Abstract
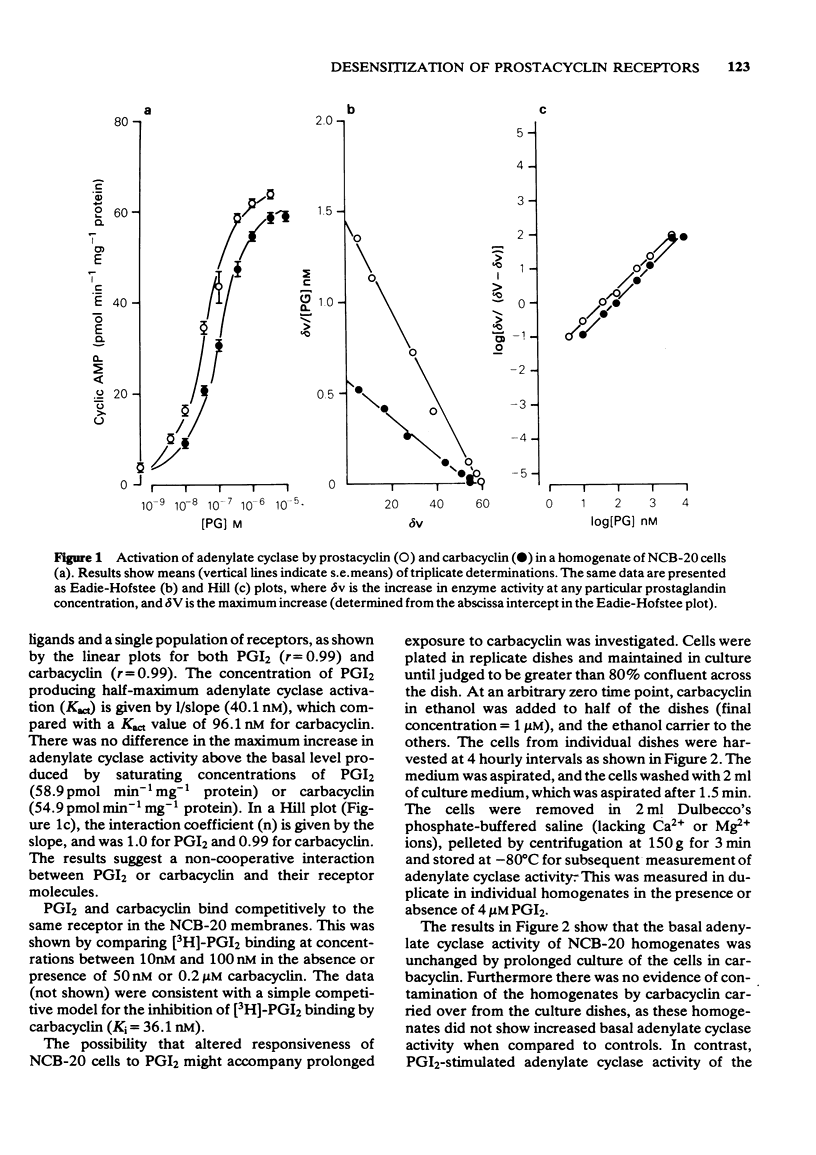
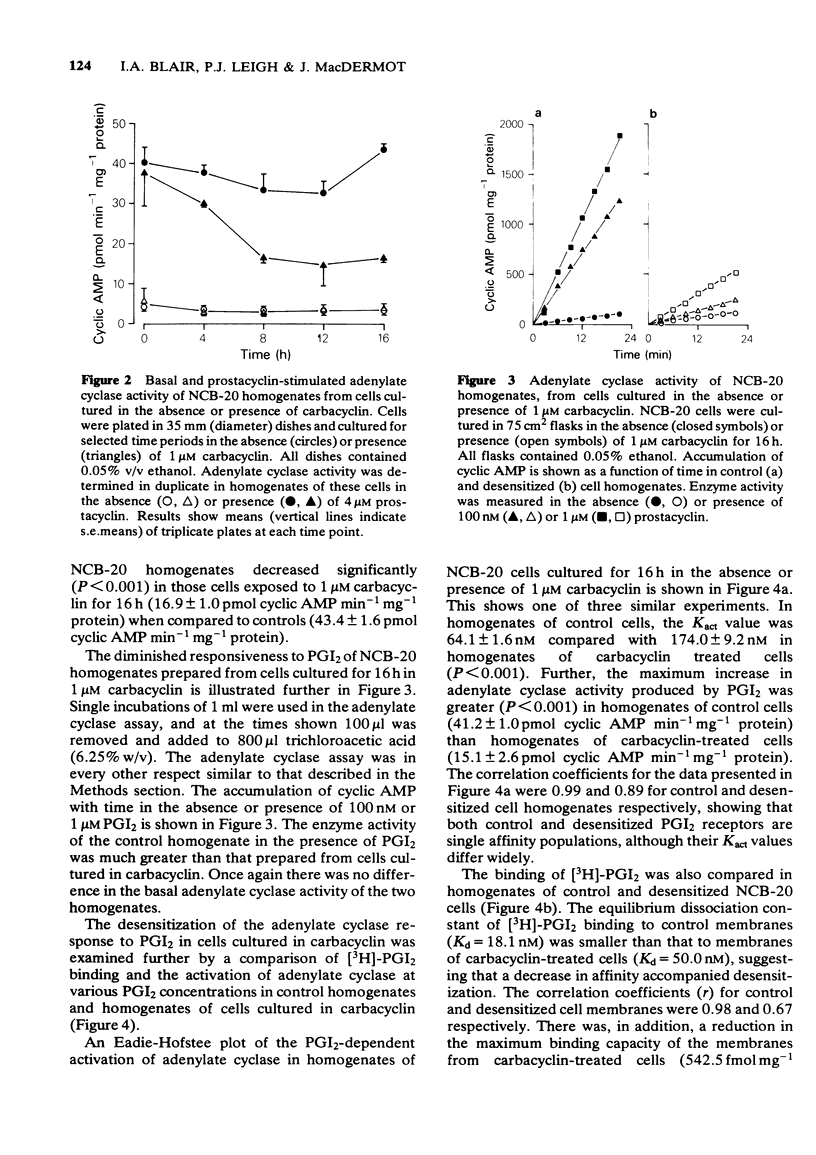
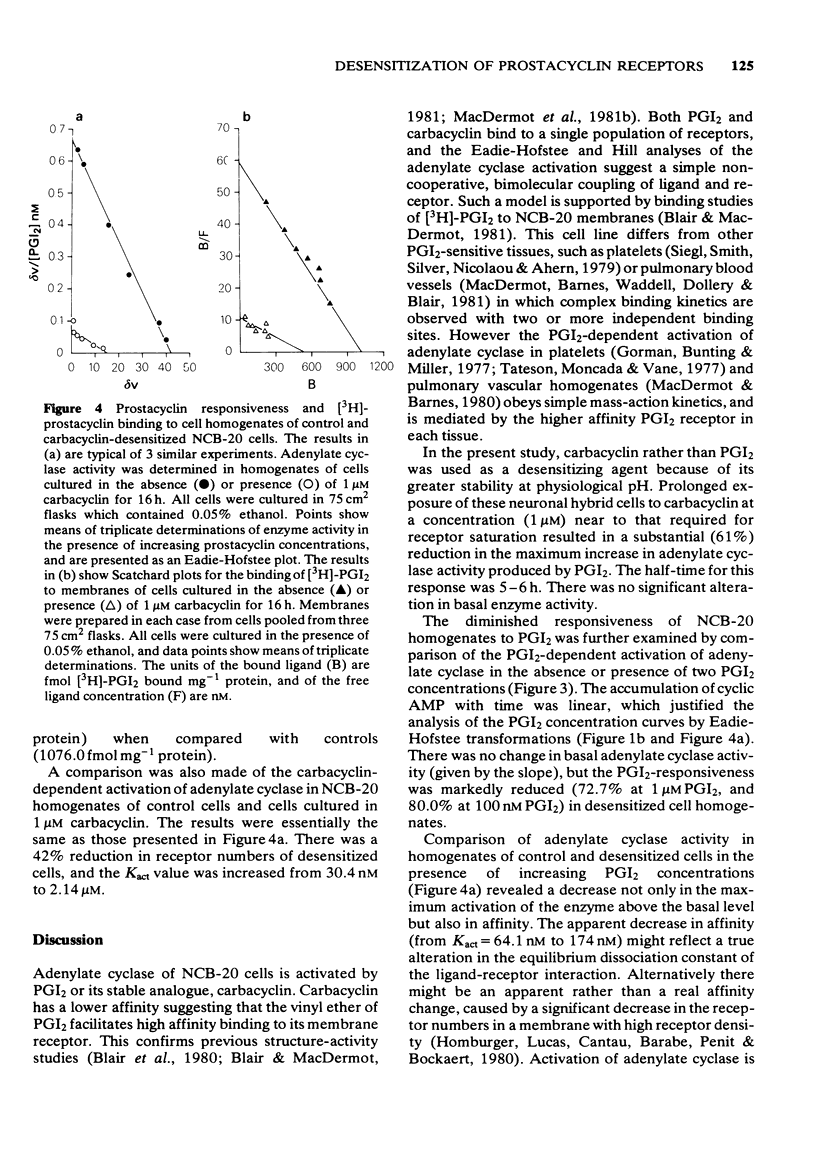
1 Prostacyclin and its stable analogue, carbacyclin, bind competitively to a single population of receptors, and activate adenylate cyclase of the NCB-20 neuronal somatic cell hybrid (Kact = 40.1 nM and 96.1 nM respectively). 2 Culture of NCB-20 cells in the presence of 1 microM carbacyclin for 4 to 16 h results in a progressive decrease in the prostacyclin-dependent activation of adenylate cyclase in cell homogenates with an increase at 16 h of the Kact from 64.1 nM to 174.0 nM and decrease in the maximum adenylate cyclase activation from 41.2 to 15.1 pmol cyclic AMP min-1 mg-1 protein. 3 The prediction that the apparent decrease in affinity in the prostacyclin-dependent activation of adenylate cyclase was secondary to a reduction in receptor numbers was tested directly by measuring binding of [3H]-prostacyclin to membranes of cells exposed to carbacyclin for 16 h. This showed an actual decrease in affinity of the prostacyclin-receptor interaction, as well as a decrease in the total receptor numbers. Thus prolonged exposure of NCB-20 cells to carbacyclin caused reductions in both receptor numbers and affinity, reflected by measurements both of binding and adenylate cyclase activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair I. A., Hensby C. N., MacDermot J. Prostacyclin-dependent activation of adenylate cyclase in a neuronal somatic cell hybrid: prostanoid structure-activity relationships. Br J Pharmacol. 1980 Jul;69(3):519–525. doi: 10.1111/j.1476-5381.1980.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair I. A., MacDermot J. The binding of [3H]-prostacyclin to membranes of a neuronal somatic hybrid. Br J Pharmacol. 1981 Mar;72(3):435–441. doi: 10.1111/j.1476-5381.1981.tb10994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B., Schafer A. I., Puchalsky D., Handin R. I. Desensitization of prostaglandin-activated platelet adenylate cyclase. Prostaglandins. 1979 Apr;17(4):561–571. doi: 10.1016/0090-6980(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Homburger V., Lucas M., Cantau B., Barabe J., Penit J., Bockaert J. Further evidence that desensitization of beta-adrenergic-sensitive adenylate cyclase proceeds in two steps. Modification of the coupling and loss of beta-adrenergic receptors. J Biol Chem. 1980 Nov 10;255(21):10436–10444. [PubMed] [Google Scholar]
- Kenimer J. G., Nirenberg M. Desensitization of adenylate cyclase to prostaglandin E1 or 2-chloroadenosine. Mol Pharmacol. 1981 Nov;20(3):585–591. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Wood C. L., Gore T. B., Mukherjee C. Regulation of prostaglandin receptors by prostaglandins and guanine nucleotides in frog erythrocytes. J Biol Chem. 1977 Aug 10;252(15):5295–5303. [PubMed] [Google Scholar]
- MacDermot J., Barnes P. J. Activation of guinea pig pulmonary adenylate cyclase by prostacyclin. Eur J Pharmacol. 1980 Oct 31;67(4):419–425. doi: 10.1016/0014-2999(80)90183-1. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Barnes P. J., Waddell K. A., Dollery C. T., Blair I. A. Prostacyclin binding to guinea pig pulmonary receptors. Eur J Pharmacol. 1981 Oct 22;75(2-3):127–130. doi: 10.1016/0014-2999(81)90071-6. [DOI] [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Newcombe D. S., Ciosek C. P., Jr, Ishikawa Y., Fahey J. V. Human synoviocytes: activation and desensitization by prostaglandins and 1-epinephrine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3124–3128. doi: 10.1073/pnas.72.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. Properties of opiate-receptor binding in rat brain. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2243–2247. doi: 10.1073/pnas.70.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzinger H., Silberbauer K., Horsch A. K., Gall A. Decreased sensitivity of human platelets to PGI2 during long-term intraarterial prostacyclin infusion in patients with peripheral vascular disease--a rebound phenomenon? Prostaglandins. 1981 Jan;21(1):49–51. doi: 10.1016/0090-6980(81)90195-7. [DOI] [PubMed] [Google Scholar]
- Su Y. F., Cubeddu L., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):257–270. [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Moncada S., Whiting F., Vane J. R. Carbacyclin--a potent stable prostacyclin analogue for the inhibition of platelet aggregation. Prostaglandins. 1980 Apr;19(4):605–627. doi: 10.1016/s0090-6980(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Steel G., Boughton-Smith N. K. Gastrointestinal actions of carbacyclin, a stable mimic of prostacyclin. J Pharm Pharmacol. 1980 Aug;32(8):603–604. doi: 10.1111/j.2042-7158.1980.tb13013.x. [DOI] [PubMed] [Google Scholar]


