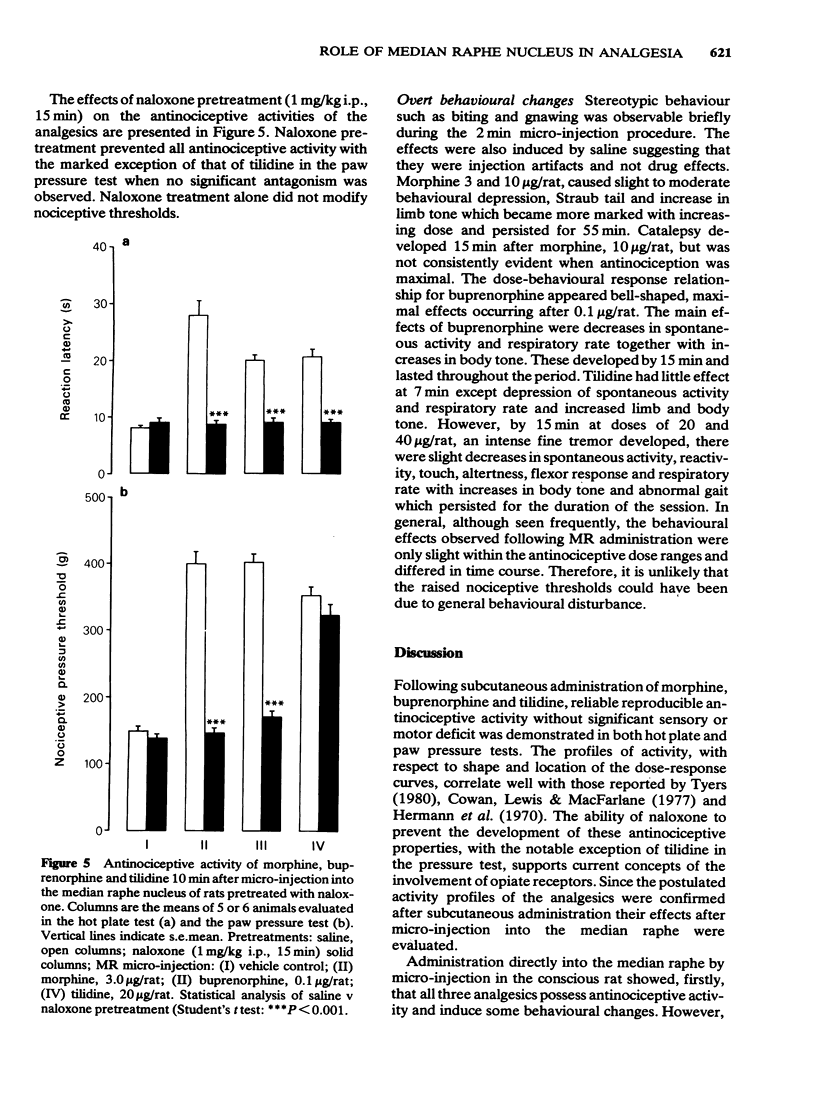
Abstract
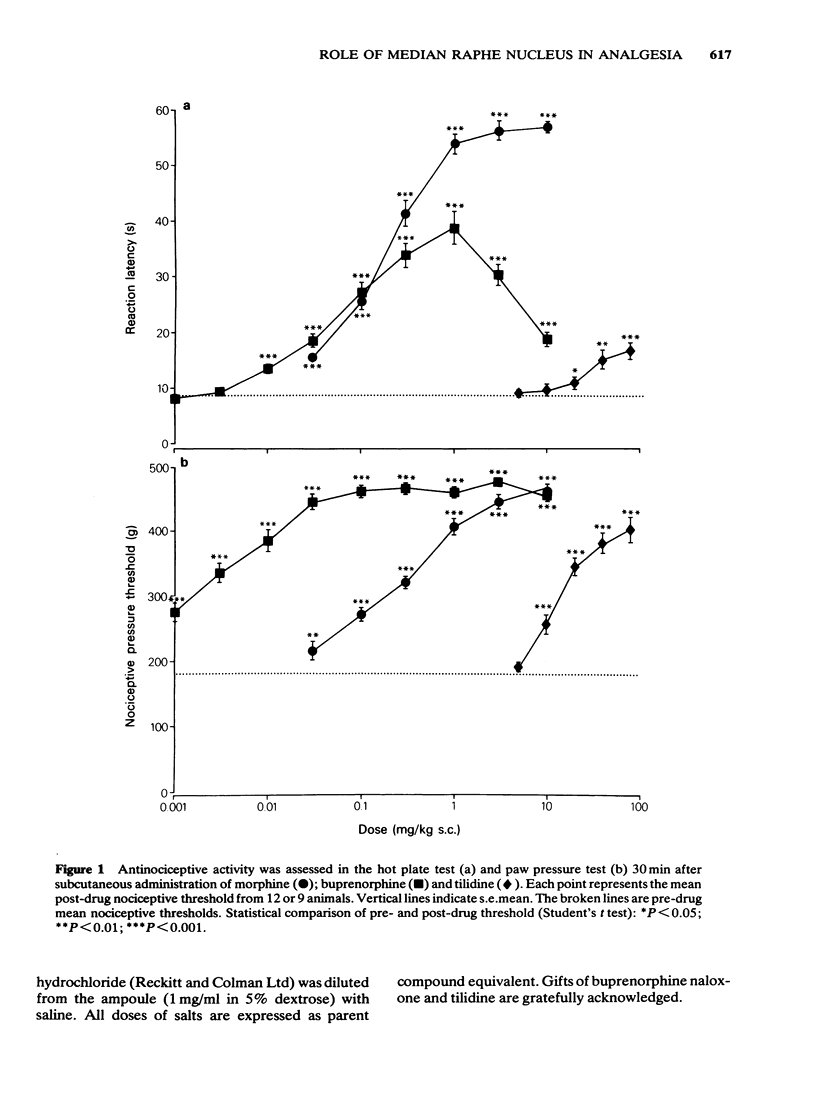
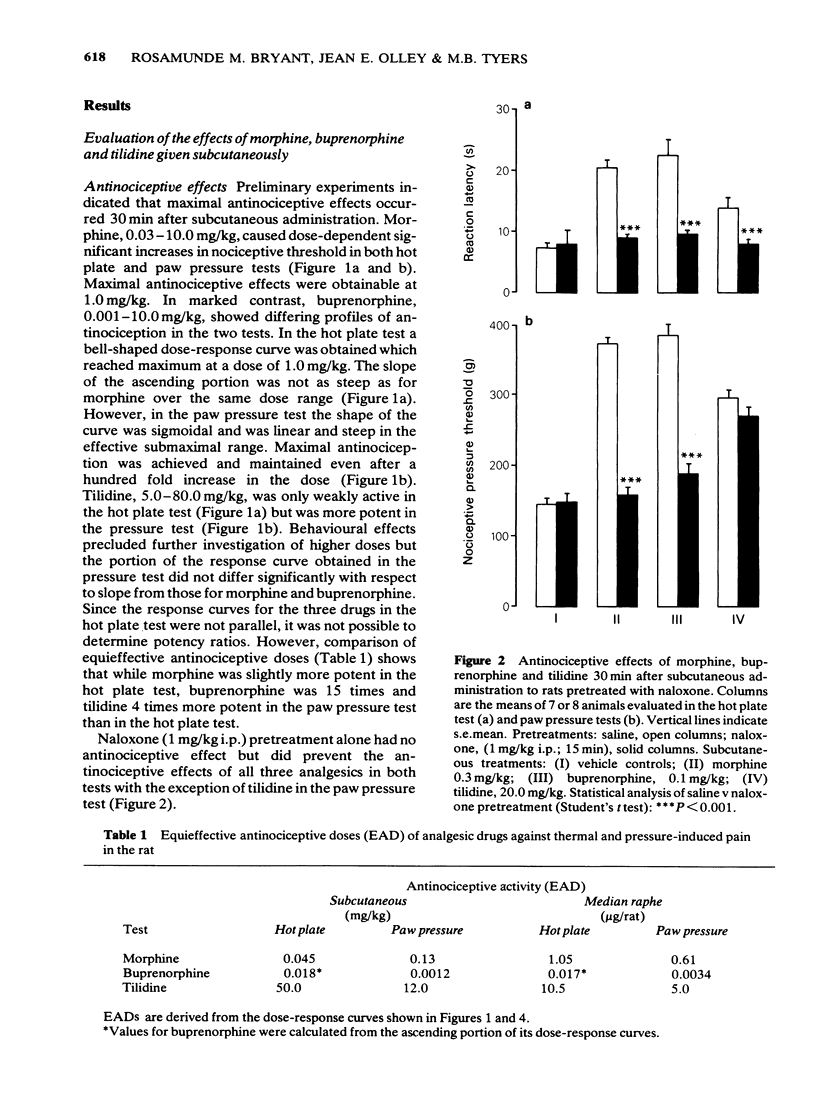
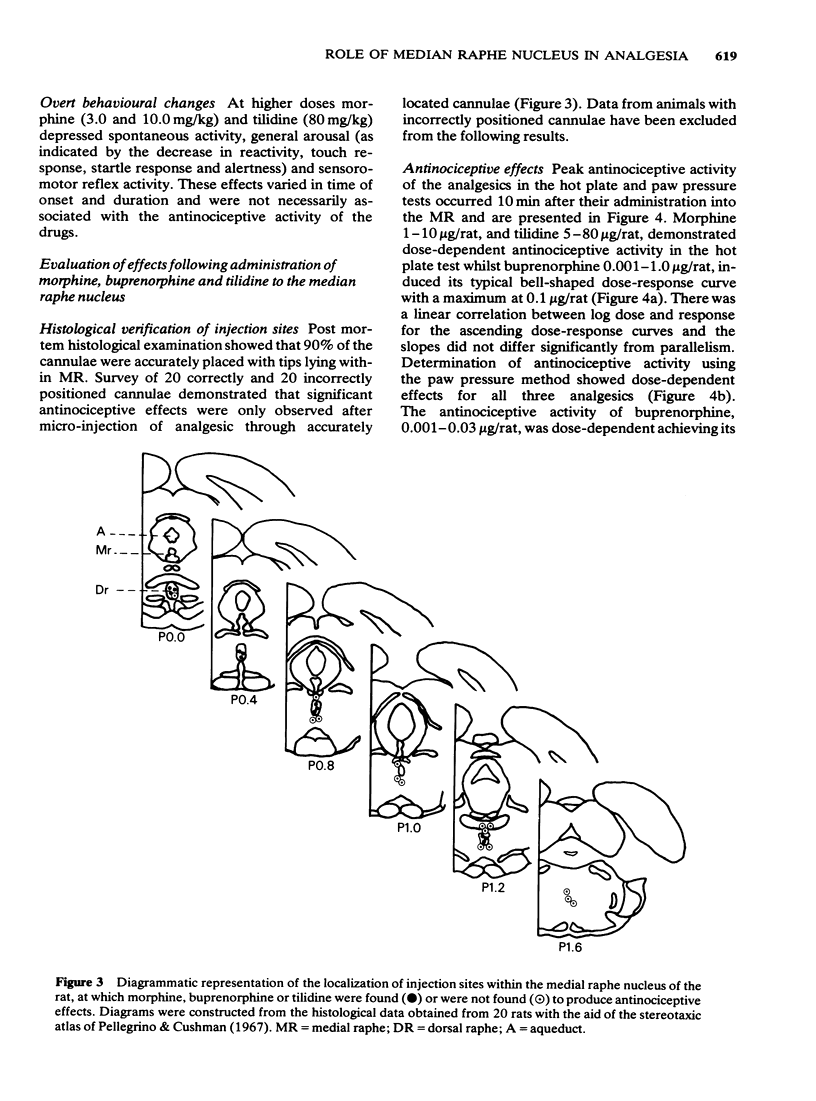
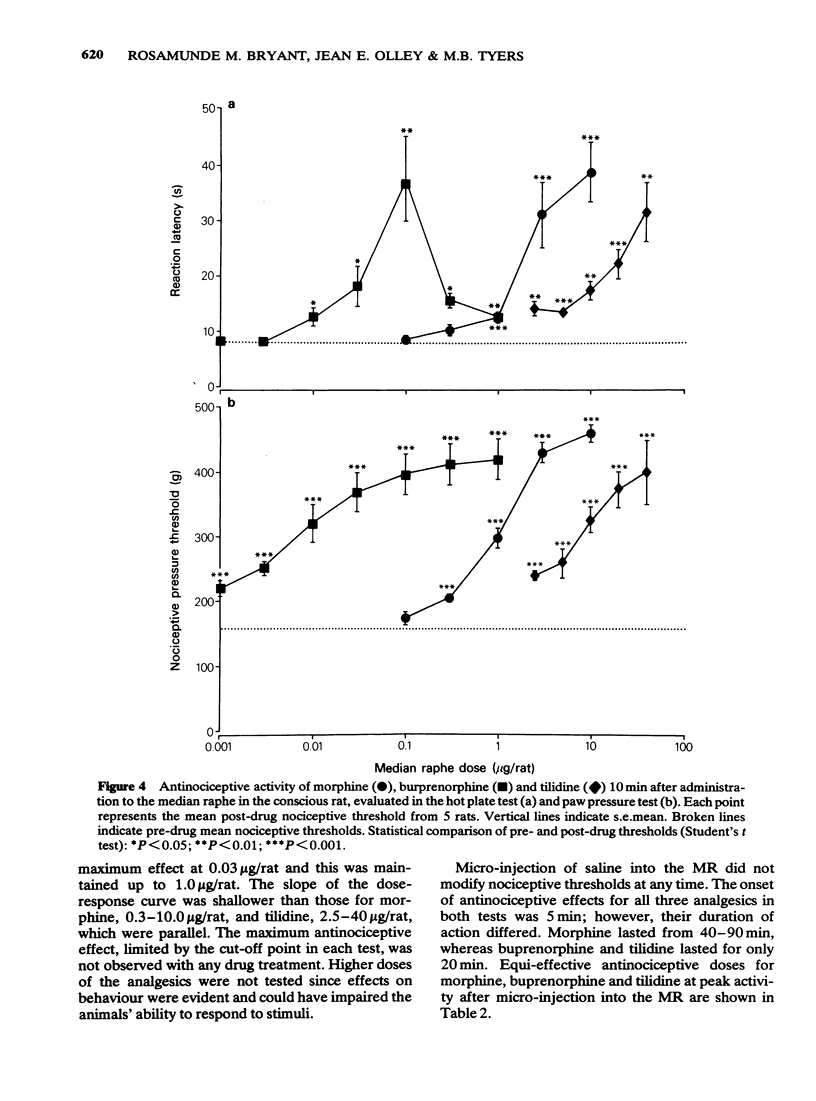
1 Antinociception induced by three analgesics with differing profiles of activity, morphine, buprenorphine and tilidine, have been evaluated in the hot plate and paw pressure tests after administration by the subcutaneous route and directly into the median raphe nucleus in the conscious rat. 2 Behavioural and neurological effects of the three analgesics were also assessed. 3 The typical profiles of antinociceptive activity induced by the three analgesics were qualitatively similar after either route of administration. Morphine induced naloxone-sensitive dose-dependent effects in both tests. Buprenorphine showed naloxone-sensitive effects with a bell-shaped dose-response curve in the thermal test but dose-dependent activity in the pressure test. Tilidine induced naloxone-sensitive dose-dependent effects in the thermal test but demonstrated naloxone-insensitive activity in the paw pressure test. 4 The behavioural and neurological effects of the analgesics in the dose range used would not have affected the animals' ability to respond to the nociceptive stimuli. 5 The results suggest that the median raphe could participate in drug-induced antinociception. The mechanisms by which this might occur are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Kostowski W., Recchia M., Samanin R. Anatomical specificity as the critical determinant of the interrelationship between raphe lesions and morphine analgesia. Eur J Pharmacol. 1975 May;32(1):39–44. doi: 10.1016/0014-2999(75)90320-9. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K., Wang R. Y. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977 Feb 18;122(2):229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978 Jun 1;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bobillier P., Pettijean F., Salvert D., Ligier M., Seguin S. Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradiography. Brain Res. 1975 Feb 28;85(2):205–210. doi: 10.1016/0006-8993(75)90071-2. [DOI] [PubMed] [Google Scholar]
- Bowsher D. Role of the reticular formation in responses to noxious stimulation. Pain. 1976 Dec;2(4):361–378. doi: 10.1016/0304-3959(76)90079-8. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Leonard C. M., Pfaff D. W. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974 Jul;156(2):179–205. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- Cowan A., Ghezzi D., Samanin R. Effect of midbrain raphe lesion and of 6-hydroxydopamine on the antinociceptive action of buprenorphine in rats. Arch Int Pharmacodyn Ther. 1974 Apr;208(2):302–305. [PubMed] [Google Scholar]
- Cowan A., Lewis J. W., Macfarlane I. R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977 Aug;60(4):537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky B., Crew M. C., Melgar M. D., Karpowicz J. K., Di Carlo F. J. Correlation of analgesia with levels of tilidine and a biologically active metabolite in rat plasma and brain. Biochem Pharmacol. 1975 Jan 15;24(2):277–281. doi: 10.1016/0006-2952(75)90288-9. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Basbaum A. I. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Jonsson G. Further mapping of central 5-hydroxytryptamine neurons: studies with the neurotoxic dihydroxytryptamines. Adv Biochem Psychopharmacol. 1974;10:1–12. [PubMed] [Google Scholar]
- Herkenham M., Nauta W. J. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979 Sep 1;187(1):19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Steinbrecher W., Heldt W. Zur Pharmakologie eines neuen stark wirksamen Analgeticums. Arzneimittelforschung. 1970 Aug;20(8):977–983. [PubMed] [Google Scholar]
- Mayer D. J., Price D. D. Central nervous system mechanisms of analgesia. Pain. 1976 Dec;2(4):379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Messing R. B., Lytle L. D. Serotonin-containing neurons: their possible role in pain and analgesia. Pain. 1977 Oct;4(1):1–21. doi: 10.1016/0304-3959(77)90083-5. [DOI] [PubMed] [Google Scholar]
- Mosko S. S., Haubrich D., Jacobs B. L. Serotonergic afferents to the dorsal raphe nucleus: evdience from HRP and synaptosomal uptake studies. Brain Res. 1977 Jan 7;119(2):269–290. doi: 10.1016/0006-8993(77)90311-0. [DOI] [PubMed] [Google Scholar]
- Oleson T. D., Twombly D. A., Liebeskind J. C. Effects of pain-attenuating brain stimulation and morphine on electrical activity in the raphe nuclei of the awake rat. Pain. 1978 Feb;4(3):211–230. doi: 10.1016/0304-3959(77)90134-8. [DOI] [PubMed] [Google Scholar]
- RANDALL L. O., SELITTO J. J. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957 Sep 1;111(4):409–419. [PubMed] [Google Scholar]
- Rance M. J., Shillingford J. S. The role of the gut in the metabolism of strong analgesics. Biochem Pharmacol. 1976 Mar 15;25(6):735–741. doi: 10.1016/0006-2952(76)90255-0. [DOI] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Schulz R., Bläsig J., Wüster M., Herz A. The opiate-like action of tilidine is mediated by metabolites. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):89–93. doi: 10.1007/BF00495543. [DOI] [PubMed] [Google Scholar]
- Tyers M. B. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980 Jul;69(3):503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978 Apr;4(4):299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Yeung J. C., Rudy T. A. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976 Sep 10;114(1):83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]


