Abstract
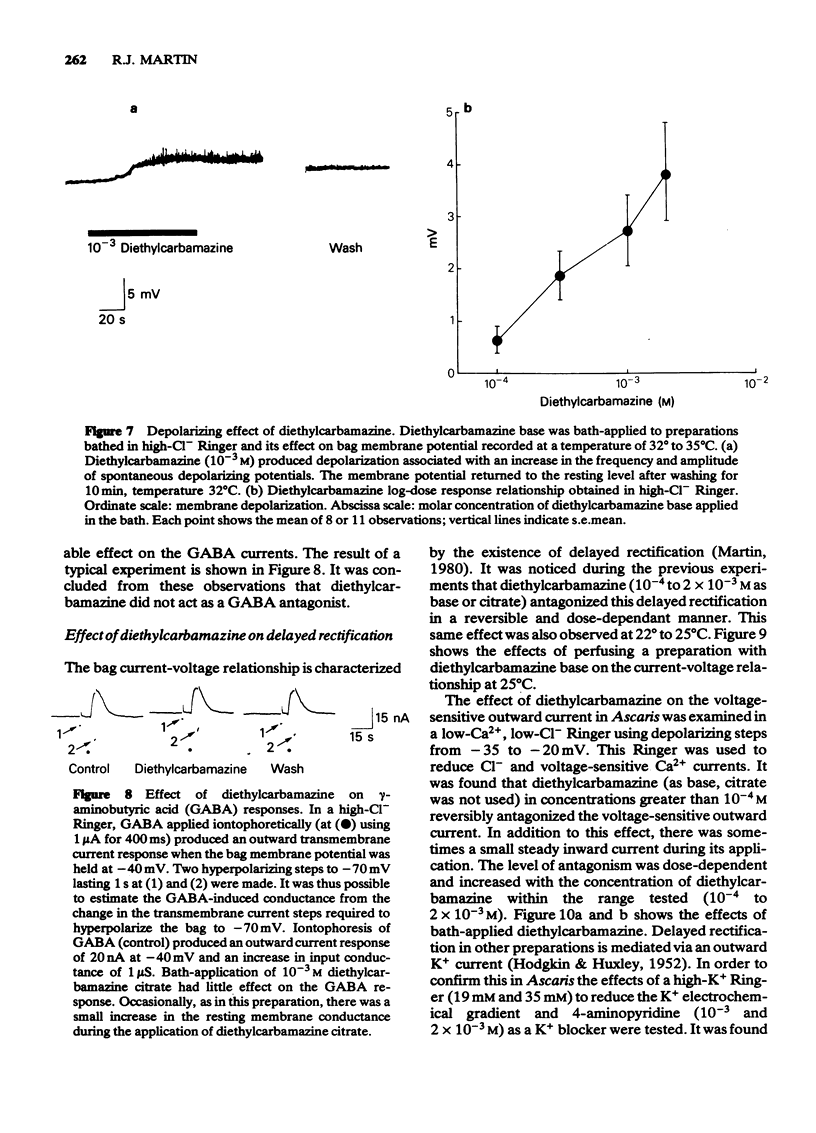
1 Electrophysiological recordings were made from the bag region of Ascaris suum muscle. Membrane potential and input conductance or membrane current under voltage clamp were measured. 2 In high-Cl- Ringer, bath-applied piperazine, at concentrations greater than 10(-4)M, produced a dose-dependent and reversible increase in input conductance associated with a hyperpolarizing potential. The increase in input conductance was reduced when the preparations were bathed in low-Cl- Ringer. Gamma-Aminobutyric acid (GABA) and piperazine reversal potentials were measured with a voltage clamp on the same cells using iontophoretic application of the agonists. The reversal potentials were the same and close to the predicted Nernst Cl- potential (-65 mV). When GABA and piperazine were applied simultaneously piperazine reversibly reduced the amplitude of the control outward GABA current response. It was concluded that piperazine acts as a GABA agonist of low potency on the extra-synaptic GABA receptors of the bag, mediating an increase in Cl- conductance. 3 Acetylcholine was applied iontophoretically within 100 micron of the bag region while the preparation was bathed in a low-Ca2+, low-Cl- Ringer. The response under voltage clamp was a dose-dependent inward current associated with an increase in input conductance. This response was reversibly antagonized by 3 X 10(-5)M tubocurarine, high concentrations of diethylcarbamazine (10(-3) to 10(-2)M) but not high concentrations of piperazine (10(-3) to 10(-2)M). It was concluded that there are extra-synaptic acetylcholine receptors on the bag region of Ascaris muscle and that diethylcarbamazine but not piperazine acts as an antagonist. 4 Bath-applied diethylcarbamazine (10(-4) to 2 X 10(-3)M) produced a reversible dose-dependent depolarization of the membrane potential which was associated with an increase in the amplitude and frequency of spontaneous depolarizing potentials in active preparations at 32 degrees C to 35 degrees C in high-Cl- Ringer. The excitatory action of diethylcarbamazine was not blocked by 3 X 10(-5)M tubocurarine. Diethylcarbamazine (10(-4) to 10(-3)M) had no effect on the outward current response to GABA iontophoresis. Diethylcarbamazine (10(-4) to 10(-2)M) reversibly antagonized in a dose-dependent manner the delayed rectification of the bag membrane. In a low-Ca2+, low-Cl- Ringer, diethylcarbamazine (10(-4) to 2 X 10(-3)M) reversibly antagonized the voltage-sensitive outward current of the bag. This effect was mimicked by high-K+ Ringer or perfusion with 4-aminopyridine (10(-3) to 2 X 10(-3)M). It was concluded that diethylcarbamazine did not react with the GABA receptor but antagonized a voltage-sensitive K+ conductance.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abaitey A. K., Parratt J. R. The effects of diethylcarbamazine citrate on smooth muscle. J Pharm Pharmacol. 1977 Jul;29(7):428–432. doi: 10.1111/j.2042-7158.1977.tb11358.x. [DOI] [PubMed] [Google Scholar]
- Aubry M. L., Cowell P., Davey M. J., Shevde S. Aspects of the pharmacology of a new anthelmintic: pyrantel. Br J Pharmacol. 1970 Feb;38(2):332–344. doi: 10.1111/j.1476-5381.1970.tb08521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Caldwell P. C. The resting membrane potential of the somatic muscle cells of Ascaris lumbricoides. J Physiol. 1971 Sep;217(3):605–624. doi: 10.1113/jphysiol.1971.sp009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. D., Constanti A., Dunn P. M., Forward A., Nistri A. The effects of piperazine on rat sympathetic neurones. Br J Pharmacol. 1981 Oct;74(2):445–454. doi: 10.1111/j.1476-5381.1981.tb09990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Nistri A. A comparative study of the action of gamma-aminobutyric acid and piperazine on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol. 1976 Jul;57(3):347–358. doi: 10.1111/j.1476-5381.1976.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W. C., MORALES T. MECHANISM OF THE PARALYSING ACTION OF PIPERAZINE ON ASCARIS MUSCLE. Br J Pharmacol Chemother. 1964 Jun;22:463–477. doi: 10.1111/j.1476-5381.1964.tb01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysels H. Immunoelectrophoresis of avian lens proteins. Experientia. 1964 Mar 15;20(3):145–146. doi: 10.1007/BF02150703. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawking F. Diethylcarbamazine and new compounds for the treatment of filariasis. Adv Pharmacol Chemother. 1979;16:129–194. doi: 10.1016/s1054-3589(08)60244-6. [DOI] [PubMed] [Google Scholar]
- Iravani J. Wechselbeziehung von Barbituraten und Piperazin mit GABA an der Membran des Krebsmuskels. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Jul 26;251(3):265–274. [PubMed] [Google Scholar]
- Martin R. J. The effect of gamma-aminobutyric acid on the input conductance and membrane potential of Ascaris muscle. Br J Pharmacol. 1980;71(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON S., DE BEER E. J. Investigations on the action of piperazine on Ascaris lumbricoides. Am J Trop Med Hyg. 1957 Sep;6(5):898–905. doi: 10.4269/ajtmh.1957.6.898. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. Comparison of the effects of ethylenediamine analogues and gamma-aminobutyric acid on cortical and pallidal neurones. Br J Pharmacol. 1982 Jan;75(1):93–99. doi: 10.1111/j.1476-5381.1982.tb08761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. L., Sturman G., West G. B. The interaction between anthelmintic drugs and histamine in Ascaris suum. Br J Pharmacol. 1976 Jul;57(3):417–420. doi: 10.1111/j.1476-5381.1976.tb07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U. K. Mechanisms of anthelmintic action. Prog Drug Res. 1975;19:147–157. doi: 10.1007/978-3-0348-7090-0_19. [DOI] [PubMed] [Google Scholar]
- Standen O. D. Chemotherapy of intestinal helminthiasis. Prog Drug Res. 1975;19:158–165. doi: 10.1007/978-3-0348-7090-0_20. [DOI] [PubMed] [Google Scholar]


