Abstract
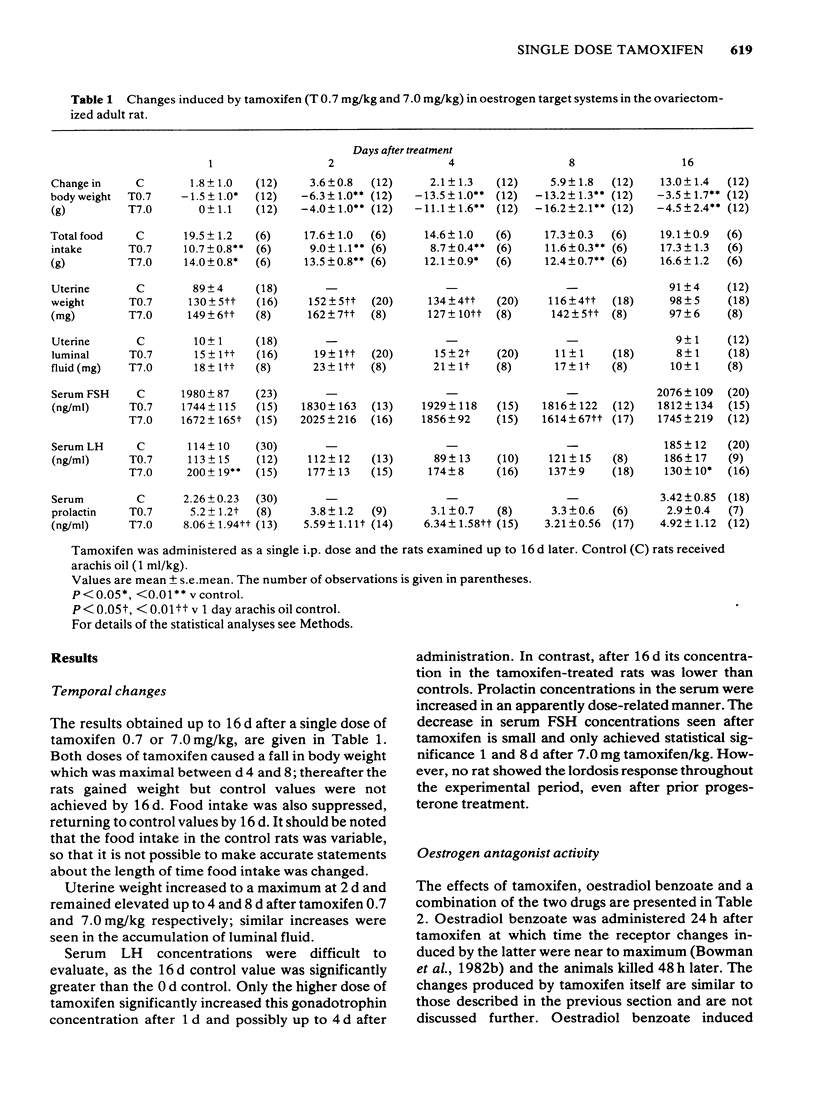
1 The peripheral and central activities of tamoxifen were studied in the ovariectomized adult rat, up to 16 d after a single dose of 7.0 or 0.7 mg/kg. 2 Food consumption and body weight were decreased; only food consumption returned to control values after 16 d. 3 The weights of the uterus and of the uterine luminal fluid were increased for up to 8 d. 4 Serum follicle-stimulating hormone (FSH) concentrations decreased, but only significantly 1 and 8 d after 7.0 mg tamoxifen/kg. Serum luteinizing hormone (LH) and prolactin concentrations were elevated for up to 4 d. 5 Lordosis behaviour was absent throughout the period studied. 6 Within 3 d of administration, tamoxifen partially antagonized the oestrogen-induced changes in uterine luminal fluid, prolactin and LH secretion and lordosis behaviour. 7 Tamoxifen did not alter oestrogen-induced changes in uterine weight, food consumption and body weight. 8 The experiments demonstrate that tamoxifen is active for up to 16 d after a single intraperitoneal dose; oestrogen agonist, partial agonist and antagonist activities were demonstrated. The duration and type of activity depends upon the dose of tamoxifen and the target tissue response examined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black L. J., Goode R. L. Uterine bioassay of tamoxifen, trioxifene and a new estrogen antagonist (LY117018) in rats and mice. Life Sci. 1980 Apr 28;26(17):1453–1458. doi: 10.1016/0024-3205(80)90049-1. [DOI] [PubMed] [Google Scholar]
- Bowman S. P., Leake A., Miller M., Morris I. D. Agonist and antagonist activity of en-clomiphene upon oestrogen-mediated events in the uterus, pituitary gland and brain of the rat. J Endocrinol. 1981 Mar;88(3):367–374. doi: 10.1677/joe.0.0880367. [DOI] [PubMed] [Google Scholar]
- Bowman S. P., Leake A., Morris I. D. Biological activity and steroid receptor interactions of cyclofenil with the oestrogen target tissues of the brain, pituitary gland and uterus of the rat. J Reprod Fertil. 1982 Jul;65(2):355–366. doi: 10.1530/jrf.0.0650355. [DOI] [PubMed] [Google Scholar]
- Bowman S. P., Leake A., Morris I. D. Hypothalamic, pituitary and uterine cytoplasmic and nuclear oestrogen receptors and their relationship to the serum concentration of tamoxifen and its metabolite, 4-hydroxytamoxifen, in the ovariectomized rat. J Endocrinol. 1982 Aug;94(2):167–175. doi: 10.1677/joe.0.0940167. [DOI] [PubMed] [Google Scholar]
- Etgen A. M. Antiestrogens: effects of tamoxifen, nafoxidine, and CI-628 on sexual behavior, cytoplasmic receptors, and nuclear binding of estrogen. Horm Behav. 1979 Oct;13(2):97–112. doi: 10.1016/0018-506x(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Heel R. C., Brogden R. N., Speight T. M., Avery G. S. Tamoxifen: a review of its pharmacological properties and therapeutic use in the treatment of breast cancer. Drugs. 1978 Jul;16(1):1–24. doi: 10.2165/00003495-197816010-00001. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Dix C. J., Rowsby L., Prestwich G. Studies on the mechanism of action of the nonsteroidal antioestrogen tamoxifen (I.C.I. 46,474) in the rat. Mol Cell Endocrinol. 1977 Apr;7(2):177–192. doi: 10.1016/0303-7207(77)90066-1. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Koerner S. Tamoxifen as an anti-tumour agent: role of oestradiol and prolactin. J Endocrinol. 1976 Feb;68(02):305–311. doi: 10.1677/joe.0.0680305. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Tsai T. S., Tatee T., Katzenellenbogen J. A. Estrogen and antiestrogen action: studies in reproductive target tissues and tumors. Adv Exp Med Biol. 1979;117:111–132. doi: 10.1007/978-1-4757-6589-2_6. [DOI] [PubMed] [Google Scholar]
- Koseki Y., Zava D. T., Chamness G. C., McGuire W. L. Estrogen receptor translocation and replenishment by the antiestrogen tamoxifen. Endocrinology. 1977 Oct;101(4):1104–1110. doi: 10.1210/endo-101-4-1104. [DOI] [PubMed] [Google Scholar]
- Labhsetwar A. P. Role of estrogens in ovulation: A study using the estrogen-antagonist, I.C.I. 46,474. Endocrinology. 1970 Sep;87(3):542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- Lunan C. B., Klopper A. Antioestrogens. A review. Clin Endocrinol (Oxf) 1975 Sep;4(5):551–572. doi: 10.1111/j.1365-2265.1975.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa H. Prolactin and human breast cancer: a review. Eur J Cancer. 1979 Mar;15(3):267–279. doi: 10.1016/0014-2964(79)90037-9. [DOI] [PubMed] [Google Scholar]
- Nagy I., Valdenegro C. A., MacLeod R. M. Effect of antiestrogens on pituitary prolactin production in normal and pituitary tumor-bearing rats. Neuroendocrinology. 1980 Jun;30(6):389–395. doi: 10.1159/000123032. [DOI] [PubMed] [Google Scholar]
- Nicholson R. I., Golder M. P. The effect of synthetic anti-oestrogens on the growth and biochemistry of rat mammary tumours. Eur J Cancer. 1975 Aug;11(8):571–579. doi: 10.1016/0014-2964(75)90129-2. [DOI] [PubMed] [Google Scholar]
- Nisker J. A., Siiteri P. K. Estrogens and breast cancer. Clin Obstet Gynecol. 1981 Mar;24(1):301–322. doi: 10.1097/00003081-198103000-00025. [DOI] [PubMed] [Google Scholar]
- Wade G. N., Blaustein J. D. Effects of an anti-estrogen on neural estradiol binding and on behaviors in female rats. Endocrinology. 1978 Jan;102(1):245–251. doi: 10.1210/endo-102-1-245. [DOI] [PubMed] [Google Scholar]


