Abstract
Mutations in the LKB1 protein kinase result in the inherited Peutz Jeghers cancer syndrome. LKB1 has been implicated in regulating cell proliferation and polarity although little is known about how this enzyme is regulated. We recently showed that LKB1 is activated through its interaction with STRADα, a catalytically deficient pseudokinase. Here we show that endogenous LKB1–STRADα complex is associated with a protein of unknown function, termed MO25α, through the interaction of MO25α with the last three residues of STRADα. MO25α and STRADα anchor LKB1 in the cytoplasm, excluding it from the nucleus. Moreover, MO25α enhances the formation of the LKB1–STRADα complex in vivo, stimulating the catalytic activity of LKB1 ∼10-fold. We demonstrate that the related STRADβ and MO25β isoforms are also able to stabilize LKB1 in an active complex and that it is possible to isolate complexes of LKB1 bound to STRAD and MO25 isoforms, in which the subunits are present in equimolar amounts. Our results indicate that MO25 may function as a scaffolding component of the LKB1–STRAD complex and plays a crucial role in regulating LKB1 activity and cellular localization.
Keywords: cell growth/mass spectrometry/Peutz Jeghers syndrome/protein interactions/signal transduction
Introduction
LKB1 is a serine/threonine protein kinase not closely related to other protein kinases (reviewed in Yoo et al., 2002; Boudeau et al., 2003c). Mutations in LKB1 have been linked to Peutz Jeghers cancer syndrome (PJS), an autosomal dominant inherited disorder (Hemminki et al., 1998; Jenne et al., 1998) characterized in those affected by the development of multiple gastro-intestinal hamartomatous polyps as well as a wide spectrum of benign and malignant tumours (Hemminki, 1999). Deletion of both alleles of the LKB1 gene in mice results in embryonic lethality at midgestation (Ylikorkala et al., 2001), but importantly LKB1 heterozygous mice develop a PJS-like disease characterized by gastro-intestinal hamartomas, and later on in life these animals also develop malignant tumours, such as hepatocellular carcinoma (Bardeesy et al., 2002; Jishage et al., 2002; Miyoshi et al., 2002; Nakau et al., 2002; Rossi et al., 2002). Overexpression of LKB1 in certain cancer cells results in growth suppression due to a G1 cell cycle arrest which is dependent on LKB1 catalytic activity (Tiainen et al., 1999), and this has been proposed to be mediated through activation of p21WAF1/CIP1 through a p53-dependent mechanism (Tiainen et al., 2002). Taken together, these findings indicate that LKB1 functions as a tumour suppressor. Recent work has indicated that both the Caenorhabditis elegans (Watts et al., 2000) and the Drosophila (Martin and St Johnston, 2003) homologues of LKB1 regulate cell polarity and, if this function is conserved in humans, loss of cell polarity in PJS patients could account for the development of hamartomas. Although LKB1 is essentially nuclear when overexpressed in cells, a low level of cytosolic localization is also frequently observed (Smith et al., 1999; Tiainen et al., 1999; Karuman et al., 2001). Significantly, mutants of LKB1 that are excluded from the nucleus retain full growth suppression activity, suggesting that the cytoplasmic localization of this enzyme is important for its tumour suppressor function (Tiainen et al., 2002). Several mutant forms of LKB1 found in PJS patients localize only in the nucleus and are not detectable in the cytoplasm (Tiainen et al., 2002; Boudeau et al., 2003b), further suggesting that cytoplasmic localization of LKB1 is important.
Relatively little is known about how LKB1 is regulated and how it functions. Recently, we have shown that LKB1 is associated with a STE20-related pseudokinase termed STRADα, which lacks catalytic activity because key residues required for the function of nearly all protein kinases are missing (Baas et al., 2003). Moreover, LKB1 binds STRADα through its catalytic domain and this interaction enhances LKB1 in vivo activity as well as promoting LKB1 cytoplasmic localization (Baas et al., 2003). LKB1 is no longer able to suppress cell growth in cells in which STRADα has been depleted using an siRNA approach, suggesting that the binding of LKB1 to STRADα plays an important role in mediating its tumour suppressor function. In this study, we identify MO25α as a novel component of the LKB1–STRADα complex and establish that MO25α plays an important role in stabilizing this complex in the cell cytoplasm as well as enhancing LKB1 catalytic activity. Our data indicate that MO25 may function as a scaffolding component of the LKB1–STRADα complex.
Results
Identification of MO25α in an LKB1 complex
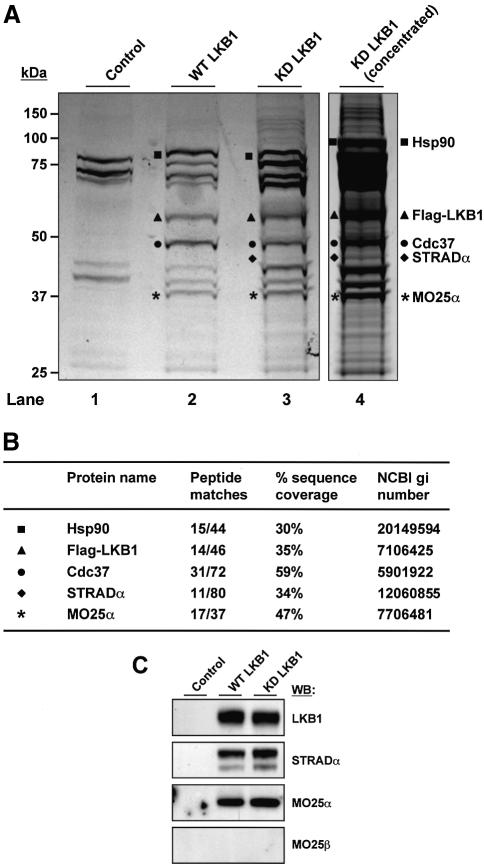
In order to identify proteins associated with LKB1, we have utilized previously described HeLa cells stably expressing low levels of either the wild type or catalytically inactive LKB1 with an N-terminal Flag epitope tag to enable facile immunopurification of LKB1-associated proteins employing the Flag antibody (Boudeau et al., 2003a). Flag-LKB1 was immunopurified from one hundred 10 cm dishes of HeLa cell lysate derived from the cells expressing wild-type LKB1 or kinase-dead LKB1, or from the control parental HeLa cell line that does not express LKB1. The preparations were subjected to electrophoresis on a polyacrylamide gel, which was stained with colloidal Coomassie Blue (Figure 1A). A sample of the kinase-dead LKB1 preparation was also concentrated further to enable better visualization of lower abundance proteins. Several bands were observed which were present in both the wild-type and kinase-dead LKB1 preparations, but were absent in the control sample (Figure 1A). The identity of the colloidal Coomassie Blue-stained bands labelled in Figure 1A was established by tryptic peptide mass-spectral fingerprinting procedures (Figure 1B), and confirmed by immunoblotting with appropriate antibodies (Figure 1C). Proteins previously established to be associated with LKB1, including the Hsp90 and Cdc37 chaperone proteins (Boudeau et al., 2003a) and STRADα (Baas et al., 2003) were detected. In addition, a protein of ∼40 kDa identified as MO25α, co-immunopurified with both wild-type and kinase-dead LKB1 and was not present in the control purification (Figure 1A and C).
Fig. 1. Association of MO25α with LKB1. (A) Cell lysates derived from control parental HeLa cells or HeLa cells stably expressing N-terminal Flag epitope-tagged wild-type (WT) or kinase-dead (KD) LKB1 were passed through an anti-Flag M2–affinity agarose column, LKB1 eluted with the Flag peptide and the samples concentrated as described in Materials and methods. The samples were electrophoresed on a polyacrylamide gel and the protein bands visualized following colloidal Coomassie Blue staining. Protein bands unique to the wild-type and kinase-dead LKB1 preparations are indicated. (B) The colloidal Coomassie Blue-stained bands labelled as indicated in (A) were excised from the gel, the proteins digested in gel with trypsin, and their identities were determined by tryptic peptide mass-spectral fingerprint. The identity of STRADα and MO25α were confirmed by LC–MS/MS sequence analysis on a Q-TOF2 mass spectrometer. The number of tryptic peptides, percentage of sequence coverage and NCBI gi accession numbers for each protein identified are indicated. (C) The samples purified in (A) were immunoblotted with the indicated antibodies. Identical results were obtained following two independent purifications of LKB1 from HeLa cells.
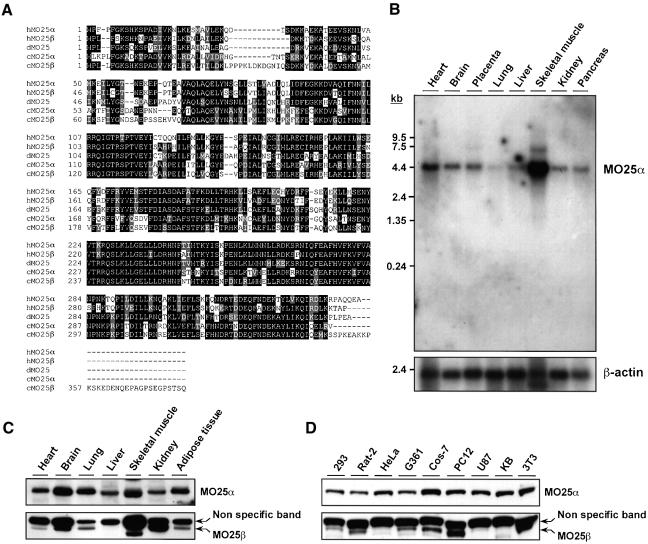
MO25α was first identified as a gene expressed at the early cleavage stage of mouse embryogenesis (Miyamoto et al., 1993) and was shown to be a highly evolutionarily conserved protein (Nozaki et al., 1996; Karos and Fischer, 1999) for which no cellular function has been ascribed. From analysis of databases, we have identified a closely related isoform of MO25α, which we termed MO25β, and a sequence alignment of both MO25 isoforms together with their putative homologues in Drosophila and C.elegans is shown in Figure 2A. Northern blot analysis indicated that MO25α is widely expressed in human tissues, with the highest levels of expression in skeletal muscle (Figure 2B). Immunoblot analysis using an antibody raised against the MO25α protein, which does not crossreact with MO25β (Figure 2C and D), and analysis of EST databases (see the Supplementary table available at The EMBO Journal Online) confirmed that MO25α is expressed in many tissues and cell lines. Although we were unable to detect significant levels of MO25β RNA by northern blot analysis, immunoblotting using an antibody raised against the MO25β protein, which does not crossreact with MO25α, suggested that MO25β is also expressed in a variety of tissues and cell lines tested (Figure 2C and D). We found that MO25β is not expressed in the liver (Figure 2C) or a number of cell lines including HeLa cells (Figure 2D), indicating that expression of MO25β may be more restricted than MO25α and explaining why MO25β was not co-purified with Flag-LKB1 in HeLa cells (Figure 1).
Fig. 2. Amino acid sequence and tissue distribution patterns of MO25α and MO25β isoforms. (A) Amino acid sequence alignment of the human MO25α (NCBI accession No. NP_057373) and MO25β (NCBI accession No. CAC37735) isoforms as well as C.elegans MO25α (NCBI accession No. CAB16486) and MO25β (NCBI accession No. NP_508691) and Drosophila MO25 (NCBI accession No. P91891) putative homologues. Conserved residues are boxed in black, and homologous residues are shaded in grey. Sequence alignments were performed using the CLUSTALW and BOXSHADE programmes at http://www.ch.embnet.org/ using standard parameters. (B) A 32P-labelled fragment of the MO25α cDNA was used to probe a northern blot containing polyadenylated RNA isolated from the indicated human tissues. The membrane was autoradiographed, and the MO25α probe was observed to hybridize to a 4.2-kb message, identical to the size predicted for the MO25α message from database analysis. As a loading control, the northern blot was hybridized with a β-actin probe. (C and D) The indicated mouse tissue (C) or cell (D) extracts containing 20 µg of total cell protein were immunoblotted with anti-MO25α and anti-MO25β antibodies.
Endogenous MO25α is present in complex with endogenous LKB1
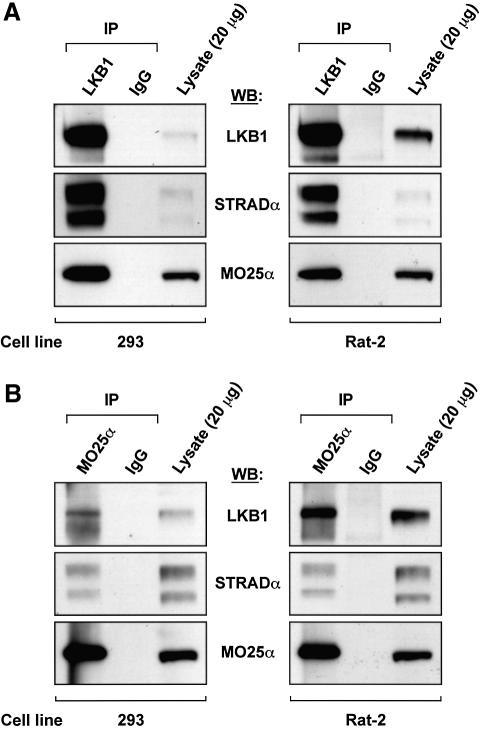
We next immunoprecipitated endogenous LKB1 from 293 cells (Figure 3A, left panel) or Rat-2 cells (Figure 3A, right panel), and immunoblotted the precipitates for LKB1, STRADα and MO25α. The experiments showed that MO25α, as well as STRADα, were co-immunoprecipitated with LKB1, but not with pre-immune IgG. We also immunoprecipitated endogenous MO25α from 293 cells (Figure 3B, left panel) or Rat-2 cells (Figure 3B, right panel), and immunoblotted for the presence of LKB1, STRADα and MO25α (Figure 3B). Endogenous LKB1 and STRADα were co-immunoprecipitated with MO25α, but not with pre-immune IgG, consistent with the notion that these proteins form a complex.
Fig. 3. Endogenous LKB1 is associated with MO25α. (A) LKB1 was immunoprecipitated from 2 mg of the indicated lysates using 10 µg of anti-LKB1 antibody covalently coupled to protein G–Sepharose, and the immunoprecipitates were immunoblotted with the indicated antibodies. As a control, immunoprecipitations were also performed in parallel experiments with pre-immune IgG antibodies covalently coupled to protein G–Sepharose. In each gel, 20 µg of total cell lysate was also immunoblotted in parallel. (B) MO25α was immunoprecipitated as above except that 15 µg of the MO25α antibody was employed. The results shown are from a single experiment that was repeated three times with similar results.
MO25 interacts with STRAD rather than with LKB1
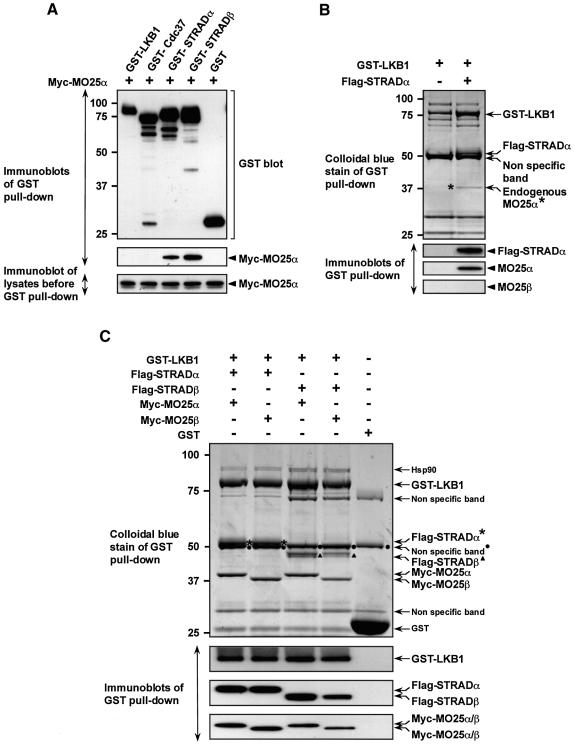
To verify which component of the LKB1–STRAD complex MO25α interacted with, we co-transfected 293 cells with constructs expressing MO25α and either N-terminal glutathione S-transferase (GST)-tagged LKB1, STRADα or the closely related STRADβ pseudokinase, as well as Cdc37, a chaperone previously shown to bind LKB1 (Boudeau et al., 2003a). A sequence alignment of STRADα and STRADβ is shown in Supplementary figure 1 and both lack the same key conserved residues required for catalysis, in subdomains VI and VII of the kinase domain. The GST-tagged proteins were affinity purified and immunoblotted for MO25α (Figure 4A). Under the rigorous conditions employed to avoid detection of weak or non-specific interactions, we found that MO25α interacts only with STRADα and STRADβ but not with LKB1 or Cdc37.
Fig. 4. MO25α is associated with LKB1 through STRAD. (A) 293 cells were transfected with plasmids encoding for the expression of the indicated GST fusion proteins together with Myc-MO25α. Thirty-six hours post-transfection, the GST-tagged proteins were affinity purified from the cell lysates using glutathione–Sepharose as described in Materials and methods. Similar amounts of the purified GST fusion proteins were subjected to SDS–PAGE and immunoblotted with anti-Myc antibody to detect co-purified Myc-MO25α, or with anti-GST antibody to ensure that comparable amounts of the GST-tagged proteins were present in each lane (upper and middle panels). Total cell lysates (5 µg) prior to affinity purification were also subjected to immunoblotting with anti-Myc antibody to ensure that Myc-MO25α was expressed at similar levels in each co-transfection (lower panel). (B) N-terminal GST-tagged LKB1 was expressed in 293 cells in the presence or absence of Flag-STRADα and purified as above. The purified proteins were subjected to SDS–PAGE and visualized by colloidal Coomassie Blue staining (upper panel). The faint protein band indicated, corresponding to an endogenous protein of 40-kDa (marked *), was identified by tryptic peptide mass-spectral fingerprint as endogenously expressed MO25α (with 38% sequence coverage). The purified proteins were also immunoblotted with the indicated antibodies (lower panels). (C) As above except that GST–LKB1 was expressed in 293 cells together with the indicated isoforms of Flag-STRAD and Myc-MO25 and the proteins visualized by colloidal Coomassie Blue staining were also immunoblotted with the indicated antibodies. For all panels, similar results were obtained in at least three separate experiments.
We next expressed LKB1 in 293 cells in the presence or absence of STRADα, and the protein composition of the affinity purified LKB1 was analysed following electrophoresis and staining with colloidal Coomassie Blue. As expected, STRADα was co-purified with LKB1 (Figure 4B). In addition, a faintly staining band corresponding to an endogenous protein of ∼40 kDa was observed in the LKB1–STRADα complex, which was not present in the uncomplexed LKB1 preparation. Tryptic peptide mass-spectral fingerprinting revealed that this protein corresponded to endogenous MO25α. This was confirmed by immunoblotting with an anti-MO25α antibody (Figure 4B), further suggesting that MO25α is associated with LKB1 through STRADα. We consistently observed that higher levels of GST–LKB1 were expressed in the presence of STRADα, indicating that binding of STRADα to LKB1 may stabilize the latter. In Figure 4C, we demonstrate by performing appropriate co-transfections, that it is possible to purify a heterotrimeric LKB1 complex in which LKB1, STRADα and MO25α are present in similar equimolar proportions. We also demonstrate that it is possible to form complexes of LKB1–STRADα–MO25β, LKB1–STRADβ–MO25α and LKB1–STRADβ–MO25β (Figure 4C), indicating that both isoforms of STRAD and MO25 are able to bind each other, as well as LKB1.
LKB1 was previously found to be phosphorylated at Ser431 by PKA and p90RSK kinases and is also farnesylated at Cys433 (Collins et al., 2000; Sapkota et al., 2001). To investigate whether these modifications affect the assembly of the LKB1–STRAD–MO25 complex, mutants of LKB1 in which Ser431 was changed to Ala or Asp, or Cys433 to Ala, were co-expressed with STRADα and MO25α. LKB1 was affinity purified and immunoblotting analysis revealed that the LKB1 mutants interacted with STRADα and MO25α similarly to wild-type LKB1 (Supplementary figure 2A). We also stimulated 293 cells with forskolin (to activate PKA) and 12-O-tetradeconylphorbol-13-acetate (to activate p90RSK) and immunoprecipitated endogenously expressed LKB1. Immunoblotting analysis of the immunoprecipitates revealed that these treatments induced phosphorylation of LKB1 at Ser431, but did not affect its association with STRADα and MO25α (Supplementary figure 2B). Consistent with (see next section) this green fluorescent protein (GFP)–LKB1[S431A] and GFP–LKB1[S431D] were localized in the cytoplasm when co-expressed with STRADα and MO25α (data not shown). This indicates that phosphorylation of Ser431 or prenylation of Cys433 do not regulate formation of the LKB1 complex.
MO25α cooperates with STRADα to localize LKB1 in the cytoplasm
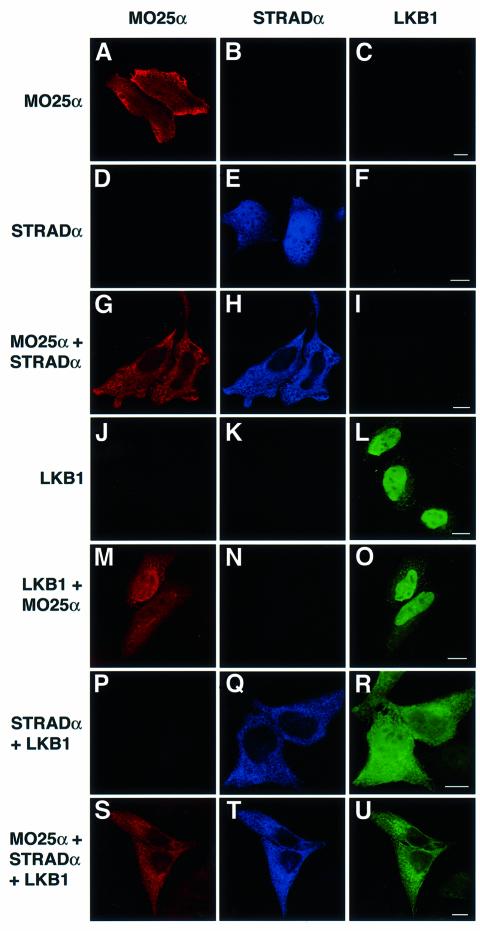
The cytoplasmic fraction of LKB1 was recently shown to conduct a G1 cell cycle arrest (Tiainen et al., 2002). Therefore, we were interested in studying how the formation of a complex of LKB1, STRADα and MO25α affected the localization of these proteins. HeLa cells were transfected with combinations of tagged MO25α, STRADα and LKB1 and these proteins were visualized in cells by confocal fluorescence microscopy in which the MO25α, STRADα and LKB1 proteins could be detected independently in the same cell. MO25α (Figure 5A) and STRADα (Figure 5E) expressed on their own were localized throughout the cytoplasm and nucleus. Strikingly however, when MO25α and STRADα were co-expressed, both proteins were re-localized to the cytoplasm and were excluded from the nucleus (Figure 5G and H). LKB1 expressed alone was mainly nuclear (Figure 5L) and consistent with MO25 not binding to LKB1, co-expression of LKB1 with MO25 did not affect LKB1 nuclear localization (Figure 5M and O). As reported previously in COS cells, co-expression of LKB1 with STRADα promoted significant cytoplasmic localization of LKB1, however significant levels of LKB1 remained in the nucleus under these conditions (Figure 5R) (Baas et al., 2003). In the presence of both MO25α and STRADα, however, LKB1 was essentially only localized in the cytoplasm and virtually excluded from the nucleus (Figure 5U). These observations indicate that MO25α and STRADα form a complex that anchors LKB1 in the cytoplasm more effectively than STRADα alone.
Fig. 5. MO25α and STRADα anchor LKB1 in the cytoplasm. HeLa cells were transfected with the indicated constructs encoding for the expression of Myc-MO25α, Flag-STRADα and GFP–LKB1. Twenty-four hours post-transfection the cells were fixed in 4% (by vol) paraformaldehyde and immunostained with anti-MO25α antibody to detect MO25α (TR anti-sheep secondary antibody, red channel) and anti-Flag to detect STRADα (Cy5 anti-mouse secondary antibody, blue channel). GFP–LKB1 localization was visualized directly through the GFP fluorescence (green channel). The cells were imaged using a Zeiss LSM 510 META confocal microscope. The cells shown are representative images obtained in three separate experiments. The scale bars correspond to 10 µm.
A mutant form of LKB1 termed SL26-LKB1 isolated from a Peutz Jeghers syndrome patient which retains normal ability to autophosphorylate, indicating that it is not catalytically deficient, in which three residues are mutated in the C-terminal region of the kinase domain (Hemminki et al., 1998), was found not to interact with STRADα (Baas et al., 2003). Consistent with this observation, the nuclear localization of SL26-LKB1 was not affected by co-expression with MO25α and STRADα (Supplementary figure 3). We also demonstrate that a catalytically inactive LKB1[D194A] mutant is anchored in the cytoplasm in the presence of MO25α and STRADα, indicating that catalytic activity of LKB1 is not essential for association with STRADα/MO25α and cytoplasmic localization (Supplementary figure 3). These findings are confirmed in Supplementary figure 4 where we demonstrate using a cell co-expression binding assay that SL26-LKB1 fails to interact with STRADα and MO25α, whereas catalytically inactive LKB1[D194A] efficiently binds these molecules.
MO25α recognizes the last three residues of STRADα
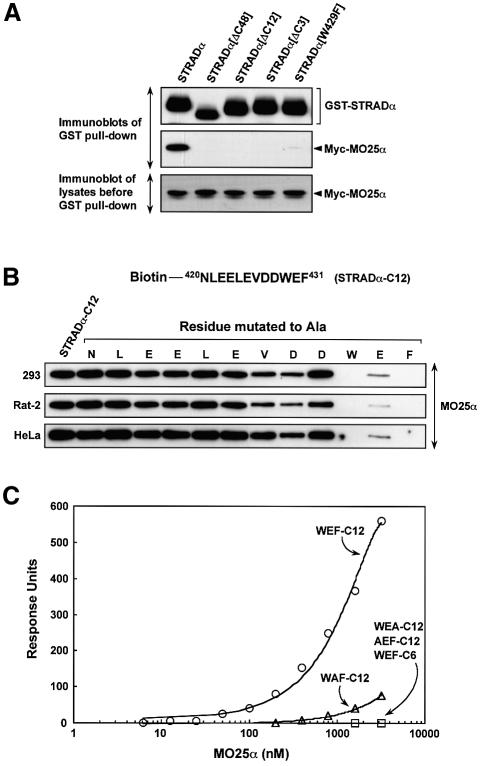
To define the binding site of MO25α on STRADα, a series of deletion mutations of STRADα were tested for their ability to interact with MO25α in a co-transfection binding assay. Strikingly, deletion of only the last three amino acids of STRADα (Trp-Glu-Phe) abolished binding of MO25α to STRADα. Moreover, mutation of the C-terminal Trp residue to Phe also vastly reduced binding of MO25α to STRADα (Figure 6A). We then tested whether it was possible to affinity purify endogenously expressed MO25α from three different mammalian cell lysates employing biotinylated peptides encompassing the C-terminal residues of STRADα conjugated to streptavidin–Sepharose. We found that a peptide encompassing the C-terminal 12 residues (Figure 6B) but not the last six residues (data not shown and Figure 6C) of STRADα efficiently purified endogenous MO25α from all cell lysates tested (Figure 6B). To further delineate the MO25α binding site on the STRADα peptide, we performed an alanine scan, mutating each residue of the peptide individually to Ala and verifying how this affected binding to MO25α (Figure 6B). Consistent with the Trp-Glu-Phe residues being required for MO25α recognition, mutation of any of these three residues in the C-terminal STRADα peptide abolished or vastly reduced MO25α binding in three different cell lysates, whereas mutation of the other residues either did not affect binding or only had a moderate effect. These findings were also confirmed in a more quantitative surface plasmon resonance BiaCore binding assay (Figure 6C).
Fig. 6. MO25α recognizes the C-terminal three residues of STRADα. (A) N-terminal GST-tagged wild-type STRADα or the indicated mutants of STRADα were expressed in 293 cells together with Myc-MO25α, and 36 h post-transfection the STRADα proteins were affinity purified from the cell lysates using glutathione–Sepharose. Similar amounts of the purified proteins were subjected to SDS–PAGE and immunoblotting with anti-Myc antibody to detect co-purified Myc-MO25α, or with anti-GST antibody to ensure that comparable amounts of the GST-tagged proteins were present in each lane (upper and middle panels). Five micrograms of the total cell lysates prior to affinity purification were also subjected to immunoblotting with anti-Myc antibody to ensure that Myc-MO25α was expressed at similar levels in each condition (lower panel). (B) The indicated cell lysates (0.5 mg) were incubated with 5 µg of an N-terminal biotinylated peptide encompassing either the C-terminal 12 residues of STRADα conjugated to streptavidin–Sepharose (NLEELEVDDWEF, termed STRADα-C12) or mutants of this peptide in which the indicated residues were individually mutated to Ala. Following isolation and washing of the beads, the samples were subjected to SDS–PAGE and immunoblotted with an anti-MO25α antibody. (C) Binding of bacterially expressed MO25α to the indicated peptides was analysed by surface plasmon resonance BiaCore analysis as described in Materials and methods. Binding was analysed over a range of MO25α concentrations (6.25–3200 nM) and the response level at the steady-state binding was plotted versus the log of the MO25α concentration. The estimated Kd for the STRADα-C12 peptide was obtained by fitting the data to the formula [m1 X m0/(m0 + m2)] using Kaleidagraph software and the Kd was calculated to be 850 nM. WEF-C12 corresponds to NLEELEVDDWEF, WEA-C12 corresponds to NLEELEVDDWEA, AEF-C12 corresponds to NLEELEVDDAEF, WAF-C12 corresponds to NLEELEVDDWAF, WEF-C6 corresponds to VDDWEF.
Binding of LKB1 to STRADα creates novel binding site(s) for MO25α
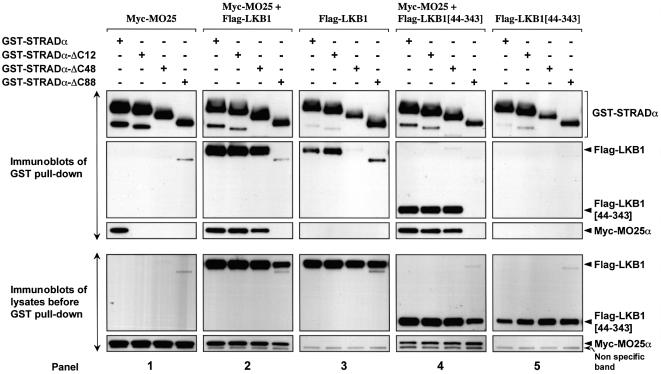
We next investigated how binding of LKB1 to STRADα affected the interaction with MO25α using a co-expression binding assay in 293 cells in which full-length wild type and deletion mutants of GST–STRADα were co-expressed in the presence or absence of MO25α and LKB1. Consistent with previous findings, STRADα mutants that lack either the C-terminal 12 or 48 residues did not bind MO25α in the absence of LKB1 (Figure 7, panel 1). Strikingly though, when these STRADα mutants were co-expressed with LKB1, MO25α was recovered in the purified complexes (Figure 7), indicating that the binding of LKB1 to STRADα generates additional binding site(s) for MO25α within the LKB1–STRADα complex. We also consistently noticed that the amount of LKB1 recovered in the GST–STRADα pull downs was significantly higher in the presence of MO25α (Figure 7, compare panels 2 and 3), suggesting that MO25α might stabilize the formation of a heterotrimeric LKB1 complex. Moreover, a catalytic fragment of LKB1 encompassing only the kinase domain (residues 44–343) interacted much more efficiently with STRADα in the presence of MO25α (Figure 7, compare panels 4 and 5). These observations indicate that MO25α could be stabilizing the LKB1–STRADα complex by generating additional MO25α binding site(s) on STRADα and/or LKB1. A mutant of STRADα lacking the C-terminal 88 residues (STRADα-ΔC88) that truncates a region of the pseudokinase domain, was no longer able to interact with LKB1 or MO25α when these proteins were co-expressed (Figure 7). This suggested that either a secondary MO25α binding site is located towards the C-terminus of STRADα or that the integrity of the pseudokinase domain, which could be disrupted by truncation of the 88 C-terminal residues of STRADα, is required in enabling formation of the LKB1–STRADα–MO25α heterotrimeric complex. It should also be noted that the levels of LKB1 that were expressed in the presence of the STRADα-ΔC88 mutant were noticeably reduced, suggesting that binding of STRADα to LKB1 enhances LKB1 expression or stability.
Fig. 7. Binding of LKB1 to STRADα creates novel binding site(s) for MO25α. The indicated forms of GST–STRADα were co-expressed in 293 cells together with Myc-MO25α and/or wild-type Flag-LKB1 or the isolated LKB1 catalytic domain (LKB1[44–343]). Thirty-six hours post-transfection, the GST–STRADα proteins were affinity purified, subjected to SDS–PAGE and immunoblotting with anti-Myc antibody to detect Myc-MO25α, anti-Flag antibody to detect co-purified Flag-LKB1 and Flag-LKB1[44–343], or with anti-GST antibody to detect STRADα forms (upper panels). Five micrograms of total cell lysates prior to affinity purification were also subjected to immunoblotting with anti-Myc and anti-Flag antibodies to ensure that MO25α and LKB1 were expressed at similar levels in each co-transfection (lower panels). Similar results were obtained in three separate experiments.
Further evidence that MO25 stabilizes the LKB1–STRAD complex
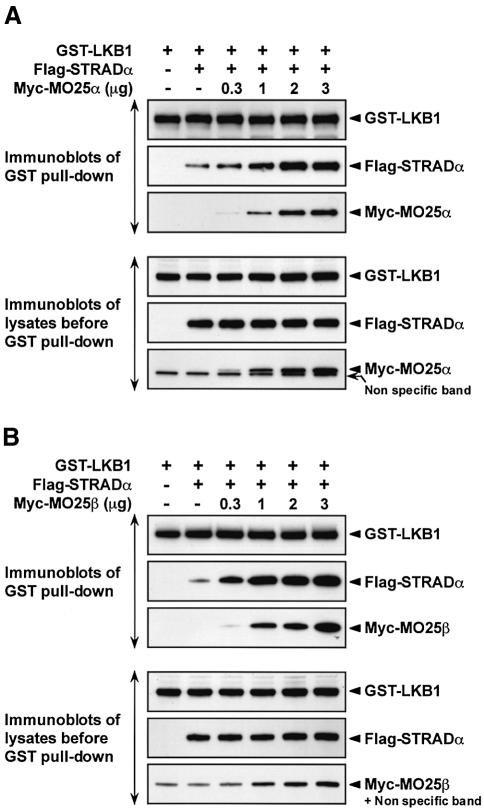
To obtain further evidence that MO25α could stabilize the binding of LKB1 to STRADα, 293 cells were co-transfected with constructs expressing LKB1 and STRADα in the absence or presence of increasing amounts of the MO25α DNA construct. LKB1 was purified and the levels of associated STRADα and MO25α were measured by immunoblotting. As a control, the levels of expression of LKB1, STRADα and MO25α were also measured in the cell lysates prior to affinity purification of LKB1. In the absence of MO25α, a moderate amount of STRADα was associated with LKB1, which was markedly enhanced by increasing expression of MO25α in a dose-dependent manner (Figure 8A, upper panel). Increased association of STRADα with LKB1 was not due to increased expression of LKB1 or STRADα as these were expressed in cell lysates at similar levels in the absence or presence of increasing amounts of MO25α (Figure 8A, lower panel). MO25β was similarly able to enhance the interaction between LKB1 and STRADα (Figure 8B).
Fig. 8. MO25 isoforms stabilize the LKB1–STRADα complex. (A) 293 cells were transfected with 3 µg of the DNA construct encoding for expression of GST–LKB1, in the presence or absence of 3 µg of the Flag-STRADα construct and in the absence or presence of the indicated amounts of Myc-MO25α construct. Thirty-six hours post- transfection, GST–LKB1 was affinity purified from the cell lysates and immunoblotted with appropriate epitope antibodies to detect LKB1, STRADα and MO25α. Five micrograms of total cell lysates prior to affinity purification were also immunoblotted with the indicated antibodies (lower panels). (B) As above except that the MO25β construct was employed instead of the MO25α construct. Similar results were obtained in three separate experiments.
We also investigated how MO25α and MO25β stabilized the binding of LKB1 to STRADβ. No detectable association of STRADβ was observed to LKB1 in the absence of MO25, whereas co-expression of MO25α or MO25β resulted in a dose-dependent increase in STRADβ binding to LKB1 (Supplementary figure 5). However, in contrast to STRADα, the levels of STRADβ expression in 293 cells were low in the absence of MO25 isoforms and significantly increased in their presence. Similar results were obtained when STRADβ was expressed as a GST fusion protein from a distinct expression vector (data not shown).
Stabilization of the LKB1–STRAD complex by MO25 isoforms correlates with increased activity
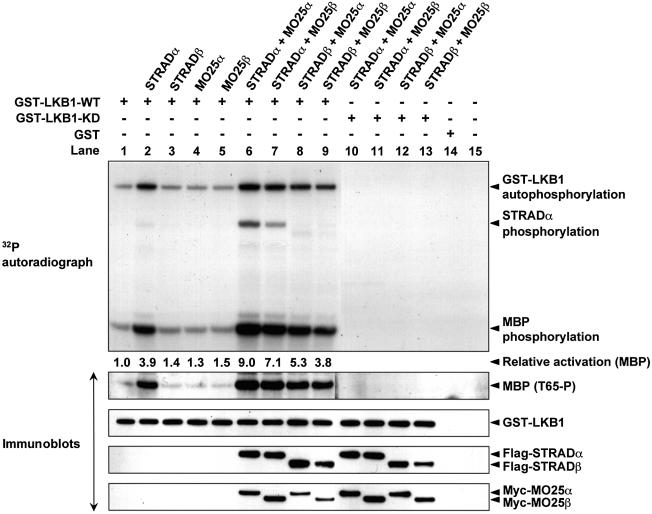
To determine how the binding of MO25α and MO25β to the LKB1–STRADα or LKB1–STRADβ complex affected LKB1 activity, 293 cells were transfected with wild-type GST–LKB1 in the presence or absence of STRAD and/or MO25 isoforms. The activity of equal amounts of affinity purified GST–LKB1 was evaluated by measuring autophosphorylation activity as well as the transphosphorylation of myelin basic protein (MBP), a non-specific exogenous substrate of LKB1, in the presence of [γ-32P]ATP. We also monitored MBP phosphorylation using a phospho-specific antibody that recognizes the major LKB1 phosphorylation site on MBP (Thr65) (Baas et al., 2003). The different LKB1 activity assays gave comparable results (Figure 9). In the absence of STRAD isoforms, LKB1 was poorly active (Figure 9, lane 1) and, as shown previously (Baas et al., 2003), co-expression with STRADα induced an ∼4-fold increase in LKB1 activity (Figure 9, lane 2). In the presence of STRADα and either MO25α or MO25β, LKB1 activity was increased a further ∼2-fold so that LKB1 became ∼9-fold more active than LKB1 expressed alone (Figure 9, lanes 6 or 7). LKB1 was only activated by STRADβ in the presence of MO25 isoforms (Figure 9, compare lane 3 with lanes 8 and 9), consistent with the observation that LKB1 is barely associated with STRADβ in the absence of MO25 (Supplementary figure 5). LKB1 was previously shown to phosphorylate STRADα (Baas et al., 2003), and we show that the MO25-mediated increase in the association of STRADα to LKB1 results in increased STRADα phosphorylation (Figure 9, compare lane 2 with lanes 6 and 7). In contrast to STRADα, STRADβ was not significantly phosphorylated by LKB1 in the presence of MO25 (Figure 9, lanes 8 and 9). LKB1 phosphorylates STRADα at Thr329 and Thr419 (Baas et al., 2003), sites that are not conserved in STRADβ (Supplementary figure 1). We also showed that catalytically inactive LKB1 is able to associate with both STRAD isoforms in the presence of either isoform of MO25 (Figure 9, lanes 10–13) consistent with the immunofluorescence results (Supplementary figure 3B). However, as expected, catalytically inactive LKB1 is unable to autophosphorylate or phosphorylate STRADα or MBP (Figure 9, lanes 10–13). Consistent with the SL26-LKB1 mutant not binding to STRADα and MO25α (Supplementary figure 4), the SL26-LKB1 mutant was not activated by co-expression with these proteins (data not shown).
Fig. 9. Activation of LKB1 by association with STRAD and MO25 isoforms. 293 cells were transfected with constructs encoding GST–LKB1 in the presence or absence of constructs encoding the indicated isoforms of STRAD and MO25. Thirty-six hours post-transfection, GST–LKB1 was affinity purified and assayed for autophosphorylation and transphosphorylation of MBP as described in the Materials and methods. The reactions were electrophoresed and the gel autoradiographed (top panel). Phosphorylation of MBP by LKB1 at Thr65 was also monitored by immunoblotting with a phosphospecific antibody (T65-P), which recognizes MBP phosphorylated by LKB1 at this residue. Each reaction was also immunoblotted with the appropriate epitope tag antibodies to monitor levels of LKB1, STRAD and MO25 isoforms. Similar results were obtained in three separate experiments.
Knockdown of MO25α destabilizes the endogenous LKB1–STRAD complex
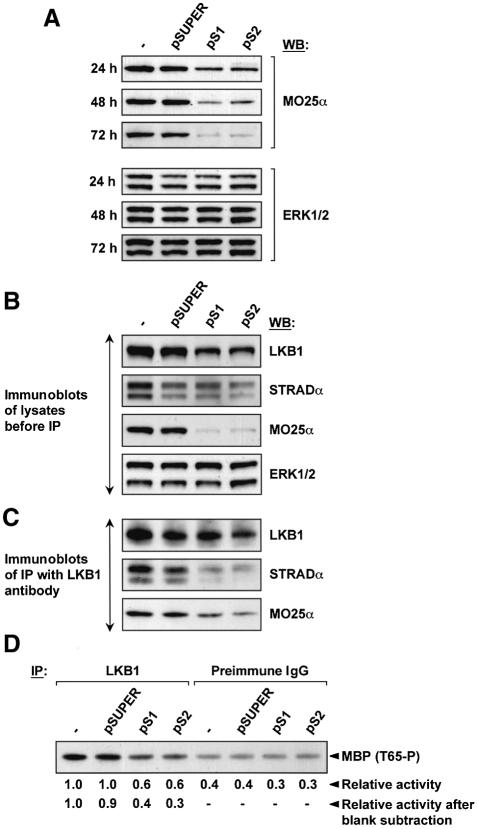
To examine the role of MO25α in stabilizing the endogenous LKB1–STRADα complex, we employed a specific small interfering RNA (siRNA) approach, by taking advantage of the pSUPER (pS) technology (Brummelkamp et al., 2002). We evaluated several siRNA sequences for their ability to reduce MO25α protein levels over a 72 h period in 293 cells. Two of these sequences (pS1 and pS2) reduced the level of endogenous MO25α by 80–90% over a 48–72 h period, without affecting levels of ERK kinases (Figure 10A). We next transfected cells for 72 h with the empty pSUPER vector or the pS1 and pS2 siRNA constructs and immunoblotted cell lysates for LKB1, STRADα and MO25α. Both the pS1 and pS2 RNAi reduced MO25α levels by ∼90%, which was accompanied by a ∼50% decrease in the levels of LKB1, whereas the levels of STRADα remained unchanged (Figure 10B). LKB1 was next immunoprecipitated from control as well as pS1 and pS2 transfected cells and the immunoprecipitates immunoblotted for the presence of LKB1, STRADα and MO25α. This revealed that although similar levels of LKB1 were immunoprecipitated from these cells, significantly reduced amounts of STRADα and MO25α were associated with LKB1 derived from the pS1 and pS2-treated cells (Figure 10C). We also assayed the activity of the immunoprecipitated endogenous LKB1 employing MBP as a substrate. To account for background non-specific levels of MBP phosphorylation, immunoprecipitations were also carried out in parallel using a pre-immune antibody. The results demonstrate that reducing MO25α levels decreased LKB1 MBP kinase activity by ∼3-fold (Figure 10D). Taken together the results from the siRNA studies support the notion that MO25α stabilizes the LKB1–STRAD complex, resulting in increased activity of LKB1.
Fig. 10. siRNA knockdown of endogenous MO25α destabilizes the LKB1–STRADα complex. (A) 293 cells were either not transfected or transfected with 8 µg of the empty pSUPER vector or the pSUPER vector encoding the pS1 or pS2 siRNA sequences. At the indicated times post-transfection, cells were lysed and 20 µg of lysate immunoblotted with the indicated antibodies. (B and C) As above except that 72 h post-transfection cells were lysed and the lysates were either immunoblotted with the indicated antibodies (B) or LKB1 was immunoprecipitated from 0.5 mg of the indicated lysates using 5 µg of anti-LKB1 antibody covalently coupled to protein G–Sepharose (C). The immunoprecipitates were immunoblotted with the indicated antibodies. (D) As above except that 72 h post-transfection cells were lysed and LKB1 immunoprecipitated from 0.5 mg of the indicated lysates using 5 µg of anti-LKB1 antibody or 5 µg of pre-immune antibody covalently coupled to protein G–Sepharose. The immunoprecipitates were assayed for transphosphorylation of MBP as described in the Materials and methods. The reactions were electrophoresed and phosphorylation of MBP by LKB1 at Thr65 was monitored by immunoblotting with a phosphospecific antibody (T65-P), which recognizes MBP phosphorylated by LKB1 at this residue. Similar results were obtained in three separate experiments.
Discussion
One of the major findings of this study is the discovery that the highly conserved MO25α protein, for which no function had been ascribed to previously, is present in a complex with STRADα and LKB1 in vivo. Interestingly, in the absence of LKB1, MO25α specifically recognizes the last three amino acids of STRADα. In this regard, MO25α functions similarly to a PDZ domain, which also recognizes the extreme C-terminal residues of its protein binding partners (Songyang et al., 1997). However, there is no homology between a PDZ domain and MO25α and, to our knowledge, other domains characterized thus far do not possess this property. The C-terminal non- pseudokinase domain ∼50 residues of STRADα and STRADβ are poorly conserved but, significantly, both terminate with the Trp-Glu-Phe sequence (Supplementary figure 1), emphasizing that STRADα and STRADβ have probably evolved to interact specifically with MO25 isoforms. The most closely related proteins to STRADα and STRADβ are the active STE20 member kinases termed SPAK (Johnston et al., 2000) and OSR1 (Tamari et al., 1999), which possess all residues required for protein kinase catalysis (Supplementary figure 1) and are therefore active enzymes. Although neither SPAK nor OSR1 terminate in a Trp-Glu-Phe sequence, both possess a Phe-Glu-Phe sequence at a similar region of their C-terminal non-catalytic domain (Supplementary figure 1). This observation prompted us to mutate the C-terminus Trp-Glu-Phe sequence of STRADα to Phe-Glu-Phe and we found that this prevented MO25α from binding to STRADα (Figure 6A). This suggests that the presence of a Trp residue three residues from the end of the protein is essential for binding of STRADα to MO25α, and that MO25α will not interact with SPAK or OSR1.
Our data indicate that one of the key roles of MO25α is to stabilize the interaction between LKB1 and STRADα. This is based on the observation that the amount of STRADα associated with LKB1 in cells is significantly enhanced by the expression of MO25α (Figure 8) and siRNA-mediated knockdown of MO25α reduces the amount of endogenous STRADα that is associated with LKB1 (Figure 10). Moreover, we provide evidence that expression of STRAD and MO25 increased levels of the LKB1 protein in cells. This conclusion is also supported by the finding that siRNA-induced knockdown of MO25α reduced LKB1 expression (Figure 10). Moreover expression of STRADα in cells increased expression of co-expressed LKB1 (Figure 3B) and conversely expression of a STRADα-Δ88 mutant that fails to bind LKB1 and MO25 resulted in a reduced expression of LKB1 (Figure 7). The finding that STRADα lacking the C-terminal Trp-Glu-Phe residues can form a complex with MO25α in the presence of LKB1 (Figure 7), indicates that the interaction of LKB1 with STRADα generates novel binding site(s) for MO25α, which could explain how MO25α stabilizes the association of LKB1 with STRADα. Our findings do not enable us to discriminate whether the additional MO25α binding site(s) are located within STRADα and/or LKB1. Our observations are consistent with the notion that MO25α or MO25β may function as a scaffolding component of the LKB1–STRAD complex.
It has previously been shown that co-expression of STRADα with LKB1 enhanced LKB1 in vitro activity (Baas et al., 2003) and here we showed that the association of LKB1–STRAD with MO25 isoforms further enhanced LKB1 activity 2-fold (Figure 9). These results indicate that stabilization of the LKB1–STRADα complex by MO25 is accompanied by activation of LKB1. Importantly, however, it should be noted that the complex of LKB1 and STRADα purified from mammalian 293 cells overexpressing tagged LKB1 and STRADα is also associated with significant levels of endogenous MO25α (Figure 4B), which could contribute to the activation of this complex.
We were able to isolate from mammalian cells a heterotrimeric complex in which LKB1, STRAD and MO25 subunits are present at a similar stoichiometry (Figure 4C), suggesting a relatively high affinity of the individual subunits for each other. We have attempted to combine Escherichia coli-expressed LKB1, STRADα and MO25α to see whether we could form a complex in which LKB1 could be activated. However, this approach was unsuccessful (J.Boudeau, data not shown), which indicate that the association of LKB1 with STRADα and MO25α is a complicated process that may require additional factors and/or that this complex may need to be assembled in vivo.
We also demonstrated that MO25α and STRADα, when expressed alone in cells, are both localized in the cytoplasm and nucleus, but they are only localized in the cytoplasm and excluded from the nucleus when expressed together (Figure 5). The molecular mechanism underlying this observation has not been investigated. It is possible that the interaction of MO25α and STRADα leads to the masking of a nuclear localization signal, exposure of a novel nuclear export signal or cytoplasmic anchoring motif, or that the STRADα–MO25α complex is too large to freely translocate into the nucleus. Under the conditions we have performed our localization studies in HeLa cells, we found that MO25α significantly cooperates with STRADα to localize LKB1 in the cytoplasm of cells, excluding it from the nucleus, as the nuclear exclusion and cytoplasmic localization of LKB1 observed following expression of LKB1 and STRADα was markedly enhanced in the presence of MO25α (Figure 5). Cytoplasmic localization of LKB1 may be important, as mutants of LKB1 that can not enter the nucleus still suppress cell growth (Tiainen et al., 2002). Additional mechanisms may also exist to maintain LKB1 in the cell cytoplasm. The interaction of LKB1 with the LIP1 protein has been shown to induce LKB1 cytoplasmic localization (Smith et al., 2001). It has yet to be tested whether LIP1 can interact with the heterotrimeric LKB1–STRAD–MO25 complex, but LIP1 has not been reported to influence LKB1 activity. LKB1 is also prenylated at its C-terminus (Collins et al., 2000; Sapkota et al., 2001), which could promote interactions with cell membranes. Recent work has indicated that prenylation of the Drosophila LKB1 homologue is important for association of LKB1 with membranes in controlling cell polarity (Martin and St Johnston, 2003).
Putative MO25 homologues are found not only in mammals, Drosophila and C.elegans but also in fission yeast (NCBI accession No. NP_594685, 51% identity to human MO25α), budding yeast (HYM1, NCBI accession No. P32464, 33% identity to human MO25α) and plant (NCBI accession No. Q9M0M4, 43% identity to human MO25α). To our knowledge, only the putative budding yeast MO25 homologue termed HYM1 has been investigated and apparently plays a key role in regulating polarized cell growth and cell separation (Dorland et al., 2000; Bidlingmaier et al., 2001; Jorgensen et al., 2002). LKB1 and STRAD homologues have not been reported in yeast and thus it is not clear whether there could be a link between LKB1 regulating polarity in C.elegans and Drosophila (Watts et al., 2000; Martin and St Johnston, 2003) and HYM1 regulating cell polarity in yeast.
It is also possible that MO25 could have functions that are independent of its ability to regulate LKB1. MO25 could interact with other proteins terminating in a similar motif to STRADα/STRADβ and there are >20 human proteins that terminate in a Trp-Glu/Asp-Phe motif. It may be interesting to investigate whether any of these bind MO25 isoforms. It is also possible that STRADα and STRADβ have functions other than regulating LKB1. In this regard, recent work has shown that overexpression of STRADβ in 293 cells protected them against apoptosis by enhancing the ability of the JNK pathway to be activated by overexpression of XIAP, an upstream component of the JNK pathway (Sanna et al., 2002). Moreover, in another study, overexpression of STRADβ (termed PAPK in this study), was reported to lead to activation of JNK and p38γ MAP kinases (Nishigaki et al., 2003). Interestingly, in this study STRADβ/PAPK immunoprecipitated from 293 cells, was shown to phosphorylate MBP albeit weakly and mutation of a conserved lysine residue (Lys89), that would be expected to abolish kinase activity only reduced MBP kinase activity by ∼30% (Nishigaki et al., 2003). Although this may indicate that STRADβ/PAPK has a very weak basal activity, an alternative explanation is that the immunoprecipitated STRADβ/PAPK was associated with LKB1, which was responsible for part or all of the phosphorylation of MBP. The mechanism by which overexpression of STRADβ/PAPK enhances JNK/p38γ activation is unknown but it would be of interest to test whether LKB1 and/or MO25 are involved in these processes.
A significant number of inherited forms of PJS found in certain families do not exhibit mutations in the LKB1 genes (Buchet-Poyau et al., 2002; Resta et al., 2002), indicating that there is a second causative locus for PJS. Genetic analysis to date has excluded LIP1 and provided no evidence that other proteins reported to interact with LKB1 are mutated in these patients (Buchet-Poyau et al., 2002). The results in this study indicate that it would be worthwhile to verify whether mutations in the genes encoding STRAD or MO25 isoforms occur in any of the PJS families that do not have mutations in the LKB1 gene. In conclusion, we define MO25 as a novel component of the LKB1 complex in vivo. Our results indicate that isoforms of MO25 function to stabilize the interaction of LKB1 with the catalytically inactive STRAD pseudokinase isoforms. This markedly enhances LKB1 activity and its cytoplasmic localization.
Materials and methods
A detailed Materials and methods section is provided in the Supplementary data available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Gursant Kular for assistance with the BiaCore analysis, Gopal Sapkota for helpful advice, John Rouse for discussion, Nicoletta Resta for the human LKB1 cDNA, Moustapha Aoubala and Jane Leitch for preparation of antibodies, the Sequencing Service (School of Life Sciences, University of Dundee, Scotland) for DNA sequencing and the Post Genomics and Molecular Interactions Centre for the BiaCore and Mass Spectrometry facilities. We thank the Association for International Cancer Research, Centre for Biomedical Genetics, Diabetes UK, the Medical Research Council and the pharmaceutical companies supporting the Division of Signal Transduction Therapy Unit in Dundee (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novo-Nordisk and Pfizer) for support.
References
- Baas A.F., Boudeau,J., Sapkota,G.P., Smit,L., Medema,R., Morrice,N.A., Alessi,D.R. and Clevers,H.C. (2003) Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J., 22, 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N., Sinha,M., Hezel,A.F., Signoretti,S., Hathaway,N.A., Sharpless,N.E., Loda,M., Carrasco,D.R. and DePinho,R.A. (2002) Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature, 419, 162–167. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S., Weiss,E.L., Seidel,C., Drubin,D.G. and Snyder,M. (2001) The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol., 21, 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Deak,M., Lawlor,M.A., Morrice,N.A. and Alessi,D.R. (2003a) Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem. J., 370, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J., Kieloch,A., Alessi,D.R., Stella,A., Guanti,G. and Resta,N. (2003b) Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in Peutz-Jeghers syndrome patients. Hum. Mutat., 21, 172. [DOI] [PubMed] [Google Scholar]
- Boudeau J., Sapkota,G. and Alessi,D.R. (2003c) LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett., 546, 159–165. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]
- Buchet-Poyau K., Mehenni,H., Radhakrishna,U. and Antonarakis,S.E. (2002) Search for the second Peutz-Jeghers syndrome locus: exclusion of the STK13, PRKCG, KLK10 and PSCD2 genes on chromosome 19 and the STK11IP gene on chromosome 2. Cytogenet. Genome Res., 97, 171–178. [DOI] [PubMed] [Google Scholar]
- Collins S.P., Reoma,J.L., Gamm,D.M. and Uhler,M.D. (2000) LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J., 345, 673–680. [PMC free article] [PubMed] [Google Scholar]
- Dorland S., Deegenaars,M.L. and Stillman,D.J. (2000) Roles for the Saccharomyces cerevisiae SDS3, CBK1 and HYM1 genes in transcriptional repression by SIN3. Genetics, 154, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A. (1999) The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell. Mol. Life Sci., 55, 735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A. et al. (1998) A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature, 391, 184–187. [DOI] [PubMed] [Google Scholar]
- Jenne D.E., Reimann,H., Nezu,J., Friedel,W., Loff,S., Jeschke,R., Muller,O., Back,W. and Zimmer,M. (1998) Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet., 18, 38–43. [DOI] [PubMed] [Google Scholar]
- Jishage K.I. et al. (2002) Role of Lkb1, the causative gene of Peutz-Jegher’s syndrome, in embryogenesis and polyposis. Proc. Natl Acad. Sci. USA, 99, 8903–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.M., Naselli,G., Gonez,L.J., Martin,R.M., Harrison,L.C. and DeAizpurua,H.J. (2000) SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene, 19, 4290–4297. [DOI] [PubMed] [Google Scholar]
- Jorgensen P., Nelson,B., Robinson,M.D., Chen,Y., Andrews,B., Tyers,M. and Boone,C. (2002) High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics, 162, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karos M. and Fischer,R. (1999) Molecular characterization of HymA, an evolutionarily highly conserved and highly expressed protein of Aspergillus nidulans. Mol. Gen. Genet., 260, 510–521. [DOI] [PubMed] [Google Scholar]
- Karuman P. et al. (2001) The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell, 7, 1307–1319. [DOI] [PubMed] [Google Scholar]
- Martin S.G. and St Johnston,D. (2003) A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature, 421, 379–384. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Matsushiro,A. and Nozaki,M. (1993) Molecular cloning of a novel mRNA sequence expressed in cleavage stage mouse embryos. Mol. Reprod. Dev., 34, 1–7. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Nakau,M., Ishikawa,T.O., Seldin,M.F., Oshima,M. and Taketo,M.M. (2002) Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res., 62, 2261–2266. [PubMed] [Google Scholar]
- Nakau M., Miyoshi,H., Seldin,M.F., Imamura,M., Oshima,M. and Taketo,M.M. (2002) Hepatocellular carcinoma caused by loss of heterozygosity in lkb1 gene knockout mice. Cancer Res., 62, 4549–4553. [PubMed] [Google Scholar]
- Nishigaki K., Thompson,D., Yugawa,T., Rulli,K., Hanson,C., Cmarik,J., Gutkind,J.S., Teramoto,H. and Ruscetti,S. (2003) Identification and characterization of a novel Ste-20/GCK-related kinase, PAP kinase (PAPK). J. Biol. Chem, 278, 13520–13530. [DOI] [PubMed] [Google Scholar]
- Nozaki M., Onishi,Y., Togashi,S. and Miyamoto,H. (1996) Molecular characterization of the Drosophila Mo25 gene, which is conserved among Drosophila, mouse and yeast. DNA Cell Biol., 15, 505–509. [DOI] [PubMed] [Google Scholar]
- Resta N. et al. (2002) Two novel mutations and a new STK11/LKB1 gene isoform in Peutz-Jeghers patients. Hum. Mutat., 20, 78–79. [DOI] [PubMed] [Google Scholar]
- Rossi D.J. et al. (2002) Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis. Proc. Natl Acad. Sci. USA, 99, 12327–12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna M.G., da Silva Correia,J., Luo,Y., Chuang,B., Paulson,L.M., Nguyen,B., Deveraux,Q.L. and Ulevitch,R.J. (2002) ILPIP, a novel anti-apoptotic protein that enhances XIAP-mediated activation of JNK1 and protection against apoptosis. J. Biol. Chem., 277, 30454–30462. [DOI] [PubMed] [Google Scholar]
- Sapkota G.P., Kieloch,A., Lizcano,J.M., Lain,S., Arthur,J.S., Williams,M.R., Morrice,N., Deak,M. and Alessi,D.R. (2001) Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys433, is essential for LKB1 to suppress cell growth. J. Biol. Chem., 276, 19469–19482. [DOI] [PubMed] [Google Scholar]
- Smith C.M., Radzio-Andzelm,E., Madhusudan, Akamine,P. and Taylor,S.S. (1999) The catalytic subunit of cAMP-dependent protein kinase: prototype for an extended network of communication. Prog. Biophys. Mol. Biol., 71, 313–341. [DOI] [PubMed] [Google Scholar]
- Smith D.P., Rayter,S.I., Niederlander,C., Spicer,J., Jones,C.M. and Ashworth,A. (2001) LIP1, a cytoplasmic protein functionally linked to the Peutz-Jeghers syndrome kinase LKB1. Hum. Mol. Genet., 10, 2869–2877. [DOI] [PubMed] [Google Scholar]
- Songyang Z. et al. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science, 275, 73–77. [DOI] [PubMed] [Google Scholar]
- Tamari M., Daigo,Y. and Nakamura,Y. (1999) Isolation and characterization of a novel serine threonine kinase gene on chromosome 3p22–21.3. J. Hum. Genet., 44, 116–120. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Ylikorkala,A. and Makela,T.P. (1999) Growth suppression by Lkb1 is mediated by a G1 cell cycle arrest. Proc. Natl Acad. Sci. USA, 96, 9248–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiainen M., Vaahtomeri,K., Ylikorkala,A. and Makela,T.P. (2002) Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum. Mol. Genet., 11, 1497–1504. [DOI] [PubMed] [Google Scholar]
- Watts J.L., Morton,D.G., Bestman,J. and Kemphues,K.J. (2000) The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development, 127, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Ylikorkala A., Rossi,D.J., Korsisaari,N., Luukko,K., Alitalo,K., Henkemeyer,M. and Makela,T.P. (2001) Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science, 293, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Yoo L.I., Chung,D.C. and Yuan,J. (2002) LKB1: a master tumour suppressor of the small intestine and beyond. Nat. Rev. Cancer, 2, 529–535. [DOI] [PubMed] [Google Scholar]