Abstract
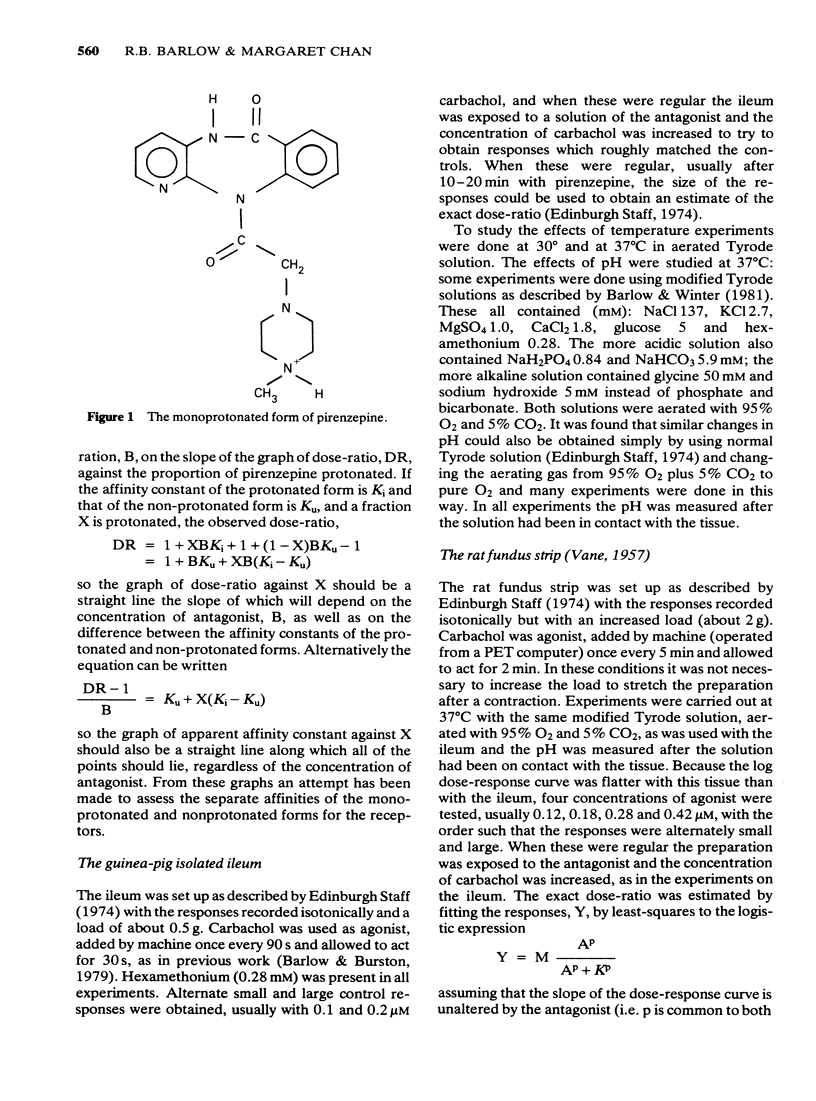
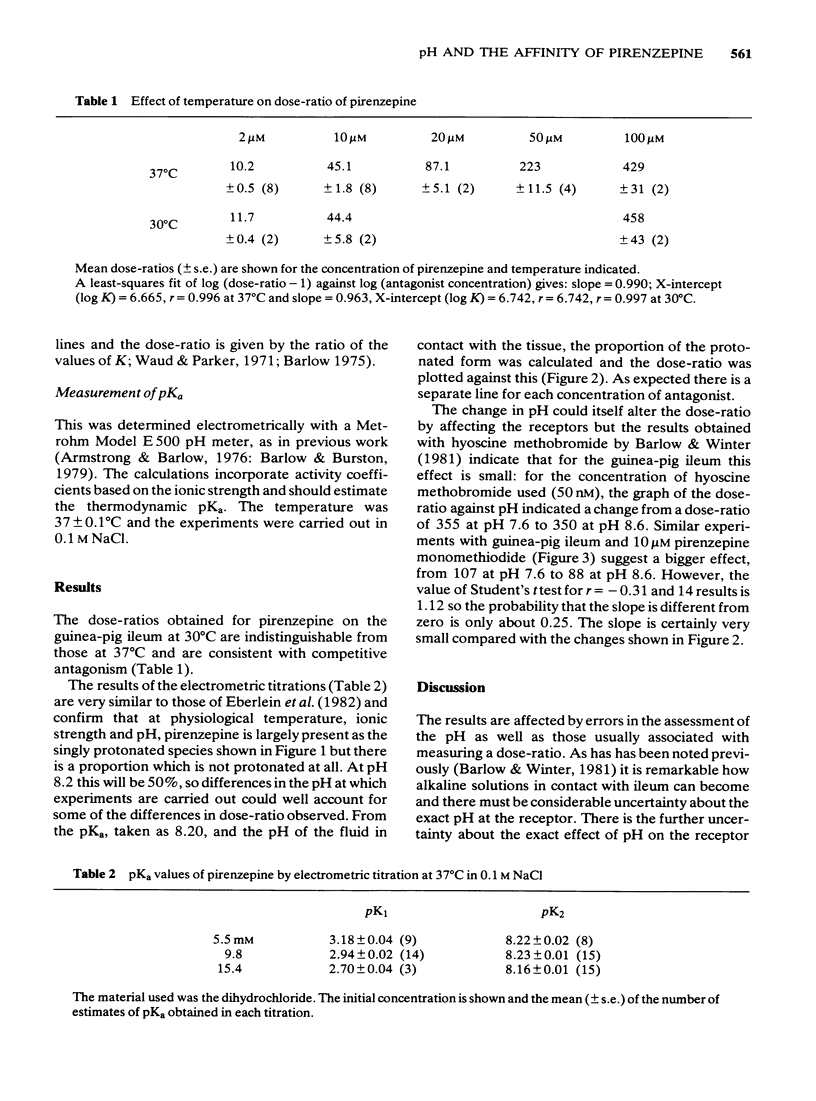
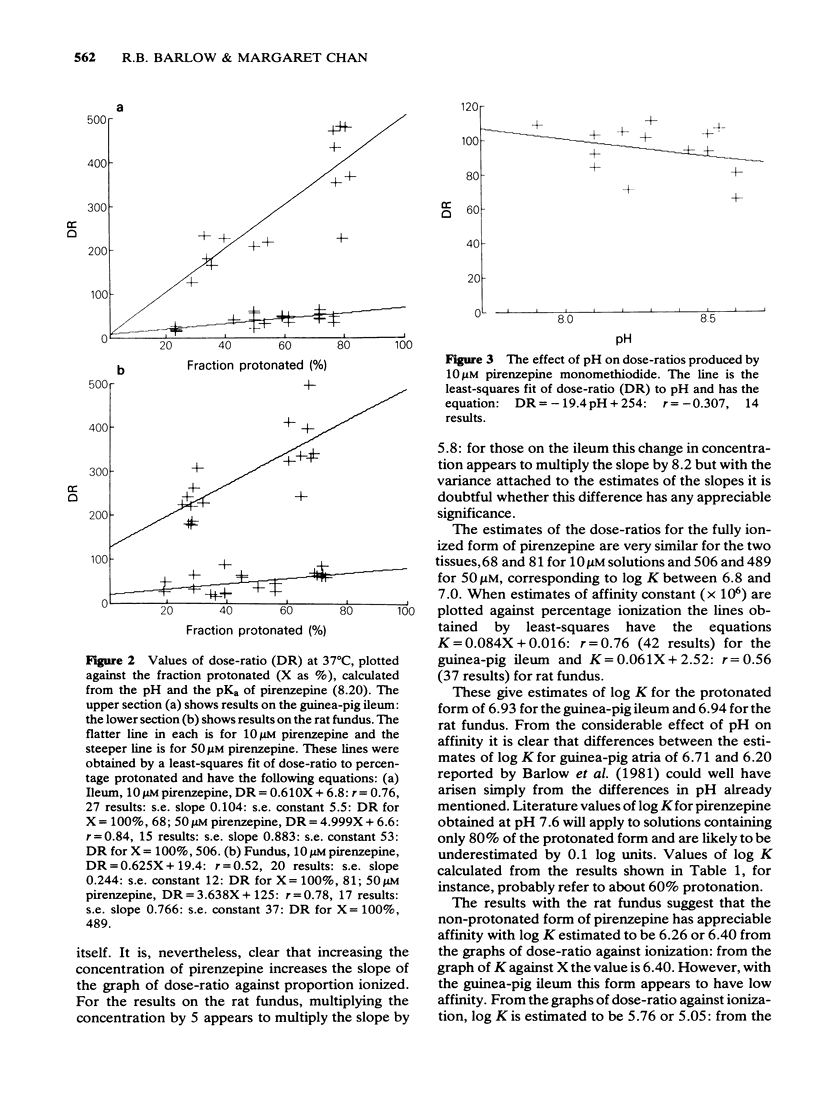
1 Dose-ratios obtained with pirenzepine on the guinea-pig ileum at 30 degrees C are indistinguishable from those obtained at 37 degrees C. 2. In 0.1 M NaCl at 37 degrees C the pKa of pirenzepine for the loss of its last ionizable proton is 8.2. The ionization of pirenzepine is therefore markedly affected by changes in pH in the physiological range. 3 In experiments with pirenzepine on guinea-pig ileum and rat fundus made over a range of pH, the dose-ratio increases with the proportion of the protonated form present. As expected, the slope of the graph of dose-ratio against proportion protonated depends on the concentration of antagonist. The changes in pH produce only small effects on dose-ratios obtained with pirenzepine monomethiodide. These effects of pH can account for some of the differences between estimates of the affinity of pirenzepine. 4 The logarithm of the affinity constant of the protonated form of pirenzepine for the receptors in guinea-pig ileum is estimated to be 6.93, compared with 6.94 for the receptors in rat fundus. However, for the non-protonated form the values appear to be below 5 for the ileum compared with about 6.4 for the rat fundus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Barlow R. B. The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants. Br J Pharmacol. 1976 Aug;57(4):501–516. doi: 10.1111/j.1476-5381.1976.tb10377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Burston K. N. Temperature coefficients of affinity and entropies of adsorption from enantiomeric pairs of compounds acting at muscarinic receptors in the guinea-pig ileum. Br J Pharmacol. 1979 Aug;66(4):581–585. doi: 10.1111/j.1476-5381.1979.tb13697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B. Use of the logistic function for the calculation of dose-ratios and potency ratios. Br J Pharmacol. 1975 Jan;53(1):139–141. doi: 10.1111/j.1476-5381.1975.tb07341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Winter E. A. Affinities of the protonated and non-protonated forms of hyoscine and hyoscine N-oxide for muscarinic receptors of the guinea-pig ileum and a comparison of their size in solution with that of atropine. Br J Pharmacol. 1981 Apr;72(4):657–664. doi: 10.1111/j.1476-5381.1981.tb09146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud D. R., Parker R. B. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. II. Competitive antagonists. J Pharmacol Exp Ther. 1971 Apr;177(1):13–24. [PubMed] [Google Scholar]


