Abstract
1 Simultaneous recordings of electrical and mechanical activity have been made from guinea-pig isolated trachealis muscle. Electrical activity was recorded both by extracellular and intracellular techniques.
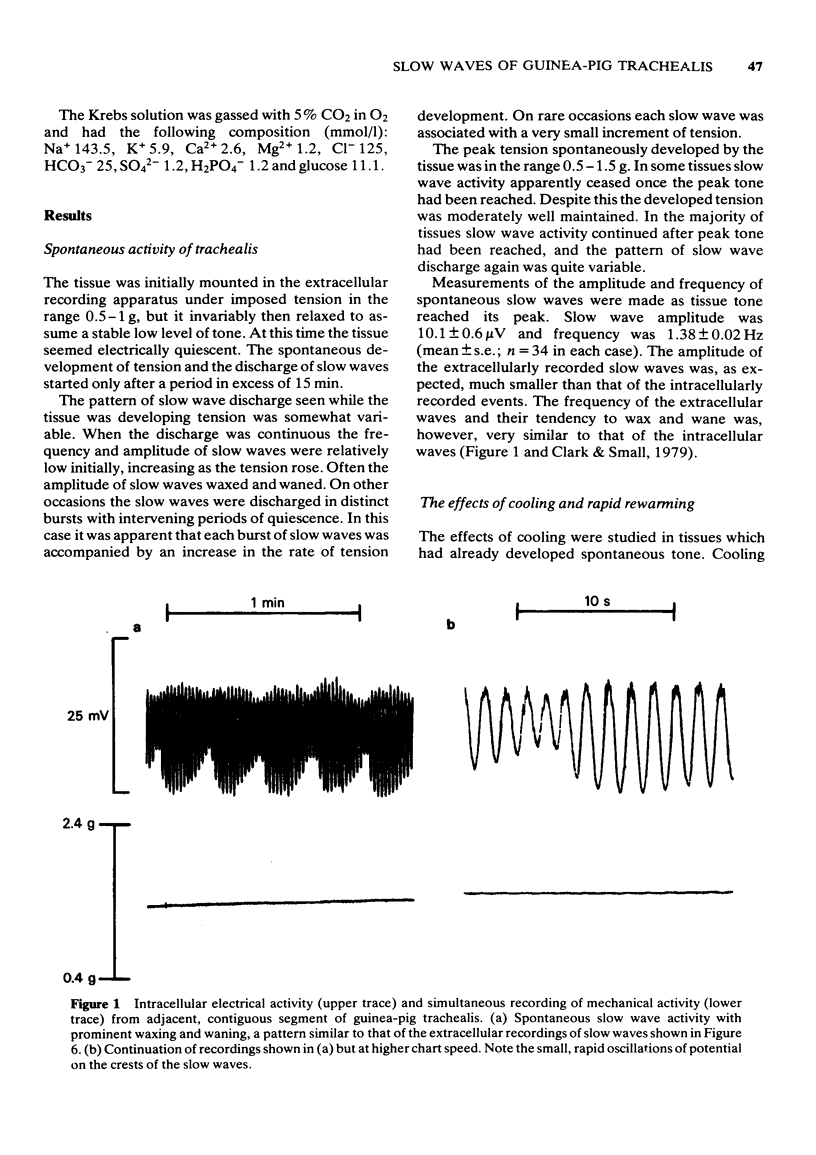
2 Extracellular studies showed that the spontaneous development of tone was accompanied by electrical slow waves which frequently exhibited pronounced waxing and waning. Intracellular recording confirmed the discharge of these slow waves in individual cells. Extracellularly-recorded slow waves were often of greatest amplitude while the tissue was developing rather than maintaining tension. Some tissues became electrically quiescent on reaching peak tone.
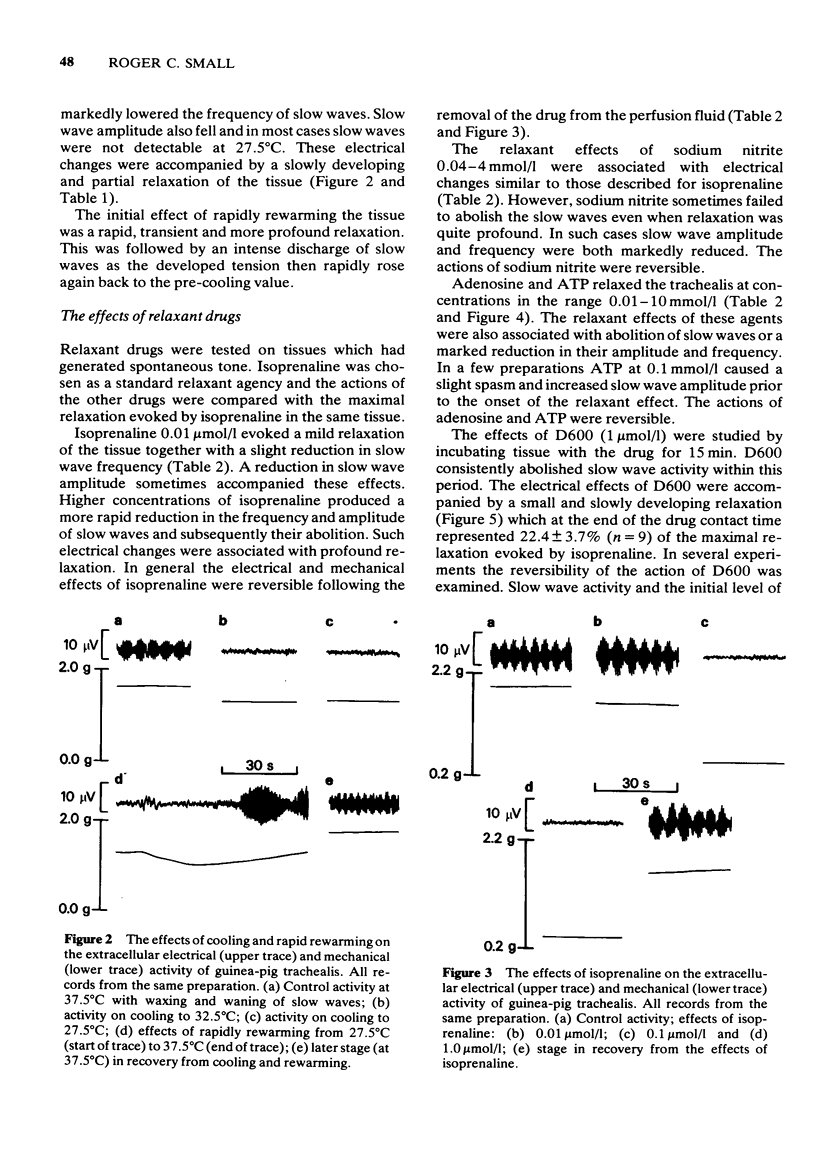
3 Cooling to 27.5°C caused some relaxation. Slow wave amplitude and frequency fell, slow waves eventually being abolished. Subsequent rapid rewarming initially evoked a more profound relaxation. An intense discharge of slow waves then occurred as the tension rapidly rose again towards the pre-cooling value.
4 Sodium nitrite, (-)-isoprenaline, adenosine and adenosine triphosphate (ATP) each evoked relaxation and reduced the frequency and amplitude of slow waves. High concentrations of these agents often abolished slow waves. The actions of these drugs were reversible.
5 Treatment with methoxyverapamil (D600) 1 μmol/l for 15 min abolished slow wave activity but only evoked partial relaxation of the tissue.
6 Acetylcholine, histamine and tetraethylammonium (TEA) each evoked contraction, but TEA was unique in consistently promoting slow waves and (in high concentration) spike activity. Spasm evoked by acetylcholine and histamine did not usually involve the initiation or promotion of slow waves. Indeed in appropriate concentration these two agents always suppressed slow wave activity. The actions of the spasmogens were reversible.
7 It is concluded that the smooth muscle cells of the trachealis are electrically coupled. While co-ordinated slow wave activity is associated with the spontaneous development of tension in trachealis, it may not be necessary for the maintenance of the major part of the spontaneous tension exhibited by the tissue or for the spasm evoked by histamine or acetylcholine. Slow wave promotion by TEA suggests that the tissue may have a high resting potassium conductance which normally attenuates the slow waves. Slow waves may be suppressed by a variety of drugs acting by different mechanisms. Since D600 suppresses slow waves of the trachealis the mechanisms underlying the waves may be similar to those underlying spike activity in other smooth muscles.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron A. R., Kirkpatrick C. T. A study of excitatory neuromuscular transmission in the bovine trachea. J Physiol. 1977 Sep;270(3):733–745. doi: 10.1113/jphysiol.1977.sp011979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Yamaguchi T. Membrane potential-dependent and-independent tension in the canine tracheal muscle. J Pharmacol Exp Ther. 1977 May;201(2):276–284. [PubMed] [Google Scholar]
- Golenhofen K., Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (Verapamil). Pflugers Arch. 1972;331(3):233–243. [PubMed] [Google Scholar]
- Golenhoffen K., von Loh D. Elektrophysiologische Untersuchungen zur normalen Spontanaktivität der isolierten Taenia coli des Meerschweinchens. Pflugers Arch. 1970;314(4):312–328. doi: 10.1007/BF00592289. [DOI] [PubMed] [Google Scholar]
- Hoyes A. D., Barber P. Innervation of the trachealis muscle in the guinea-pig: a quantitative ultrastructural study. J Anat. 1980 Jun;130(Pt 4):789–800. [PMC free article] [PubMed] [Google Scholar]
- Jetley M., Weston A. H. Some effects of sodium nitroprusside, methoxyverapamil (D600) and nifedipine on rat portal vein. Br J Pharmacol. 1980 Feb;68(2):311–319. doi: 10.1111/j.1476-5381.1980.tb10420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Kannan M. S., Daniel E. E. Ultrastructural study of guinea pig tracheal smooth muscle and its innervation. Can J Physiol Pharmacol. 1980 Aug;58(8):974–983. doi: 10.1139/y80-148. [DOI] [PubMed] [Google Scholar]
- Kannan M. S., Daniel E. E. Formation of gap junctions by treatment in vitro with potassium conductance blockers. J Cell Biol. 1978 Aug;78(2):338–348. doi: 10.1083/jcb.78.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger E. A., Stephens N. L. Effect of tetraethylammonium on tonic airway smooth muscle: initiation of phasic electrical activity. Am J Physiol. 1975 Feb;228(2):633–636. doi: 10.1152/ajplegacy.1975.228.2.633. [DOI] [PubMed] [Google Scholar]
- Small R. C., Weston A. H. Simultaneous long-term recording of the mechanical and intracellular electrical activity of smooth muscles. J Pharmacol Methods. 1980 Jan;3(1):33–38. doi: 10.1016/0160-5402(80)90062-5. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E. Effect of hypoxia on airway smooth muscle mechanics and electrophysiology. J Appl Physiol. 1970 May;28(5):630–635. doi: 10.1152/jappl.1970.28.5.630. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]


