Abstract
1 Atenolol reduces sympathetic efferent discharge and attenuates the responses of the sympathetic nerves to changes in blood pressure. The present experiments were carried out to determine whether these changes were mediated by cardiopulmonary receptors whose afferents lie in the vagal nerves.
2 Cats were anaesthetized with α-chloralose and artificially ventilated. In one group of cats recordings were made of sympathetic efferent discharge from few-fibre preparations from the lumbar trunk, splanchnic or renal nerves over a range of blood pressures. In a second group of cats changes in heart rate and blood pressure in response to bilateral occlusion of the common carotid arteries were investigated. In all cats the influence of vagal afferent fibres was removed by cooling both vagal nerves in the neck, both before and after administration of atenolol (3 mg kg-1 i.v.).
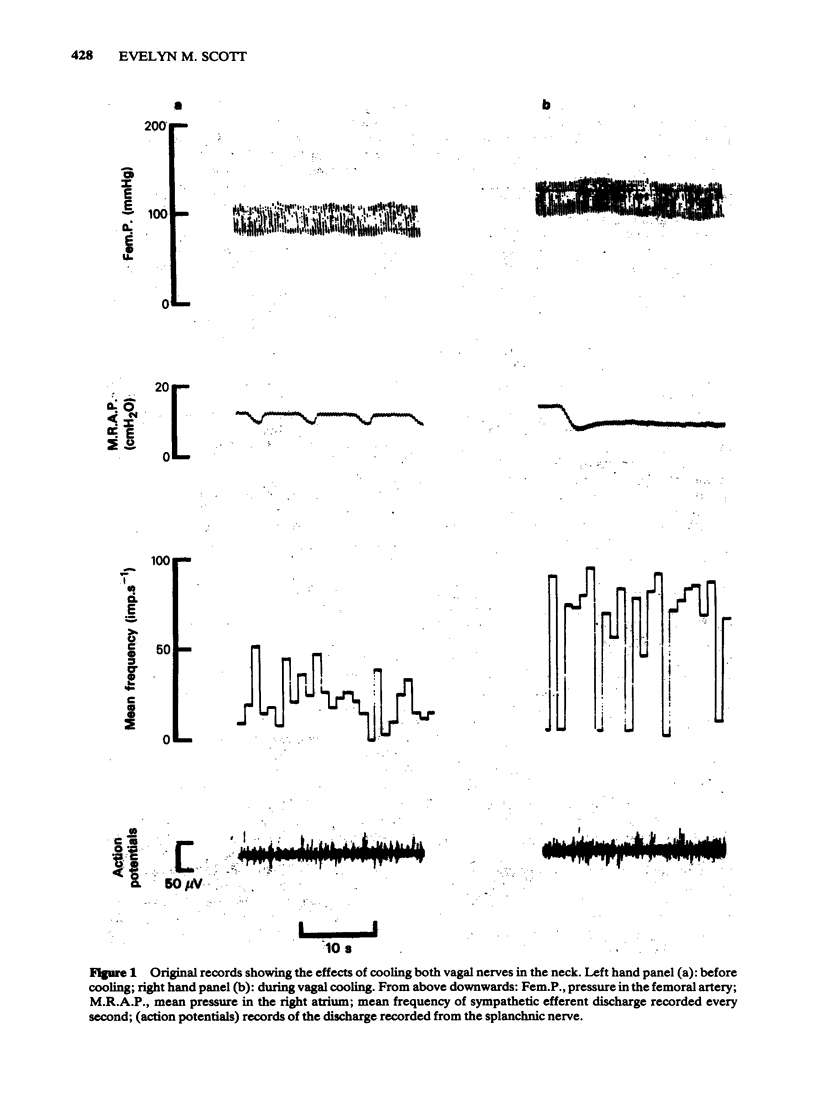
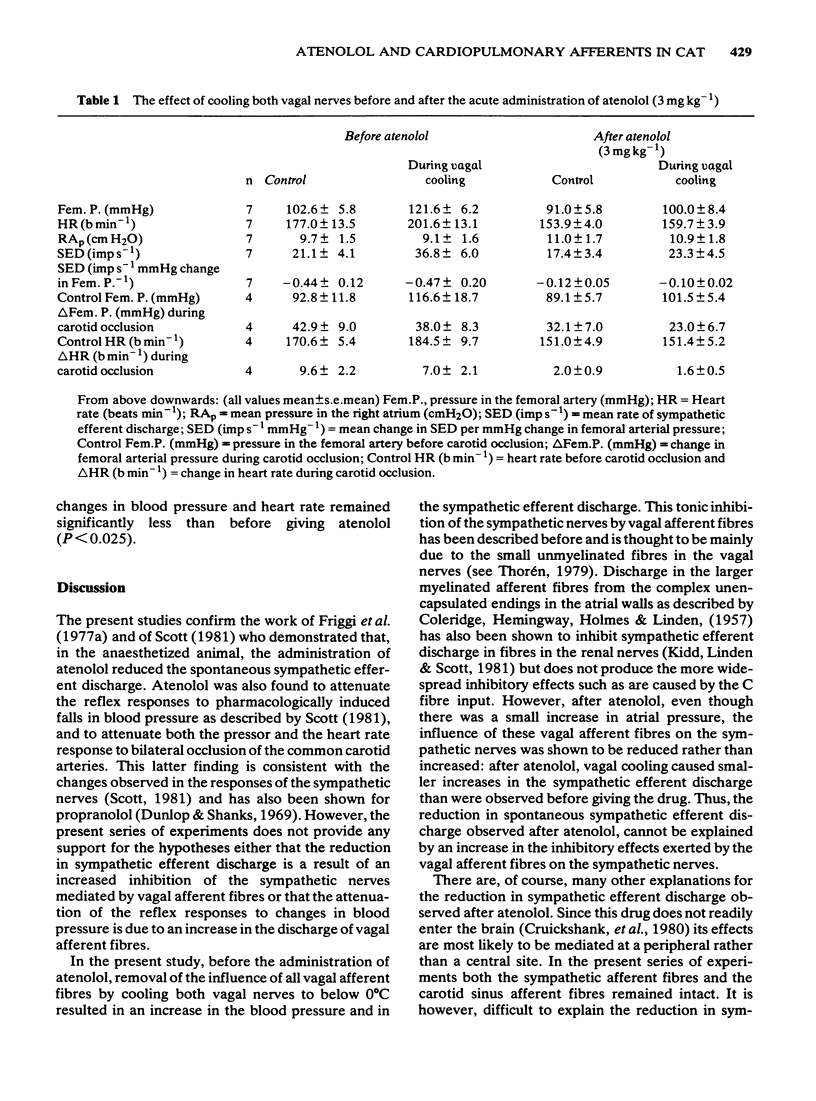
3 Cooling both vagal nerves produced significant increases in blood pressure, heart rate and spontaneous sympathetic efferent discharge but did not affect the relationship between sympathetic efferent discharge and mean blood pressure or the responses to carotid occlusion. Atenolol significantly reduced blood pressure, heart rate and sympathetic efferent discharge but the change in sympathetic efferent discharge on vagal cooling was less than before giving the drug. Atenolol also attenuated the reflex responses of the sympathetic nerves to changes in blood pressure and reduced responses to carotid occlusion. This attenuation was not removed by vagal cooling.
4 Thus, neither the reduction in spontaneous sympathetic efferent discharge nor the attenuation of the baroreceptor reflex seen after atenolol, are due to an increased input to the brain from vagal afferent fibres. Other possible mechanisms whereby atenolol might exert its effects on the sympathetic nerves are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aars H. Central interaction between reflex responses to activity in aortic nerve A- and C-fibres. Acta Physiol Scand. 1980 Nov;110(3):315–320. doi: 10.1111/j.1748-1716.1980.tb06669.x. [DOI] [PubMed] [Google Scholar]
- Abboud F. M. Integration of reflex responses in the control of blood pressure and vascular resistance. Am J Cardiol. 1979 Oct 22;44(5):903–911. doi: 10.1016/0002-9149(79)90221-2. [DOI] [PubMed] [Google Scholar]
- COLERIDGE J. C., HEMINGWAY A., HOLMES R. L., LINDEN R. J. The location of atrial receptors in the dog: a physiological and histological study. J Physiol. 1957 Apr 3;136(1):174–197. doi: 10.1113/jphysiol.1957.sp005750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank J. M., Neil-Dwyer G., Cameron M. M., McAinsh J. beta-Adrenoreceptor-blocking agents and the blood-brain barrier. Clin Sci (Lond) 1980 Dec;59 (Suppl 6):453s–455s. doi: 10.1042/cs059453s. [DOI] [PubMed] [Google Scholar]
- Dunlop D., Shanks R. G. Inhibition of the carotid sinus reflex by the chronic administration of propranolol. Br J Pharmacol. 1969 May;36(1):132–143. doi: 10.1111/j.1476-5381.1969.tb08310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi A., Chevalier-Cholat A. M., Bodard H. Effets d'un inhibiteur béta-adrénergique, l'aténolol, sur l'activité efférente du nerf rénal chez le lapin. Experientia. 1977 Sep 15;33(9):1207–1208. doi: 10.1007/BF01922332. [DOI] [PubMed] [Google Scholar]
- Kappagoda C. T., Linden R. J., Snow H. M. An approach to the problems of acid-base balance. Clin Sci. 1970 Aug;39(2):169–182. doi: 10.1042/cs0390169. [DOI] [PubMed] [Google Scholar]
- Kendrick J. E., Matson G. L., Lalley P. M. Central interaction between the baroreceptor reflexes from the carotid sinus and aortic arch. Am J Physiol. 1979 Jan;236(1):H127–H133. doi: 10.1152/ajpheart.1979.236.1.H127. [DOI] [PubMed] [Google Scholar]
- Kidd C., Linden R. J., Scott E. M. Reflex responses of single renal sympathetic fibres to stimulation of atrial receptors and carotid baro- and chemoreceptors. Q J Exp Physiol. 1981 Jul;66(3):311–320. doi: 10.1113/expphysiol.1981.sp002561. [DOI] [PubMed] [Google Scholar]
- Koike H., Mark A. L., Heistad D. D., Schmid P. G. Influence of cardiopulmonary vagal afferent activity on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ Res. 1975 Oct;37(4):422–429. doi: 10.1161/01.res.37.4.422. [DOI] [PubMed] [Google Scholar]
- Lewis P. J., Haeusler G. Reduction in sympathetic nervous activity as a mechanism for hypotensive effect of propranolol. Nature. 1975 Jul 31;256(5516):440–440. doi: 10.1038/256440a0. [DOI] [PubMed] [Google Scholar]
- Lombardi F., Malliani A., Pagani M. Nervous activity of afferent sympathetic fibers innervating the pulmonary veins. Brain Res. 1976 Aug 20;113(1):197–200. doi: 10.1016/0006-8993(76)90019-6. [DOI] [PubMed] [Google Scholar]
- Scott E. M. The effects of atenolol on spontaneous and reflex activity of the sympathetic nerves in the anaesthetized cat. Br J Pharmacol. 1981 Jul;73(3):609–616. doi: 10.1111/j.1476-5381.1981.tb16795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorén P. Role of cardiac vagal C-fibers in cardiovascular control. Rev Physiol Biochem Pharmacol. 1979;86:1–94. doi: 10.1007/BFb0031531. [DOI] [PubMed] [Google Scholar]


