be statistically significant.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
References
- Antos C.L., McKinsey,T.A., Frey,N., Kutschke,W., McAnally,J., Shelton,J.M. Richardson,J.A. Hill,J.A. and Olson,E.N. (2002). Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc. Natl Acad. Sci. USA, 99, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J.C., Bueno,O.F., Liang,Q., Wilkins,B.J., Dai,Y.-S., Braunwart,J., Glascock,B.J., Klevitsky,R., Kimball,T.F. et al. (2003). Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin–NFAT signaling. J. Clin. Invest., 111, 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno O.F., Brandt,E.B., Rothenberg,M.E. and Molkentin,J.D. (2002a). Defective T cell development and function in Calcineurin A-deficient mice. Proc. Natl Acad. Sci. USA, 99, 9398–9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno O.F., Wilkins,B.J., Tymitz,K.M., Glascock,B.J., Kimball,T.F., Lorenz,J.N. and Molkentin,J.D. (2002b). Impaired cardiac hypertrophic response in Calcineurin A beta-deficient mice. Proc. Natl Acad. Sci. USA, 99, 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun G., Hajjar,R., Kyriakis,J.M., Bonventre,J.V., Rosenzweig,A. and Force,T. (1998). Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J. Clin. Invest., 102, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun G., Hajjar,R., Fry,S., del Monte,F., Haq,S., Guerrero,J.L., Picard,M., Rosenzweig,A. and Force,T. (1999). Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH2-terminal kinases. J. Clin. Invest., 104, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.W., Rincon,M., Cavanagh,J., Dickens,M. and Davis,R.J. (1997). Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science, 278, 1638–1641. [DOI] [PubMed] [Google Scholar]
- Claycomb W.C. (1977). Cardiac-muscle hypertrophy. Differentiation and growth of the heart cell during development. Biochem. J., 168, 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R. (1999). Generic signals and specific outcomes: signaling through Ca2+, calcineurin and NF-AT, Cell, 96, 611–614. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R. and Olson,E.N. (2002). NFAT signaling: choreographing the social lives of cells. Cell, 109, S67–S79. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000). Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- De Windt L.J., Lim,H.W., Haq,S., Force,T. and Molkentin,J.D. (2000) Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J. Biol. Chem., 275, 13571–13579. [DOI] [PubMed] [Google Scholar]
- Downward J. and Goff,S.P. (2002). Update on the big C: complexity and cross-talk between pathways. Curr. Opin. Genet. Dev., 12, 11–13. [Google Scholar]
- Esposito G., Prasad,S.V., Rapacciulo,A., Mao,L., Koch,W.J. and Rockman,H.A. (2001). Cardiac overexpression of G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation, 103, 1453–1458. [DOI] [PubMed] [Google Scholar]
- Fleming Y., Armstrong,C.G., Morrice,N., Paterson,A., Goedert,M. and Cohen,P. (2000). Synergistic activation of stress-activated protein kinase1/JNK isoforms by MAPKs MKK4 and MKK7 Biochem. J., 352, 145–154. [PMC free article] [PubMed] [Google Scholar]
- Garrington T.P and Johnson,G.L. (1999). Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell. Biol., 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Gomez del Arco P., Martinez-Martinez,S., Maldonado,J.L., Ortega-Perez,I. and Redondo,J.M. (2000). A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem., 275, 13872–13878. [DOI] [PubMed] [Google Scholar]
- Graef I.A., Chen,F., Chen,L., Kuo,A. and Crabtree,G.R. (2001). Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell, 105, 863–875. [DOI] [PubMed] [Google Scholar]
- Gupta S., Campbell,D., Derijard,B. and Davis,R.J. (1995) Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science, 267, 389–393. [DOI] [PubMed] [Google Scholar]
- Gupta S., Barrett,T., Whitmarsh,A.J., Cavanagh,J., Sluss,H.K., Derijard,B. and Davis,R.J. (1996). Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J., 15, 2760–2770. [PMC free article] [PubMed] [Google Scholar]
- He H., Li,H.L., Lin,A. and Gottlieb,R.A. (1999). Activation of the JNK pathway is important for cardiomyocyte death in response to simulated ischemia. Cell Death Differ., 6, 987–991. [DOI] [PubMed] [Google Scholar]
- Hunton D.L., Lucchesi,P.A., Pang,Y., Cheng,X., Dell’Italia,L.J. and Marchase,R.B. (2002). Capacitative calcium entry contributes to NFAT nuclear translocation and hypertrophy in cardiomyocytes. J. Biol. Chem., 277, 14266–14273. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshioka,K., Akechi,M., Yamashita,S., Takamatsu,N., Sugiyama,K., Hibi,M., Nakabeppu,Y., Shiba,T. et al. (1999). JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway. Mol. Cell. Biol., 19, 7539–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandikar M., Xu,S. and Cobb,M.H. (2000). MEKK1 binds raf-1 and the ERK2 cascade components. J. Biol. Chem., 275, 40120–40127. [DOI] [PubMed] [Google Scholar]
- Kuan C-Y., Yang,D.D., Samanta-Roy,D.R., Davis,R.J., Rakic,P. and Flavell,R.A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron, 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch,J. (1996). Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem., 271, 24313–24316. [DOI] [PubMed] [Google Scholar]
- Lei K., Nimnual,A., Zong,W.X., Kennedy,N.J., Flavell,R.A., Thompson,C.B., Bar-Sagi,D. and Davis,R.J. (2002). The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by C-Jun NH2-terminal kinase. Mol. Cell. Biol., 22, 4929–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Wiese,R.J., Bueno,O.F., Dai,Y.S., Markham,B.E. and Molkentin,J.D. (2001). The transcription factor gata4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol. Cell. Biol., 21, 7460–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P., Georgakopoulos,D., Kovacs,A., Zheng,M., Lerner,D., Pu,H., Saffitz,J., Chien,K., Xiao,R.P. et al. (2001). The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl Acad. Sci. USA, 98, 12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorell B.H. and Carabello,B.A. (2000). Left ventricular hypertrophy: pathogenesis, detection and prognosis. Circulation, 102, 470–479. [DOI] [PubMed] [Google Scholar]
- Lu Z., Xu,S., Joaseiro,C., Cobb,M.H. and Hunter,T. (2002). The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol. Cell., 9, 945–956. [DOI] [PubMed] [Google Scholar]
- Minamino T., Yujiri,T., Terada,N., Taffet,G.E., Michael,L.H., Johnson,G.L. and Schneider,M.D. (2002). MEKK1 is essential for cardiac hypertrophy and dysfunction induced by Gq. Proc. Natl Acad. Sci. USA, 99, 3866–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J.D., Lu,R.J., Antos,C.L., Markham,B.E., Richardson,J., Robbins,J., Grant,S.R. and Olson,E.N. (1998). A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell, 93, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muda M., Theodosiou,A., Rodrigues,N., Boschert,U., Camps,M., Gillieron,C., Davies,K., Ashworth,A. and Arkinstall,S. (1996). The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J. Biol. Chem., 271, 27205–27208. [DOI] [PubMed] [Google Scholar]
- Nicol R.L., Frey,N., Pearson,G., Cobb,M., Richardson,J. and Olson,E.N. (2001). Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J., 20, 2757–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich B.G., Gong,X., Lerner,D.L., Wang,X., Brown,J.H., Saffitz,J.E. and Wang,Y. (2002). C-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ. Res., 91, 640–647. [DOI] [PubMed] [Google Scholar]
- Porter C.M., Havens,M.A. and Clipstone,N.A. (2000). Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J. Biol. Chem., 275, 3543–3551. [DOI] [PubMed] [Google Scholar]
- Ramirez M.T., Sah,V.P., Zhao,X.L., Hunter,J.J., Chien,K.R. and Brown,J.H. (1997). The MEKK–JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J. Biol. Chem., 272, 14057–14061. [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Jochum,W., Hochedlinger,K., Chang,L., Karin,M. and Wagner,E.F. (1999). Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev., 89, 115–124. [DOI] [PubMed] [Google Scholar]
- Sadoshima J. et al. (2002). The MEKK1–JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J. Clin. Invest., 110, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias,P., Muller,M.M. and Schaffner,W. (1989). Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res., 17, 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Su,B., Sah,V.P., Brown,J.H., Han,J. and Chien,K.R. (1998). Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J. Biol. Chem., 273, 5423–5426. [DOI] [PubMed] [Google Scholar]
- Weston C.R. and Davis,R.J. (2002). The JNK signal transduction pathway. Curr. Opin. Genet. Dev., 12, 14–21. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A.J. and Davis,R.J. (2001). Analyzing JNK and p38 mitogen-activated protein kinase activity. Methods Enzymol., 332, 319–336. [DOI] [PubMed] [Google Scholar]
- Wilkins B.J. and Molkentin,J.D. (2002). Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J. Physiol., 541, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B.J., De Windt,L.J., Bueno,O.F., Braz,J.C., Glascock,B.J., Kimball,T.F. and Molkentin,J.D. (2002). Targeted disruption of NFATc3, but not NFATc4 reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol. Cell. Biol., 22, 7603–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.D., Kuan,C.-Y., Whitmarsh,A.J., Rincon,M., Zheng,T.S., Davis,R.J., Rakic,P. and Flavell,R.A. (1997). Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature, 389, 865–870. [DOI] [PubMed] [Google Scholar]
- Yang T.T.C., Xiong,Q., Enslen,H., Davis,R.J. and Chow,C.-W. (2002). Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol. Cell. Biol., 22, 3892–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]