Abstract
1 The actions of carbachol on the membrane potential and conductance of smooth muscle of the guinea-pig intestine were investigated using microelectrode recording and the double sucrose-gap method in solutions in which calcium was the only cation creating an inwardly-directed electrochemical gradient.
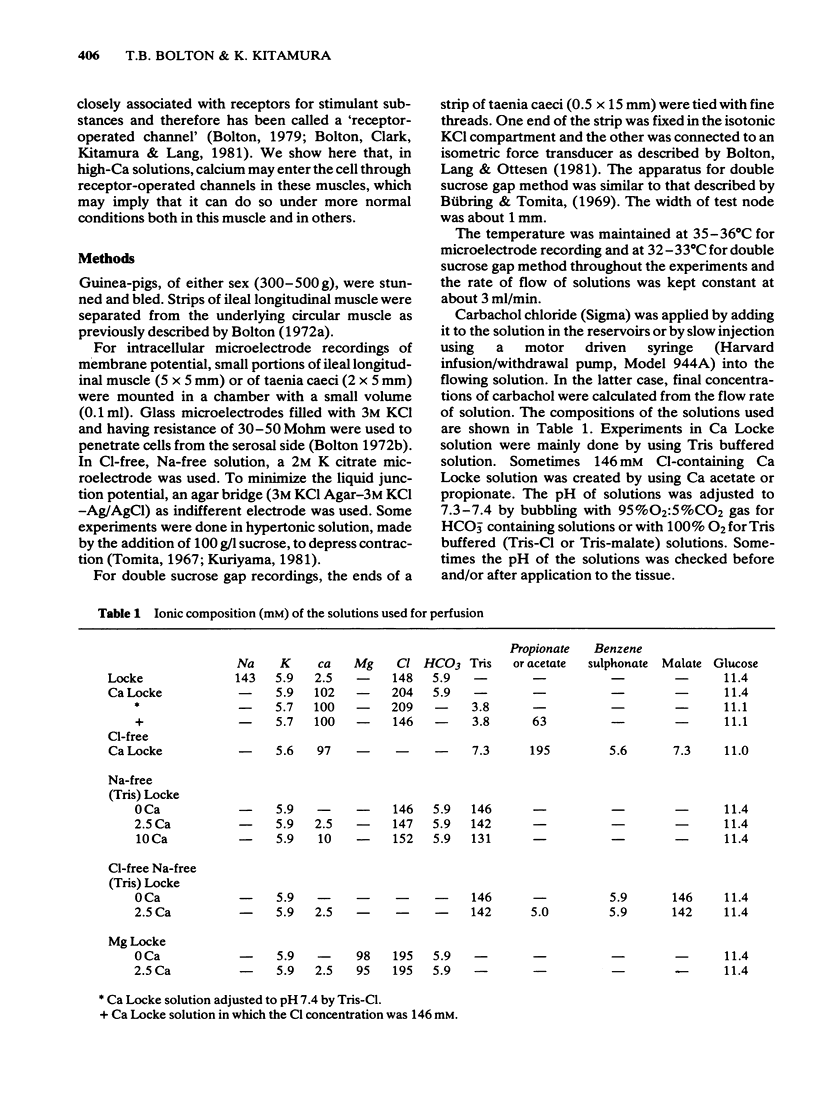
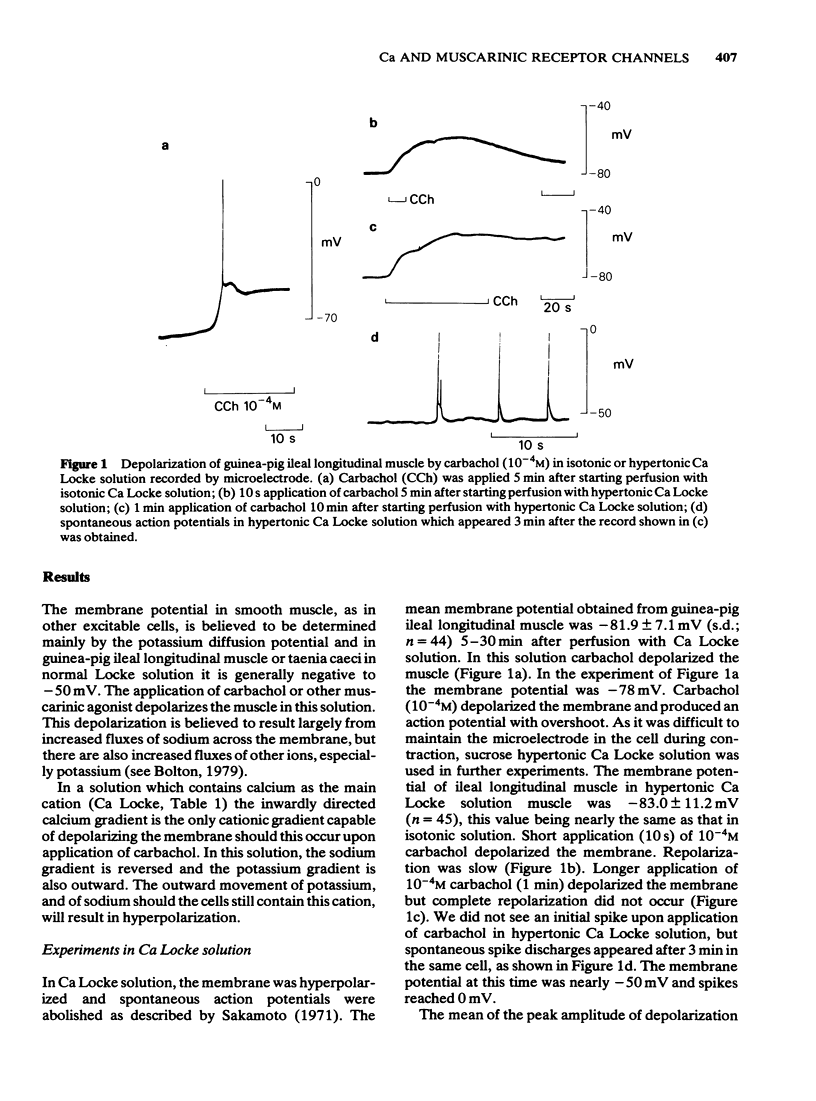
2 In a calcium chloride solution containing a small amount of potassium but no sodium and buffered to physiological pH (Ca Locke) the membrane was hyperpolarized to more than -80 mV. Carbachol (2 × 10-7-10-4M) depolarized the membrane and increased the membrane conductance.
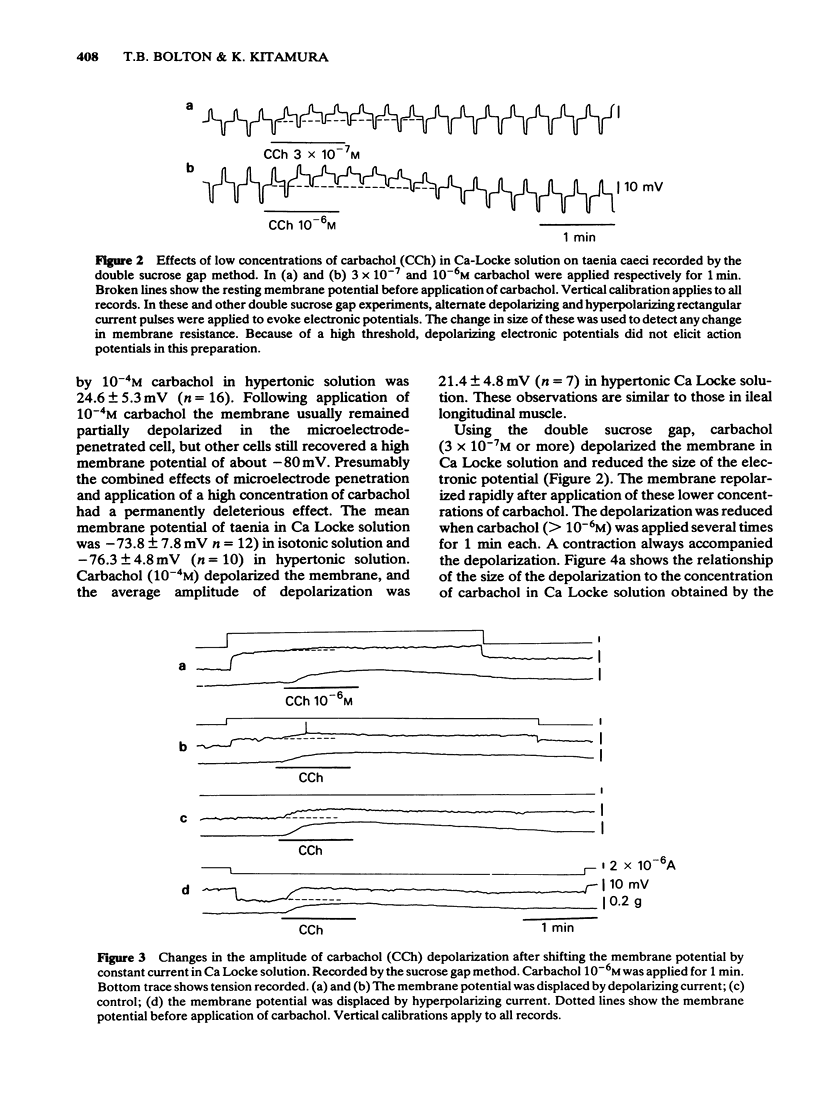
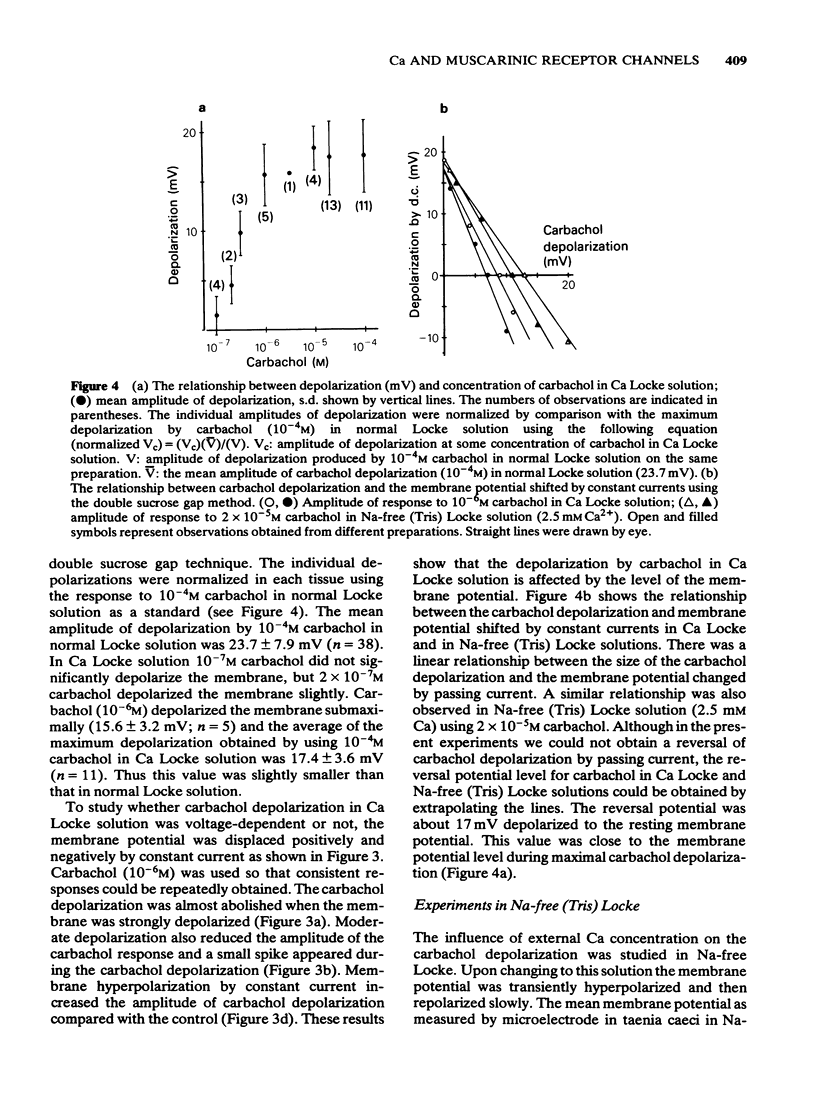
3 By passing current the membrane potential of the smooth muscle cells could be varied. In Ca Locke the depolarization produced by carbachol was shown to be reduced if the membrane was depolarized. The relationship between the size of the carbachol depolarization and the membrane depolarization was linear, giving an apparent reversal potential for carbachol depolarization some 20 mV positive to the resting membrane potential, as measured by extracellular electrodes in the sucrose gap.
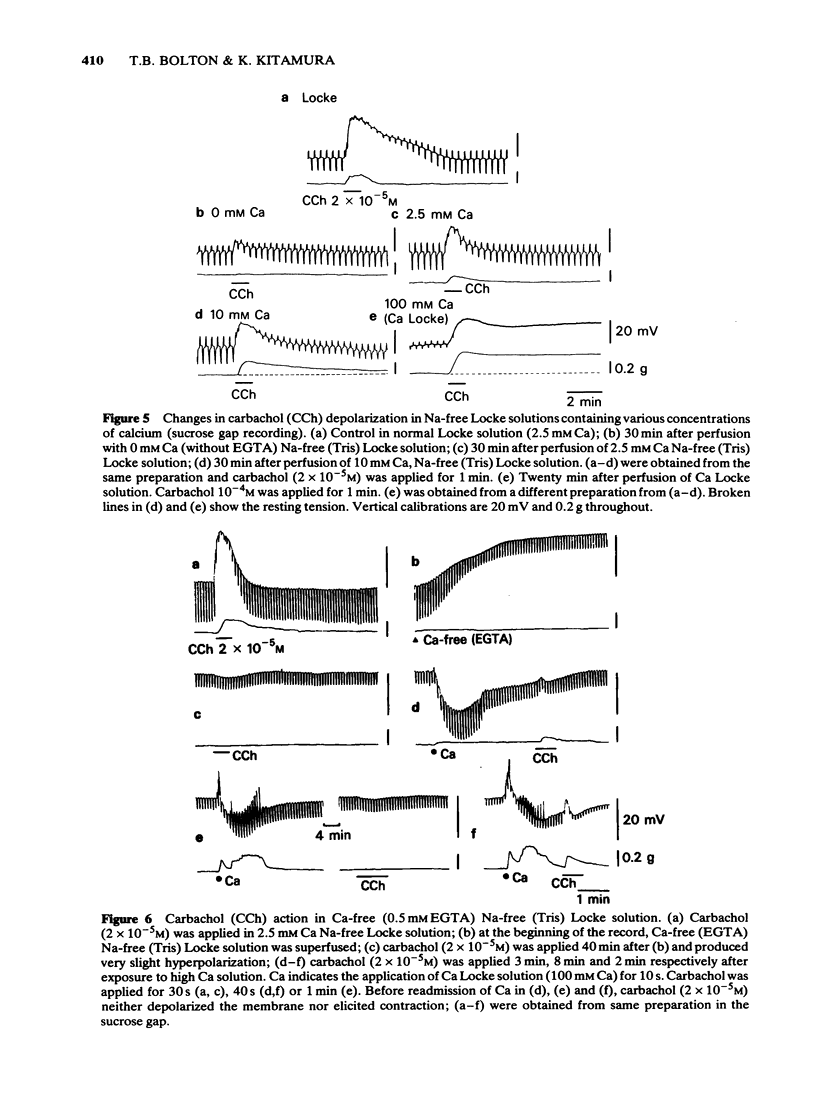
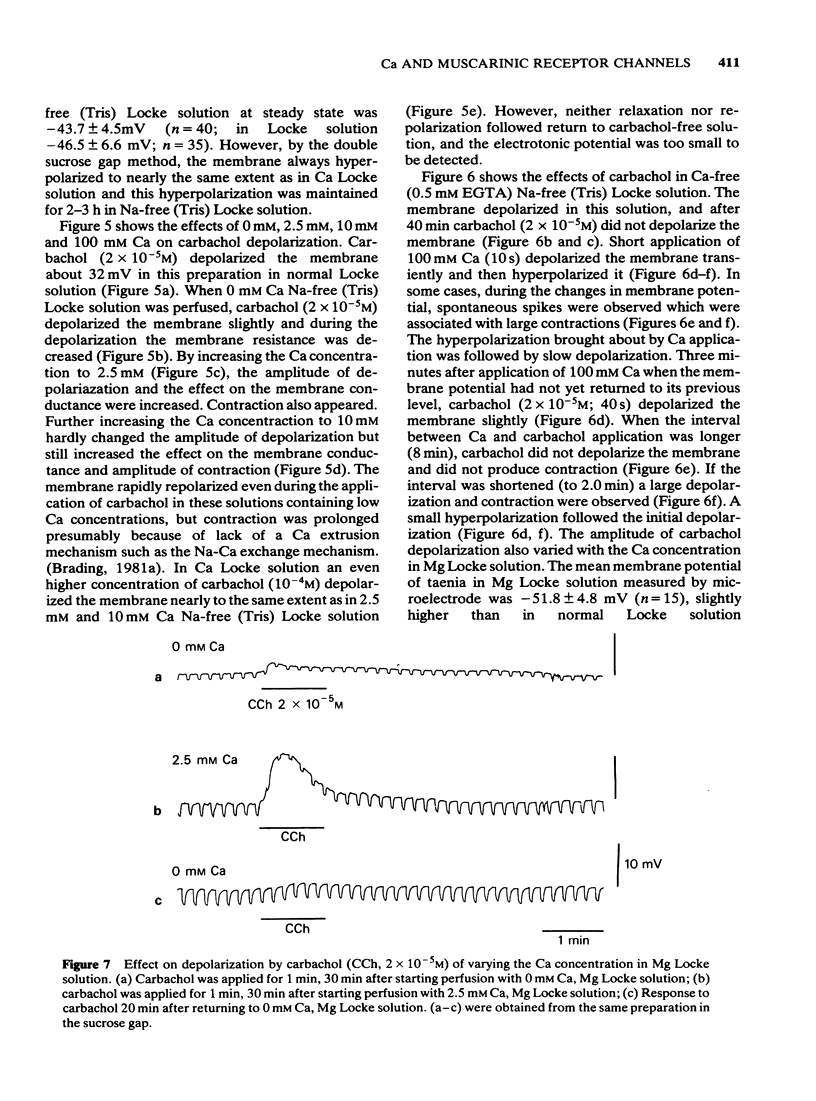
4 Carbachol depolarization was reduced if the calcium concentration was reduced below 2.5 mM by replacing calcium with Tris, but the depolarization in 2.5 mM Ca and in Ca Locke (100 mM Ca) were of similar size. In Ca-free Na-free solution with EGTA, carbachol depolarization was soon abolished.
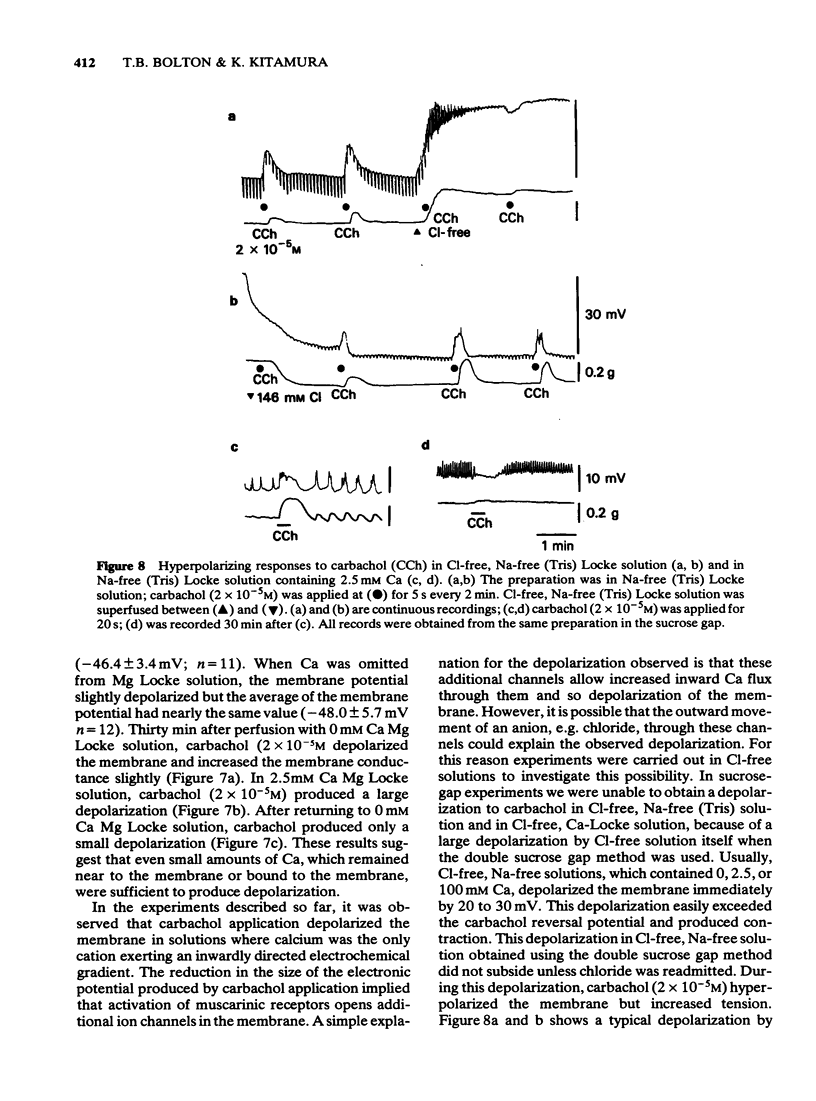
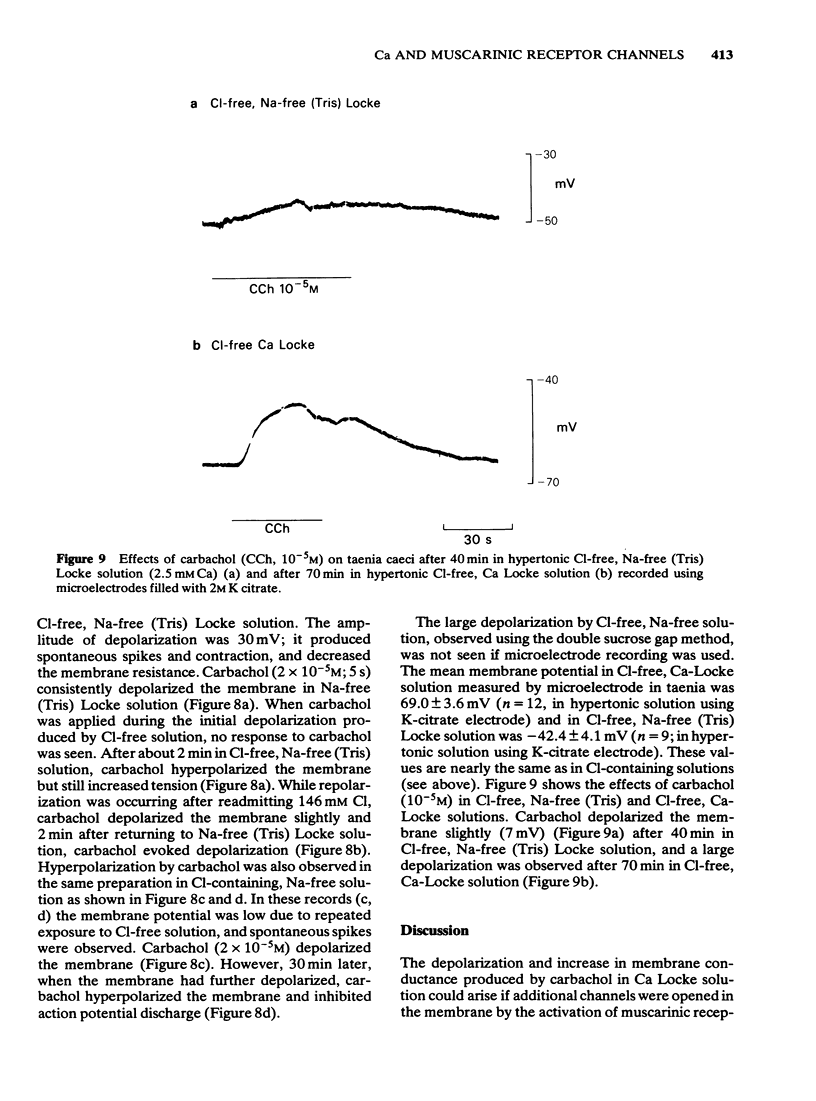
5 In the sucrose-gap when the chloride gradient across the cell membrane was reversed by replacing the chloride of Ca Locke by an impermeant anion the membrane depolarized. Carbachol now contracted the muscle but produced a hyperpolarization.
6 These results are consistent with the hypothesis that activation of muscarinic receptors opens ionic channels which at least in the solutions used, can admit sufficient calcium ions to depolarize the cell and cause tension development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Ottesen B. Mechanism of action of vasoactive intestinal polypeptide on myometrial smooth muscle of rabbit and guinea-pig. J Physiol. 1981 Sep;318:41–55. doi: 10.1113/jphysiol.1981.sp013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The effects of varying the concentrations of ions in the external solution on the oscillations of the membrane potential (slow waves) produced by carbachol in longitudinal ileal miscle. Pflugers Arch. 1972;335(2):85–96. doi: 10.1007/BF00592036. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Szurszewski J. H. The stimulant action of noradrenaline (alpha-action) on guinea-pig myometrium compared with that of acetylcholine. Proc R Soc Lond B Biol Sci. 1974 Jan 29;185(1079):225–262. doi: 10.1098/rspb.1974.0018. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Itoh T., Kuriyama H. Effects of external cations on calcium efflux from single cells of the guinea-pig taenia coli and porcine coronary artery. J Physiol. 1981 Jan;310:321–336. doi: 10.1113/jphysiol.1981.sp013552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisheri K. D., Hwang O., van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981 Mar 15;59(1):19–25. doi: 10.1007/BF01870817. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y. Electrical activity of guinea-pig taenia coli in calcium Locke solution. Jpn J Physiol. 1971 Jun;21(3):295–306. doi: 10.2170/jjphysiol.21.295. [DOI] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Hwang O., Meisheri K. D. The mechanism of inhibitory action of diltiazem on vascular smooth muscle contractility. J Pharmacol Exp Ther. 1981 Aug;218(2):459–463. [PubMed] [Google Scholar]
- van Breemen C., Mangel A., Fahim M., Meisheri K. Selectivity of calcium antagonistic action in vascular smooth muscle. Am J Cardiol. 1982 Feb 18;49(3):507–510. doi: 10.1016/s0002-9149(82)80003-9. [DOI] [PubMed] [Google Scholar]


