Abstract
Alterations of the epidermal growth factor receptor (EGFR) gene occur frequently in human malignant gliomas. The most common of these is deletion of exons 2–7, resulting in truncation of the extracellular domain (ΔEGFR or EGFRvIII), which occurs in a large fraction of de novo malignant gliomas (but not in progressive tumors or those lacking p53 function) and enhances tumorigenicity, in part by decreasing apoptosis through up-regulation of Bcl-XL. Here, we demonstrate that the ΔEGFR concomitantly confers resistance to the chemotherapeutic drug cisplatin (CDDP) by suppression of CDDP-induced apoptosis. Expression of Bcl-XL was elevated in U87MG.ΔEGFR cells prior to and during CDDP treatment, whereas it decreased considerably in CDDP-treated parental cells. CDDP-induced activation of caspase-3-like proteases was suppressed significantly in U87MG.ΔEGFR cells. These responses were highly specific to constitutively kinase-active ΔEGFR, because overexpression of kinase-deficient ΔEGFR (DK) or wild-type EGFR had no such effects. Correspondingly, ΔEGFR specific tyrosine kinase inhibitors reduced Bcl-XL expression and potentiated CDDP-induced apoptosis in U87MG.ΔEGFR cells. Ectopic overexpression of Bcl-XL in parental U87MG cells also resulted in suppression of both caspase activation and apoptosis induced by CDDP. These results may have important clinical implications for the use of CDDP in the treatment of those malignant gliomas expressing ΔEGFR.
Persistent invasion of malignant glioma tumor cells into the adjacent normal brain parenchyma renders surgical resection incomplete and necessitates adjuvant treatments such as radiation and chemotherapy (1). However, most gliomas eventually become drug-resistant, limiting the effectiveness of chemotherapy. A number of mechanisms may contribute to cellular drug resistance, including reduced intracellular drug concentrations, rapid inactivation of the drug, and increased rate of DNA repair (2). Inhibition of apoptosis, a genetically controlled form of cell death, may also be important for drug resistance because the primary mechanism by which most chemotherapeutic agents having disparate modes of action and cellular targets induce cell death appears to be apoptosis (3). The observations that tumors which were either deficient in the p53 tumor suppressor gene or those in which expression of the antiapoptotic protein Bcl-2 was elevated, were resistant to apoptosis and showed poor response to radiotherapy and chemotherapy (4, 5) suggest that tumor-specific genetic lesions may bestow this property to tumor cells, resulting in a survival advantage.
The malignant progression of gliomas involves accumulation of genetic alterations that inactivate tumor suppressor genes such as p53, p16, RB, and PTEN, or activate oncogenes including the epidermal growth factor receptor (EGFR), CDK4, CDK6, and MDM2 genes (6, 7). EGFR gene amplification occurs frequently in gliomas, is restricted to high-grade tumors that are usually of the de novo type and express wild-type p53 (8), and occurs at a frequency of 40–50% of all grade IV gliomas (9, 10). Several clinical and histopathological studies have shown that the presence of EGFR amplification correlates with a shorter interval to disease relapse and lower rates of survival in patients receiving adjuvant therapies, suggesting that it may affect responsiveness of malignant gliomas to treatment (10). The majority of such EGFR gene amplifications also include rearrangements (9, 11), the most common being a genomic deletion of exons 2–7, resulting in a mutant receptor truncated in its extracellular domain (ΔEGFR or EGFRvIII) (11). This specific genetic alteration has also been found frequently in lung and breast cancers (12, 13). Introduction of ΔEGFR into the U87MG human glioma cell line resulted in cell surface expression of a truncated receptor having a ligand-independent, weak but constitutively active, and unattenuated kinase and enhanced tumorigenicity in nude mice (14), which was mediated by both an increase in proliferation and a decrease in apoptosis of tumor cells. In contrast, overexpression of wild-type (wt) EGFR did not confer a similar growth advantage (15, 16). Bcl-XL, an inhibitor of the Bcl-2 family of apoptotic proteins, was up-regulated in U87MG.ΔEGFR tumors, which was inversely correlated with their reduced apoptotic rate (16). Overexpression of Bcl-XL has been shown to confer drug resistance in some tumor cells (17) and also to suppress activation of caspases, the cysteine proteases that play a key role in the execution phase of apoptosis (18).
Here we report that ΔEGFR expression in glioma cells confers resistance to some commonly utilized chemotherapeutic agents. The resistance was associated with suppression of drug-induced apoptosis, which was largely mediated by increased expression of Bcl-XL and subsequent inhibition of caspase-3-like protease activation. These effects required constitutive signaling by ΔEGFR, because overexpression of kinase-deficient ΔEGFR (DK) or wt EGFR had no such effects. Moreover, suppression of ΔEGFR enzymatic function by specific inhibitors sensitized the cells to drug treatment. These results suggest a new treatment strategy for glioma in which ΔEGFR inhibition could be effectively combined with chemotherapy.
MATERIALS AND METHODS
Cells.
The human glioma cell line U87MG, which expresses a low amount of wt EGFR, and its sublines, U87MG.ΔEGFR, U87MG.DK, and U87MG.wtEGFR, which overexpress ΔEGFR, a kinase-deficient mutant of ΔEGFR (DK), and exogenous wt EGFR, respectively, were described previously (15). U87MG cells were transfected with either pSFFVneo-bcl-XL or its control vector pSFFV-neo plasmids (gifts of S. J. Korsmeyer, Washington University, St. Louis) by using the calcium phosphate precipitation method and selected in the presence of 400 μg/ml of G418 (GIBCO/BRL). Clones expressing high levels of Bcl-XL were used for experiments. All cells were cultured as described (16). To determine the level of resistance of the cells to the chemotherapeutic agents cisplatin [cis-diamminedichloroplatinum(II), CDDP], paclitaxel (Taxol), and vincristine (Sigma), in the presence or absence of tyrosine kinase inhibitors, tyrphostins AG1478 (Calbiochem), AG1479, AG1517, and AG1536 (chemically synthesized at The Hebrew University of Jerusalem), colony-forming efficiency assays were performed as described (19).
In Situ Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) of Apoptotic DNA Fragmentation.
Apoptotic cells were detected by using TUNEL of apoptotic DNA strand breaks as described (16).
Caspase Activity Assay.
Protease activity was assayed as described previously (20) with minor modifications. One unit of protease activity was defined as the amount of enzyme required to release 1 pmol p-nitroanilide (pNA)/min at 37°C.
Western Blotting.
Analysis was as described previously (16) and utilized antibodies to Bcl-XL (Transduction Laboratories, Lexington, KY), poly(ADP-ribose) polymerase (PARP) (C2–10, Enzyme Systems Products, Livermore, CA), EGFR (C13) (15), or phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY).
RESULTS
ΔEGFR Confers CDDP Resistance in U87MG Human Glioma Cells.
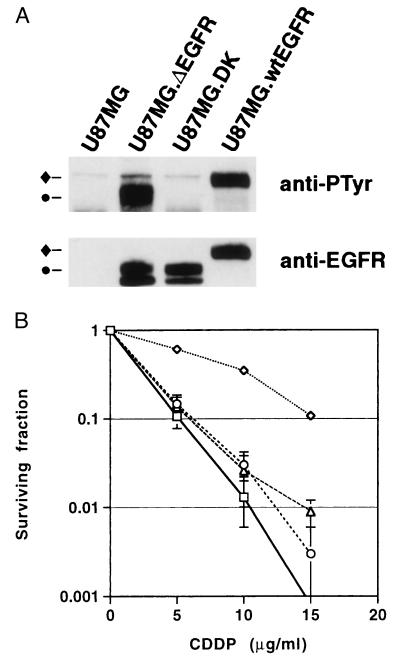
Because ΔEGFR expression in glioma cells has been shown to decrease the rate of apoptosis both under serum-starved culture conditions and in implanted tumors derived from such cells (16), we tested the sensitivity of ΔEGFR-expressing glioma cells to the chemotherapeutic drug, CDDP, a DNA-damaging agent known to induce apoptosis in tumor cells and that often has been used in glioma therapy (21, 22). Endogenous and exogenous wt EGFR were moderately autophosphorylated under the culture condition used (10% serum), suggesting that wt EGFR was constantly stimulated by ligand in the serum (Fig. 1A). Consistent with previous studies, ΔEGFR was also constitutively autophosphorylated while a kinase-deficient mutant of ΔEGFR (DK) was devoid of any significant tyrosine phosphorylation (15). CDDP-treated U87MG.ΔEGFR, but not U87MG.DK or U87MG.wtEGFR, cells had a significantly higher survival rate (4.3-fold greater IC50 value) than parental U87MG cells (Fig. 1B). This relative resistance was also observed in mice bearing xenografts of the various cell types when treated with CDDP (data not shown). Similar results were obtained when cells were treated in vitro with the new and more traditional chemotherapeutic agents Taxol and vincristine, respectively (data not shown) (23).
Figure 1.
(A) Quantitation of EGFR species in cells. Western blot analysis of expression (Lower) and autophosphorylation (Upper) of wt EGFR (♦) and ΔEGFR (•) in U87MG, U87MG.ΔEGFR, U87MG.DK, and U87MG.wtEGFR cells grown in medium containing 10% serum. Low and similar levels of endogenous wt EGFR expression also was detected in U87MG, U87MG.ΔEGFR, and U87MG.DK at longer exposures (data not shown). (B) Survival of U87MG (□), U87MG.ΔEGFR (⋄), U87MG.DK (○), and U87MG.wtEGFR (▵) cells in response to varying amounts of cisplatin (CDDP). Cells were plated in triplicate 60-mm dishes and treated with medium containing various concentrations of CDDP for 1 hr, followed by incubation with fresh medium for 10–12 days. Numbers of colonies were counted after Giemsa staining. Results were reproduced in four independent experiments. [Bars = SD (some bars are too small to be visualized).]
U87MG.ΔEGFR Cells Exhibit Reduced Apoptosis upon CDDP Treatment.
To determine whether the drug resistance observed in U87MG.ΔEGFR cells was associated with inhibition of drug-induced apoptosis, CDDP-treated glioma cells were examined for the morphologic changes typical of apoptotic cells and for DNA fragmentation. After treatment with CDDP for 2 days, U87MG, U87MG.DK, and U87MG.wtEGFR cells each displayed typical features of apoptosis such as a shrunken morphology with condensed and fragmented nuclei, whereas U87MG.ΔEGFR cells were relatively unaffected (data not shown). With regard to DNA fragmentation, the proportion of cells that were TUNEL-positive was lowest in CDDP-treated U87MG.ΔEGFR cells, being more than 5-fold lower than that of CDDP-treated U87MG, U87MG.DK, or U87MG.wtEGFR cells (all P < 0.001, Student’s t test) (Fig. 2). In all untreated cell lines apoptotic cells were nearly undetectable, indicating that apoptosis was induced by CDDP. Similar results were obtained in an Annexin V binding assay for apoptosis detection (data not shown). These results suggested that the constitutively active and aberrant ΔEGFR signaling may play an inhibitory role in the induction of apoptosis by the DNA-damaging agent, CDDP.
Figure 2.
Cells expressing ΔEGFR are diminished in their apoptotic response to CDDP. Cells were seeded on coverslips and treated with 5 μg/ml of CDDP for 2 days, and then TUNEL assays were performed. The percentage of TUNEL-positive cells induced by CDDP treatment was assessed by counting more than 400 cells per coverslip. □, U87MG; ▪, U87MG.ΔEGFR; ▨, U87MG.DK; ▧, U87MG.wtEGFR. Values represent means from three independent experiments, each performed in triplicate. ∗∗∗, Significantly different (P < 0.001) from the value for the U87MG.ΔEGFR cells treated with CDDP. (Bars = SE.)
Increased Expression of Bcl-XL and Inhibition of Caspase Activation Are Associated with Suppression of CDDP-Induced Apoptosis in U87MG.ΔEGFR Cells.
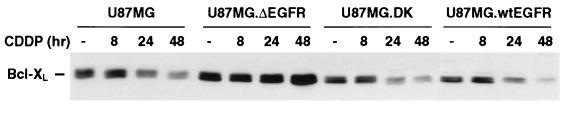
We next investigated the mechanism by which U87MG.ΔEGFR cells were more resistant than parental cells to CDDP-induced apoptosis. Because we have shown previously the up-regulation of expression of the anti-apoptotic Bcl-XL protein in U87MG.ΔEGFR cells and tumors (16), we determined whether its expression was affected by CDDP treatment. Expression levels of Bcl-XL in U87MG, U87MG.DK, and U87MG.wtEGFR cells were similar and were equally decreased by continuous exposure to CDDP for more than 24 hr, whereas that in U87MG.ΔEGFR cells was higher than other cells and remained unchanged during CDDP treatment. The Bcl-XL expression levels in these cells were inversely correlated with the proportion of cells undergoing apoptosis, consistent with the role of Bcl-XL as an inhibitor of cell death (Figs. 2 and 3). In contrast, the levels of Bcl-2 and Bax, two other members of the Bcl-2 family, showed similar basal expression in U87MG, U87MG.DK, and U87MG.ΔEGFR cells; this expression decreased in each cell type upon treatment with CDDP (data not shown).
Figure 3.
Cells expressing ΔEGFR have higher initial and sustained levels of Bcl-XL. Western blot analysis of Bcl-XL expression in U87MG, U87MG.ΔEGFR, U87MG.DK, and U87MG.wtEGFR cells was performed after CDDP treatment. Total cell lysates were prepared at the various time points indicated. For each sample, 20 μg of lysate protein was used. Results were reproduced in three independent experiments.
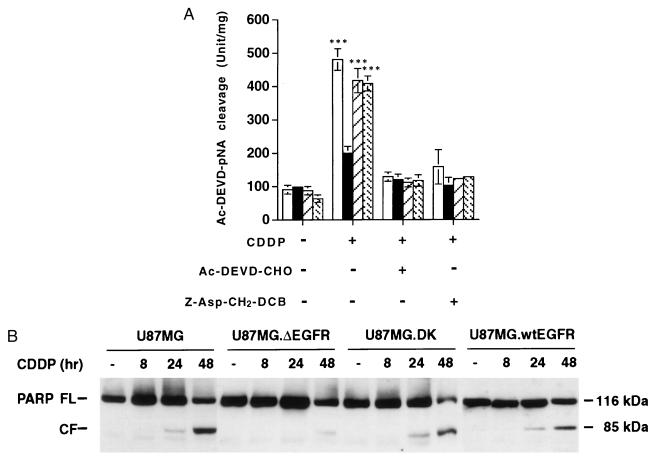
These results suggested that activation of caspases that are implicated in the execution phase of apoptosis and thought to be regulated by members of Bcl-2 family including Bcl-XL (18) may be altered in U87MG.ΔEGFR cells under CDDP treatment. To test this possibility, we measured caspase-3-like protease activity by using the tetrapeptide substrate, acetyl-Asp-Glu-Val-Asp (Ac-DEVD)-pNA (California Peptide Research, Napa, CA), because DEVD-specific caspase-3-like proteases play a crucial role as apoptosis executioners (18). Basal caspase-3-like protease activity in untreated cells was similar among all cell types (Fig. 4A), although basal Bcl-XL expression was higher in U87MG.ΔEGFR cells in the absence of an apoptotic stimulus. However, 48 hr after CDDP treatment, a substantial increase of caspase-3-like protease activity was detected in the lysates from U87MG parental cells (5.3-fold more than basal activity). In contrast, activation of the caspase activity in CDDP-treated U87MG.ΔEGFR cells was diminished substantially (only 2.0-fold higher than its basal activity), whereas the extent of caspase-3-like protease activation in U87MG.DK and U87MG.wtEGFR cells was comparable to that in parental cells (4.7- and 6.4-fold, respectively). The absolute caspase-3-like protease activity in U87MG.ΔEGFR cells after CDDP treatment was significantly lower (P < 0.001) than that in the other cells tested. The increased caspase activities were completely abolished by preincubating lysates with Ac-DEVD-aldehyde (CHO) (Bachem), a specific inhibitor of caspase-3-like protease, indicating the authenticity of the measured activity (Fig. 4A). In addition, when treated with CDDP in the presence of a plasma-membrane-permeable caspase-specific inhibitor, Z-Asp-CH2-DCB (Bachem), these cells did not exhibit an increase of caspase-3-like protease activity (Fig. 4A) and also displayed a significant inhibition of CDDP-induced apoptosis (data not shown). Consistent with this result, cleavage of the 116-kDa full-length PARP, a known cellular substrate of caspase-3-like proteases, into an inactive 85-kDa fragment was observed in U87MG, U87MG.DK, and U87MG.wtEGFR cells at 24 hr after CDDP treatment, and the degradation proceeded considerably by 48 hr, whereas in U87MG.ΔEGFR cells the cleaved product was present at only trace levels at 48 hr (Fig. 4B). These results suggested that in U87MG.ΔEGFR cells, suppression of caspase-3-like protease activation was involved in the resistance to CDDP-induced apoptosis and correlated with increased expression of Bcl-XL. Furthermore, overexpression and continuous stimulation of wt EGFR did not elicit significant effects on CDDP-induced apoptotic cell death, caspase activation, or Bcl-XL expression, further suggesting that these effects were a result of expression of the tumor-derived ΔEGFR.
Figure 4.
ΔEGFR expression causes reduced activation of caspase-3-like proteases in response to CDDP treatment. (A) Total cell lysates were prepared 2 days after treatment with 5 μg/ml of CDDP. Samples were assayed for protease activity by using the peptide substrate Ac-DEVD-pNA. For inhibition of the protease activity, 10 μM Ac-DEVD-CHO was added to the reaction mixture before the addition of substrate. Cells also were treated with the plasma membrane-soluble caspase inhibitor Z-Asp-CH2-DCB (200 μM) before and during CDDP treatment. □, U87MG; ▪, U87MG.ΔEGFR; ▨, U87MG.DK; ▧, U87MG.wtEGFR. Values represent the means of seven independent experiments (two for the Z-Asp-CH2-DCB experiment). ∗∗∗, Significantly different (P < 0.001) from the value for U87MG.ΔEGFR cells treated with CDDP. (Bars = SE.) (B) Proteolytic cleavage of PARP after CDDP treatment. For each sample, 20 μg total clarified protein lysate was loaded onto SDS/PAGE gels, electrophoresed, transferred to membranes, and probed with anti-PARP mAbs. FL, full-length; CF, cleaved fragment.
Overexpression of Bcl-XL in Parental U87MG Cells Recapitulates Suppression of both Caspase-3-Like Protease Activation and Apoptosis Induced by CDDP.
Having determined that Bcl-XL expression was associated with the CDDP-resistant phenotype of U87MG.ΔEGFR cells, we next tested whether Bcl-XL has a direct role in CDDP-induced apoptosis. Parental U87MG cells were transfected with either the Bcl-XL expression vector, pSFFVneo-bcl-XL, or its control vector, pSFFV-neo, and stable clones overexpressing various levels of Bcl-XL were established after G418 selection (Fig. 5A). After CDDP treatment, U87MG.Bcl-XL clones exhibited significantly lower caspase-3-like protease activities (Fig. 5B) and apoptosis indices (Fig. 5C) in an expression level-dependent manner, relative to those observed in U87MG cells (P < 0.001). The caspase activity and apoptotic index in clone-12, a clone having Bcl-XL overexpression at a level similar to that of U87MG.ΔEGFR cells, were also lower than in parental cells after CDDP treatment, but slightly higher than those of U87MG.ΔEGFR cells. Control vector transfectants (U87MG.SFFV) did not demonstrate inhibition of apoptosis or caspase-3-like protease activation.
Figure 5.
Overexpression of Bcl-XL inhibits the caspase-3-like protease activation and induction of apoptosis caused by CDDP treatment. (A) Western blot analysis showing expression levels of Bcl-XL in U87MG Bcl-XL-overexpressing clones (Bcl-XL-6, -9, -13, -11, -12, and -8) and empty vector-transfected clones (SFFV-2 and -5). U87MG and U87MG.ΔEGFR cells were treated with 5 μg/ml of CDDP for 2 days. For each sample, 20 μg total cell lysate was used. (B) Caspase-3-like protease activity of the Bcl-XL-overexpressing clones 2 days after treatment with 5 μg/ml CDDP measured as described in Fig. 4A. Values are means of two to six independent experiments. (Bars = SE.) (C) Apoptosis rate of Bcl-XL-overexpressing clones 2 days after treatment with 5 μg/ml of CDDP determined by TUNEL assay. Values represent means from two to three independent experiments in triplicate. (Bars = SE.)
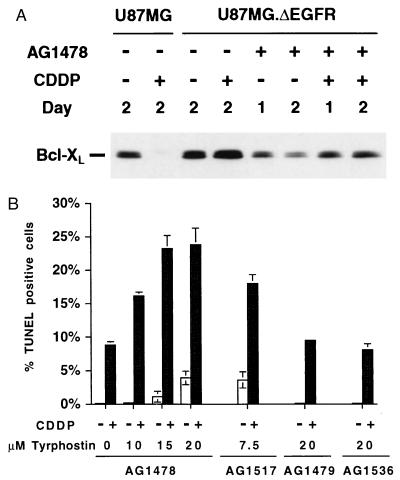
The ΔEGFR-Selective Tyrosine Kinase Inhibitor, AG1478, Modulates CDDP-Induced Apoptosis in U87MG.ΔEGFR Cells.
To confirm that ΔEGFR confers CDDP resistance through its signal transduction, we utilized the tyrosine kinase inhibitor, AG1478, which has been shown to have a more than 10-fold greater specificity for inhibition of ΔEGFR relative to wt EGFR (24). Treatment of U87MG.ΔEGFR cells with 15 μM AG1478 resulted in marked reduction of Bcl-XL expression (Fig. 6A). Their apoptosis index increased slightly after a single continuous treatment with 15–20 μM AG1478 for 2 days compared with that by the vehicle control, dimethyl sulfoxide. Thus, AG1478 is unlikely to be a direct apoptotic stimulus. Combination treatment of U87MG.ΔEGFR with the kinase inhibitor, AG1478, and the apoptotic inducer, CDDP, caused significant apoptosis in a dose-dependent and synergistic manner (P < 0.001 compared with CDDP alone) (Fig. 6B). Similar results were obtained when the more potent ΔEGFR-selective tyrphostin, AG1517, was used at the lower concentration (7.5 μM). In contrast, the nonspecific and less potent tyrphostins, AG1479 and AG1536, had no effect on apoptosis induction in these cells (Fig. 6B), indicating that the observed synergistic effects likely were specific to the kinase activity of ΔEGFR. CDDP-induced caspase activation in U87MG.ΔEGFR cells also was enhanced by cotreatment with AG1478 or AG1517, and the colony-forming efficiency of U87MG.ΔEGFR cells treated with the CDDP/AG1478 combination was significantly less than when treated with CDDP alone (data not shown). These results suggest that constitutively active ΔEGFR signaling is responsible for Bcl-XL up-regulation and inhibition of drug-induced apoptosis.
Figure 6.
Modulation of CDDP-induced apoptosis in U87MG.ΔEGFR cells by various tyrphostin-type tyrosine kinase inhibitors. (A) Reduced Bcl-XL expression upon exposure to tyrphostin AG1478. U87MG.ΔEGFR cells were treated with the ΔEGFR-selective tyrphostin AG1478 (15 μM) with or without CDDP (5 μg/ml), and total cell lysates were prepared. For each sample, 20 μg lysate was used. (B) Increased apoptosis induced by combination treatment by using ΔEGFR-selective tyrphostins (AG1478 or AG1517) and CDDP. U87MG.ΔEGFR cells were treated with or without 5 μg/ml CDDP for 2 days in the presence of the ΔEGFR-selective tyrphostins (AG1478 or AG1517) or the nonspecific, less potent tyrphostins (AG1479 or AG1536) at the concentrations indicated. For control, the vehicle (dimethyl sulfoxide) was used. Apoptosis rate was determined by TUNEL assays from triplicate coverslips. Experiments were repeated independently two to three times, with similar results.
DISCUSSION
Resistance to chemotherapy or radiotherapy is a major obstacle for the treatment of malignant gliomas that are surgically incurable because of their diffusely infiltrative nature. In addition to the conventional drug-resistance mechanisms related to pharmacodynamics, suppression of apoptosis is another means of acquiring drug resistance and could be mediated by specific genetic alterations in tumor cells, such as inactivating mutations of p53 and/or overexpression of Bcl-2 (3–5). The EGFR gene is the most frequently amplified and mutated oncogene observed in highly malignant gliomas, especially of the more common de novo type, which does not usually have p53 mutations, and here we show that overexpression of a tumor-derived mutant form of EGFR, ΔEGFR, in wt p53-expressing human U87MG glioma cells results in resistance to the chemotherapeutic drug, CDDP. Drug resistance was associated with a significant reduction in the rate of apoptosis, which could be attributed at least partially to ΔEGFR-induced, elevated Bcl-XL expression and reduced caspase activity. Elevated levels of Bcl-XL, known to inhibit apoptosis, were observed in U87MG.ΔEGFR cells before treatment but were even more manifest after CDDP treatment. Additionally, caspase activation, a core component of the apoptosis execution machinery, was inhibited post-CDDP treatment in U87MG.ΔEGFR cells, in agreement with the recent reports that Bcl-XL regulates caspase activation (18, 25). These observations were highly specific to cells expressing constitutively active ΔEGFR, because overexpression of the kinase-defective form of ΔEGFR, DK, or wt EGFR had no such effects, and inhibition of ΔEGFR kinase activity by the tyrphostin AG1478, a tyrosine kinase inhibitor with selectivity for ΔEGFR, significantly reversed these properties. Moreover, when ΔEGFR was overexpressed in U373MG cells (which are mutant for p53), no CDDP-resistance was observed.
It has been proposed that glioblastomas could be subdivided into subsets that are characterized by distinct molecular abnormalities, particularly the status of p53 mutations and EGFR alterations (8). Because some functions of wt p53 involve induction of apoptosis, and human cancer cells deficient for p53 have been shown to reacquire sensitivity to CDDP by the transfer of wt p53 gene into p53−/− cells (26), it is likely that the subset of glioblastomas with p53 mutations or inactivation of p53 by MDM2 may fail to respond adequately to cytotoxic drugs that induce apoptosis (27). The present study of U87MG cells that have a wt p53 background raises the possibility that de novo glioblastomas with wt p53 but with EGFR alterations also could develop drug resistance. In this regard, it will be intriguing to investigate clinical brain tumors in terms of the relationship between their spectra of genetic alterations and their responsiveness to chemotherapy. It will also be worth determining whether populations of glioma cells expressing ΔEGFR increase in occurrence in recurrent tumors after chemotherapy.
Previous studies have shown that the molecular and biological effects of ΔEGFR on glioma tumorigenesis differ from those of wt EGFR (15, 16). Unlike ΔEGFR, overexpression of wt EGFR was unable to confer significant growth advantages to glioma cells both in vitro and in vivo and also did not contribute to CDDP resistance. Several lines of evidence have suggested that enhanced expression of wt EGFR may be associated with CDDP resistance in other tumor types (28, 29). For example, suppression of EGFR activity by a dominant negative EGFR construct enhanced the cytotoxic effect of CDDP in pancreatic cancer cells (29). In contrast, activation of EGFR by EGF has been shown to enhance sensitivity of a variety of cancer cells to CDDP (30). Dixit et al. (31) also showed that down-regulation of EGFR expression by antisense RNA resulted in CDDP resistance of breast cancer cells expressing high levels of wt EGFR, but not in those expressing low levels. The observation that intensive stimulation of overexpressed wt EGFR in A431 cells induced apoptosis, whereas lower levels of receptor phosphorylation stimulated proliferation (32), however, implies that the activation level of overexpressed wt EGFR may be critical for its biological consequence. Because the extent of autophosphorylation per molecule of ΔEGFR was only 10% of that of wt EGFR fully activated by EGF (15), the low level of autophosphorylation of ΔEGFR and its constitutive signaling may play an important role in conferring CDDP resistance. Alternatively, ΔEGFR could transduce signals through pathways different from those of wt EGFR, because transcriptional up-regulation of Bcl-XL was observed only in cells overexpressing ΔEGFR, but not in those overexpressing wt EGFR or DK (16). It is also possible that ΔEGFR may preferentially phosphorylate tyrosine residues of molecules involved in apoptosis regulation. In this regard, we have demonstrated that ΔEGFR phosphorylated and is associated with the adaptor protein Shc (33), thus leaving open the possibility that Shc Y239 and Y240, which have been shown to mediate antiapoptotic signals (34), are potential substrates of ΔEGFR.
Here we showed that combination treatment of U87MG.ΔEGFR cells with the mutant EGFR-specific tyrosine kinase inhibitor AG1478 and CDDP synergistically induced apoptosis. Because tumor-derived ΔEGFR conferred resistance of tumor cells to drug-induced apoptosis, which was specific to ΔEGFR but not to wt EGFR, it is reasonable to consider ΔEGFR as a therapeutic target to enhance the efficacy of drug treatment. It would be of benefit to render ΔEGFR-positive tumor cells susceptible to treatment through specific targeting and combination chemotherapy. Monoclonal antibodies raised against ΔEGFR-specific epitopes have been produced for potential use in imaging and for immunotoxin delivery (35). Tyrphostin AG1478 also has been shown to preferentially inhibit constitutive autophosphorylation of ΔEGFR and growth of tumor cells expressing ΔEGFR (24). We showed that treatment of U87MG.ΔEGFR cells with AG1478 alone resulted in the reduction of resistance-related Bcl-XL expression to basal levels, but was not enough to induce apoptosis. However, tyrphostin treatment in combination with CDDP was able to induce apoptosis synergistically and more effectively in U87MG.ΔEGFR cells than was CDDP treatment alone. These results provide a fundamental basis for the development of combination treatments of the intractable malignant gliomas, especially those such as the de novo type, which express ΔEGFR. Similar approaches using other inhibitors that are not specific to ΔEGFR in combination with chemotherapeutic agents may be useful in treatment of gliomas without this specific genetic rearrangement. This contention is supported by studies that have shown that HER-2/erbB-2-expressing lung cancer cells have synergistic responses to the erbB-2-specific tyrphostin, AG825, and the chemotherapeutic agents CDDP and etoposide (36).
Acknowledgments
The authors thank Drs. S. J. Korsmeyer for the Bcl-XL construct, C. Kitanaka for helpful technical advice, and J. F. Costello and K. Knudson for critical reading of the manuscript. M.N. was supported in part by the Brain Foundation, Tokyo.
ABBREVIATIONS
- EGFR
epidermal growth factor receptor
- wt
wild type
- CDDP
cis-diamminedichloroplatinum(II)
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- PARP
poly(ADP-ribose) polymerase
References
- 1.Fine H A. J Neurooncol. 1994;20:111–120. doi: 10.1007/BF01052722. [DOI] [PubMed] [Google Scholar]
- 2.Feun L G, Savaraj N, Landy H J. J Neurooncol. 1994;20:165–176. doi: 10.1007/BF01052726. [DOI] [PubMed] [Google Scholar]
- 3.Guchelaar H J, Vermes A, Vermes I, Haanen C. Pharm World Sci. 1997;19:119–125. doi: 10.1023/a:1008654316572. [DOI] [PubMed] [Google Scholar]
- 4.Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D E, Housman D E, Jacks T. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen R, Wilander E, Oberg K. Br J Cancer. 1995;72:1324–1329. doi: 10.1038/bjc.1995.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagane M, Huang H-J S, Cavenee W K. Curr Opin Oncol. 1997;9:215–222. doi: 10.1097/00001622-199709030-00001. [DOI] [PubMed] [Google Scholar]
- 7.Furnari F B, Lin H, Huang H-J S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Brain Pathol. 1996;6:217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 9.Libermann T A, Nusbaum H R, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield M D, Ullrich A, Schlessinger J. Nature (London) 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 10.Schlegel J, Merdes A, Stumm G, Albert F K, Forsting M, Hynes N, Kiessling M. Int J Cancer. 1994;56:72–77. doi: 10.1002/ijc.2910560114. [DOI] [PubMed] [Google Scholar]
- 11.Ekstrand A J, Sugawa N, James C D, Collins V P. Proc Natl Acad Sci USA. 1992;9:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscatello D K, Holgado M M, Godwin A K, Ramirez G, Gunn G, Zoltick P W, Biegel J A, Hayes R L, Wong A J. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 13.Garcia de Palazzo I E, Adams G P, Sundareshan P, Wong A J, Testa J R, Bigner D D, Weiner L M. Cancer Res. 1993;53:3217–3220. [PubMed] [Google Scholar]
- 14.Nishikawa R, Ji X D, Harmon R C, Lazar C S, Gill G N, Cavenee W K, Huang H-J S. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H-J S, Nagane M, Klingbeil C K, Lin H, Nishikawa R, Ji X-D, Huang C-M, Gill G N, Wiley H S, Cavenee W K. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 16.Nagane M, Coufal F, Lin H, Bögler O, Cavenee W K, Huang H-J S. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 17.Minn A J, Rudin C M, Boise L H, Thompson C B. Blood. 1995;86:1903–1910. [PubMed] [Google Scholar]
- 18.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 19.Nagane M, Asai A, Shibui S, Nomura K, Kuchino Y. Neurosurgery. 1997;41:434–441. doi: 10.1097/00006123-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Datta R, Banach D, Kojima H, Talanian R V, Alnemri E S, Wong W W, Kufe D W. Blood. 1996;88:1936–1943. [PubMed] [Google Scholar]
- 21.Kondo S, Barna B P, Morimura T, Takeuchi J, Yuan J, Akbasak A, Barnett G H. Cancer Res. 1995;55:6166–6171. [PubMed] [Google Scholar]
- 22.Boiardi A, Silvani A, Milanesi I, Botturi M, Broggi G. J Neurooncol. 1991;11:165–170. doi: 10.1007/BF02390176. [DOI] [PubMed] [Google Scholar]
- 23.Levin V A, Wilson C B. Semin Oncol. 1975;2:63–67. [PubMed] [Google Scholar]
- 24.Han Y, Caday C G, Nanda A, Cavenee W K, Huang H J. Cancer Res. 1996;56:3859–3861. [PubMed] [Google Scholar]
- 25.Clem R J, Cheng E H-Y, Karp C L, Kirsch D G, Ueno K, Takahashi A, Kastan M B, Griffin D E, Earnshaw W C, Veliuona M A, Hardwick J M. Proc Natl Acad Sci USA. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara T, Grimm E A, Mukhopadhyay T, Zhang W W, Owen S L, Roth J A. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 27.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 28.Aboud P E, Hurwitz E, Pirak M E, Bellot F, Schlessinger J, Sela M. J Natl Cancer Inst. 1988;80:1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, Cao T, Lopez M E, Hope C, van Nostrand K, Kobrin M S, Fan H U, Büchler M W, Korc M. Int J Cancer. 1996;68:782–787. doi: 10.1002/(SICI)1097-0215(19961211)68:6<782::AID-IJC16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Kröning R, Jones J A, Hom D K, Chuang C C, Sanga R, Los G, Howell S B, Christen R D. Br J Cancer. 1995;72:615–619. doi: 10.1038/bjc.1995.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixit M, Yang J L, Poirier M C, Price J O, Andrews P A, Arteaga C L. J Natl Cancer Inst. 1997;89:365–373. doi: 10.1093/jnci/89.5.365. [DOI] [PubMed] [Google Scholar]
- 32.Gulli L F, Palmer K C, Chen Y Q, Reddy K B. Cell Growth Differ. 1996;7:173–178. [PubMed] [Google Scholar]
- 33.Prigent S A, Nagane M, Lin H, Huvar I, Boss G R, Feramisco J R, Cavenee W K, Huang H-J S. J Biol Chem. 1996;271:25639–25645. doi: 10.1074/jbc.271.41.25639. [DOI] [PubMed] [Google Scholar]
- 34.Gotoh N, Tojo A, Shibuya M. EMBO J. 1996;15:6197–6204. [PMC free article] [PubMed] [Google Scholar]
- 35.Hills D, Rowlinson B G, Gullick W J. Int J Cancer. 1995;63:537–543. doi: 10.1002/ijc.2910630414. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C M, Levitzki A, Wu L H, Chang K T, Cheng C C, Gazit A, Perng R P. Cancer Res. 1996;56:1068–1074. [PubMed] [Google Scholar]