Abstract
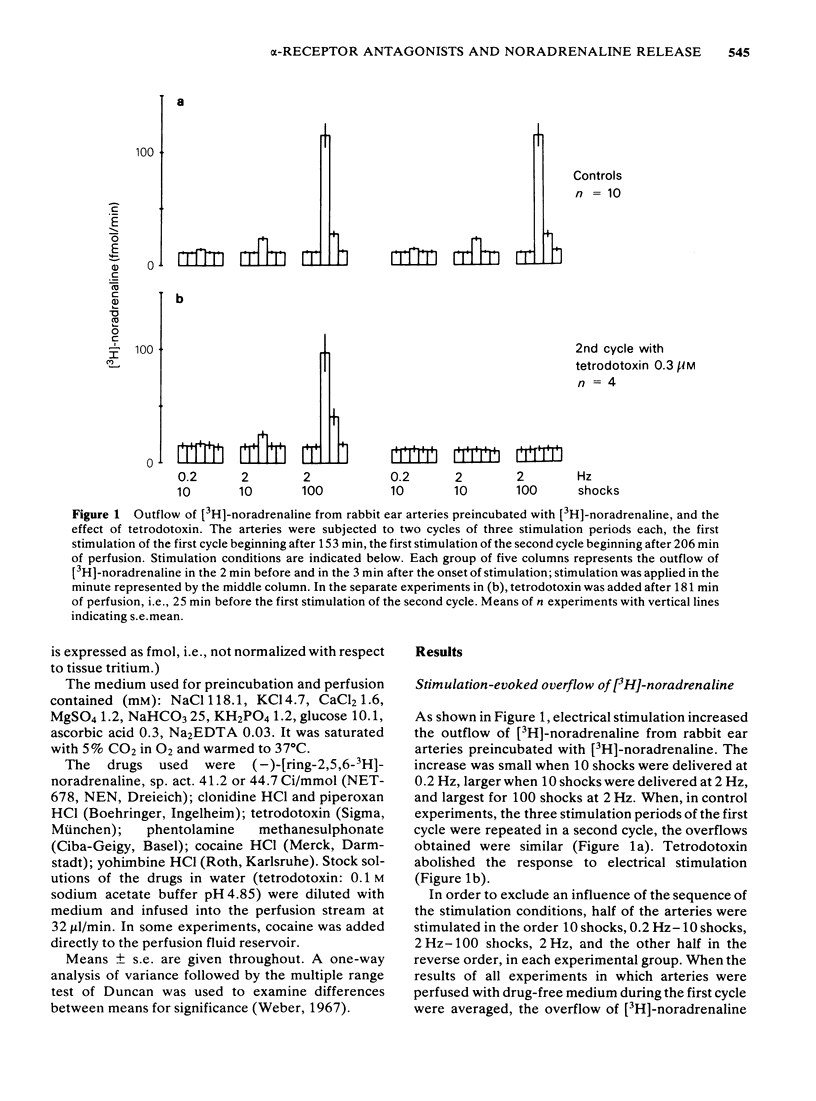
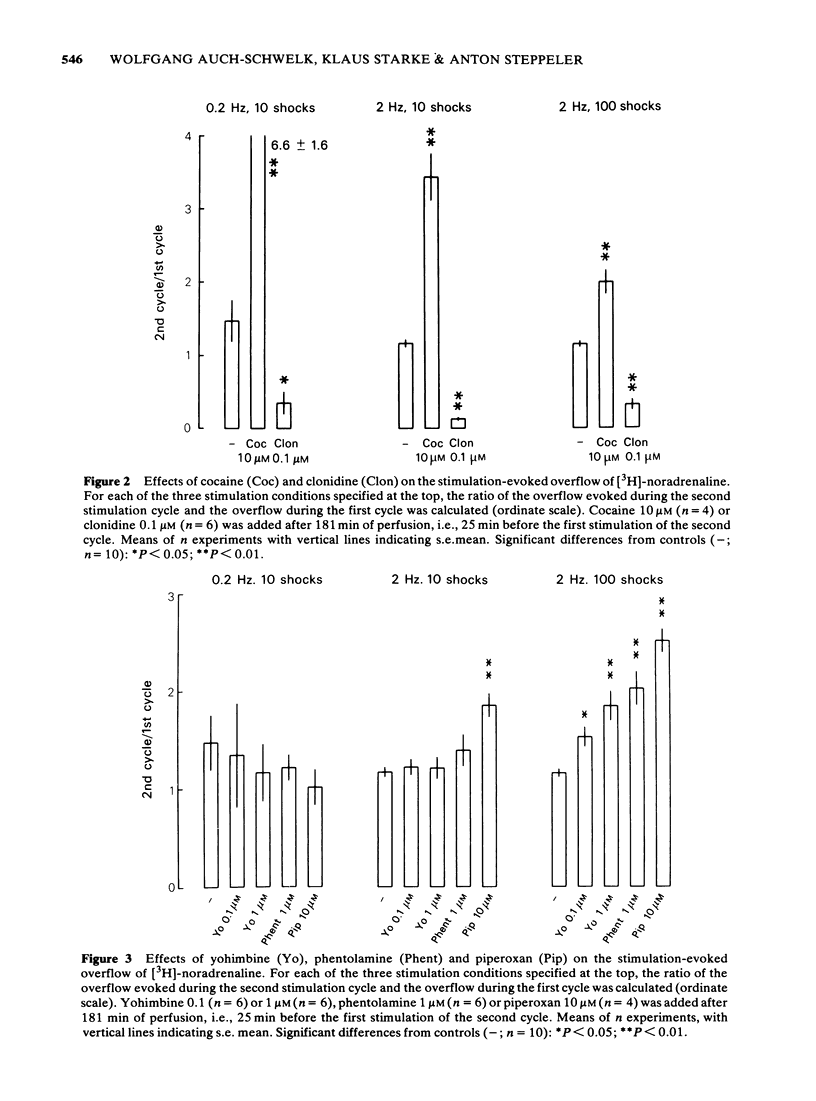
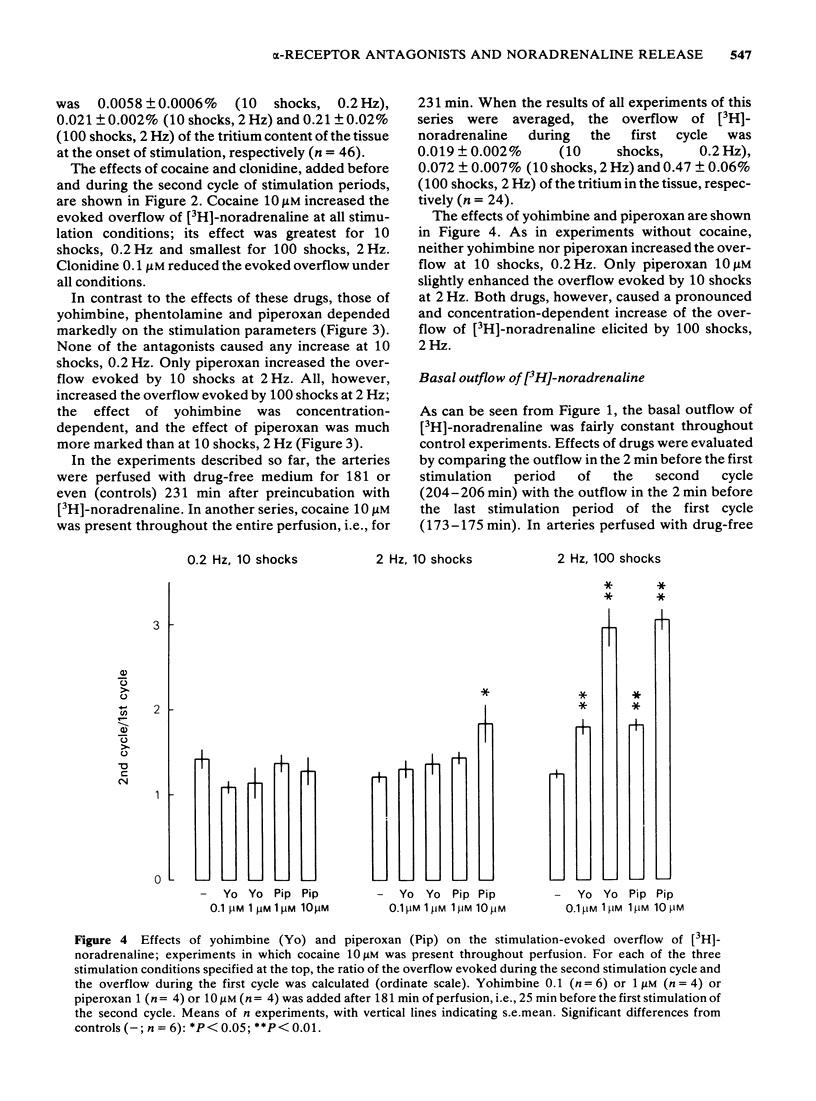
1 Segments of the rabbit ear artery were preincubated with (-)-[3H]-noradrenaline and then perfused/superfused and stimulated by transmural electrical pulses. The outflow of [3H]-noradrenaline, separated from its metabolites by column chromatography, was determined. 2 Tetrodotoxin abolished, cocaine increased, and clonidine reduced the overflow of [3H]-noradrenaline elicited by 10 shocks at 0.2 Hz, 10 shocks at 2 Hz or 100 shocks at 2 Hz. 3 The effects of yohimbine (0.1 or 1 microM), phentolamine (1 microM) and piperoxan (1 or 10 microM) depended on the stimulation conditions. No antagonist increased the overflow of [3H]-noradrenaline evoked by 10 pulses at 0.2 Hz, but all markedly increased the overflow evoked by 100 pulses at 2 Hz. Only piperoxan (10 microM) slightly enhanced the overflow at 10 pulses, 2 Hz. The effects of yohimbine and piperoxan were similar in arteries not exposed to cocaine and in those that were perfused/superfused with medium containing cocaine (10 microM) after preincubation. 4 It is concluded that yohimbine, phentolamine and piperoxan increase the release of noradrenaline only when the concentration of noradrenaline in the biophase of the ear artery is sufficiently high. The effect is, hence, an anti-noradrenaline effect and due to the blockade of presynaptic alpha-adrenoceptors. A second prerequisite for the release-enhancing effect appears to be a sufficient length of the pulse train, indicating that the alpha-adrenergic auto-inhibition develops relatively slowly.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Graschinsky E., Langer S. Z., Rubio M. C. Metabolism of norepinephrine released by phenoxybenzamine in isolated guinea-pig atria. J Pharmacol Exp Ther. 1972 Feb;180(2):286–301. [PubMed] [Google Scholar]
- Allen G. S., Rand M. J., Story D. F. Techniques for studying adrenergic transmitter release in an isolated perfused artery. Cardiovasc Res. 1973 May;7(3):423–428. doi: 10.1093/cvr/7.3.423. [DOI] [PubMed] [Google Scholar]
- Bacq Z. M. Les contröles de la libération des médiateurs aux terminaisons des nerf adrénergiques. J Physiol (Paris) 1976;72(4):371–473. [PubMed] [Google Scholar]
- Borowski E., Starke K., Ehrl H., Endo T. A comparison of pre- and postsynaptic effects of alpha-adrenolytic drugs in the pulmonary artery of the rabbit. Neuroscience. 1977;2(2):285–296. doi: 10.1016/0306-4522(77)90095-1. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Kalsner S. An examination of the negative feedback function of presynaptic adrenoceptors in a vascular tissue. Br J Pharmacol. 1979 Nov;67(3):401–407. doi: 10.1111/j.1476-5381.1979.tb08694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu L. X., Weiner N. Release of norepinephrine and dopamine-beta-hydroxylase by nerve stimulation. V. Enhanced release associated with a granular effect of a benzoquinolizine derivative with reserpine-like properties. J Pharmacol Exp Ther. 1975 Jun;193(3):757–775. [PubMed] [Google Scholar]
- Graffe K. H., Stefano F. J., Langer S. Z. Preferential metabolism of (-) 3 H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol. 1973 May 15;22(10):1147–1160. doi: 10.1016/0006-2952(73)90231-1. [DOI] [PubMed] [Google Scholar]
- Hieble J. P., Pendleton R. G. Effects of ring substitution on the pre- and postjunctional alpha-adrenergic activity of aryliminoimidazolidines. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(3):217–224. doi: 10.1007/BF00504753. [DOI] [PubMed] [Google Scholar]
- Hope W., Law M., McCulloch M. W., Rand M. J., Story D. F. Effects of some catecholamines on noradrenergic transmission in the rabbit ear artery. Clin Exp Pharmacol Physiol. 1976 Jan-Feb;3(1):15–28. [PubMed] [Google Scholar]
- Hope W., McCulloch M. W., Rand M. J., Story D. F. Modulation of noradrenergic transmission in the rabbit ear artery by dopamine. Br J Pharmacol. 1978 Dec;64(4):527–537. doi: 10.1111/j.1476-5381.1978.tb17314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Langer S. Z. Effects of phenoxybenzamine on the uptake and metabolism of noradrenaline in the rat heart and vas deferens. Br J Pharmacol. 1969 Nov;37(3):627–637. doi: 10.1111/j.1476-5381.1969.tb08501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Adrenergic presynaptic receptors: examination of a hypothesis in guinea pig vas deferens. Can J Physiol Pharmacol. 1979 Jul;57(7):717–724. doi: 10.1139/y79-108. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Limitations of presynaptic adrenoceptor theory: the characteristics of the effects of noradrenaline and phenoxybenzamine on stimulation-induced efflux of [3H]noradrenaline in vas deferens. J Pharmacol Exp Ther. 1980 Feb;212(2):232–239. [PubMed] [Google Scholar]
- Kalsner S. Single pulse stimulation of guinea-pig vas deferens and the presynaptic receptor hypothesis. Br J Pharmacol. 1979 Jun;66(2):343–349. doi: 10.1111/j.1476-5381.1979.tb13686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S., Suleiman M., Dobson R. E. Adrenergic presynaptic recpetors: an overextended hypothesis? J Pharm Pharmacol. 1980 Apr;32(4):290–292. doi: 10.1111/j.2042-7158.1980.tb12914.x. [DOI] [PubMed] [Google Scholar]
- Kalsner S. The role of calcium in the effects of noradrenaline and phenoxybenzamine on adrenergic transmitter release from atria: no support for negative feedback of release. Br J Pharmacol. 1981 Jun;73(2):363–371. doi: 10.1111/j.1476-5381.1981.tb10430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Langer S. Z. Selective metabolic pathways for noradrena-line in the peripheral and in the central nervous system. Med Biol. 1974 Dec;52(6):372–383. [PubMed] [Google Scholar]
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1981;21:7–30. doi: 10.1146/annurev.pa.21.040181.000255. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Story D. F., McCulloch M. W., Rand M. J., Standford-Starr C. A. Conditions required for the inhibitory feedback loop in noradrenergic transmission. Nature. 1981 Sep 3;293(5827):62–65. doi: 10.1038/293062a0. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Westfall T. C. Local regulation of adrenergic neurotransmission. Physiol Rev. 1977 Oct;57(4):659–728. doi: 10.1152/physrev.1977.57.4.659. [DOI] [PubMed] [Google Scholar]


