Abstract
1 [3H]-amezinium is taken up selectively into noradrenergic axons and their transmitter-storing vesicles and is released from these axons by action potentials. We used it as a non-α-adrenergic marker in order to study the α-adrenergic autoinhibition of noradrenaline release.
2 Rat occipitocortical slices were preincubated with [3H]-amezinium 0.03 μM and then superfused and stimulated electrically (3 Hz for 3 min). The stimulation-evoked overflow of tritium was measured in six groups of slices: from saline-pretreated rats; from saline-pretreated rats, the slices being exposed to exogenous noradrenaline before preincubation with [3H]-amezinium; from saline-treated rats, slices from which were exposed simultaneously to noradrenaline and cocaine before preincubation with [3H]-amezinium; from rats in which noradrenaline stores had been depleted by pretreatment with α-methyltyrosine (α-MT); from α-MT-treated rats, the slices being exposed to noradrenaline before preincubation with [3H]-amezinium; and from α-MT-treated rats, slices from which were exposed to noradrenaline plus cocaine before preincubation with [3H]-amezinium.
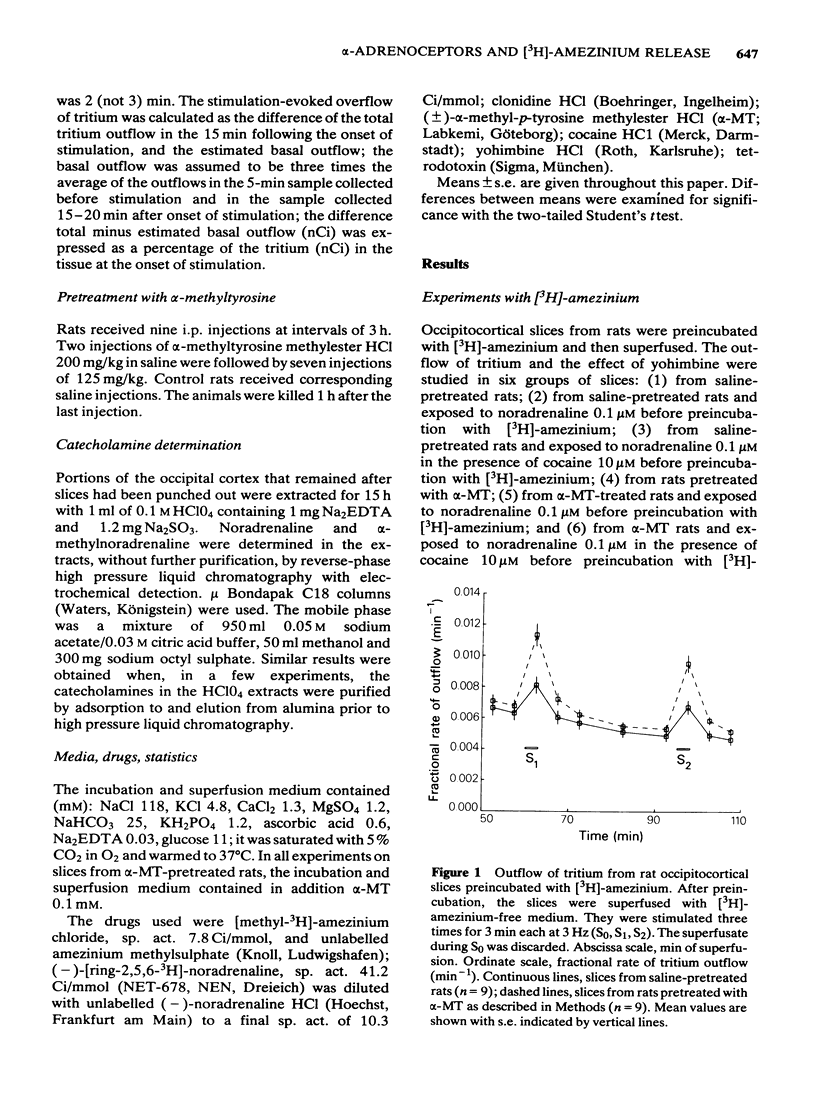
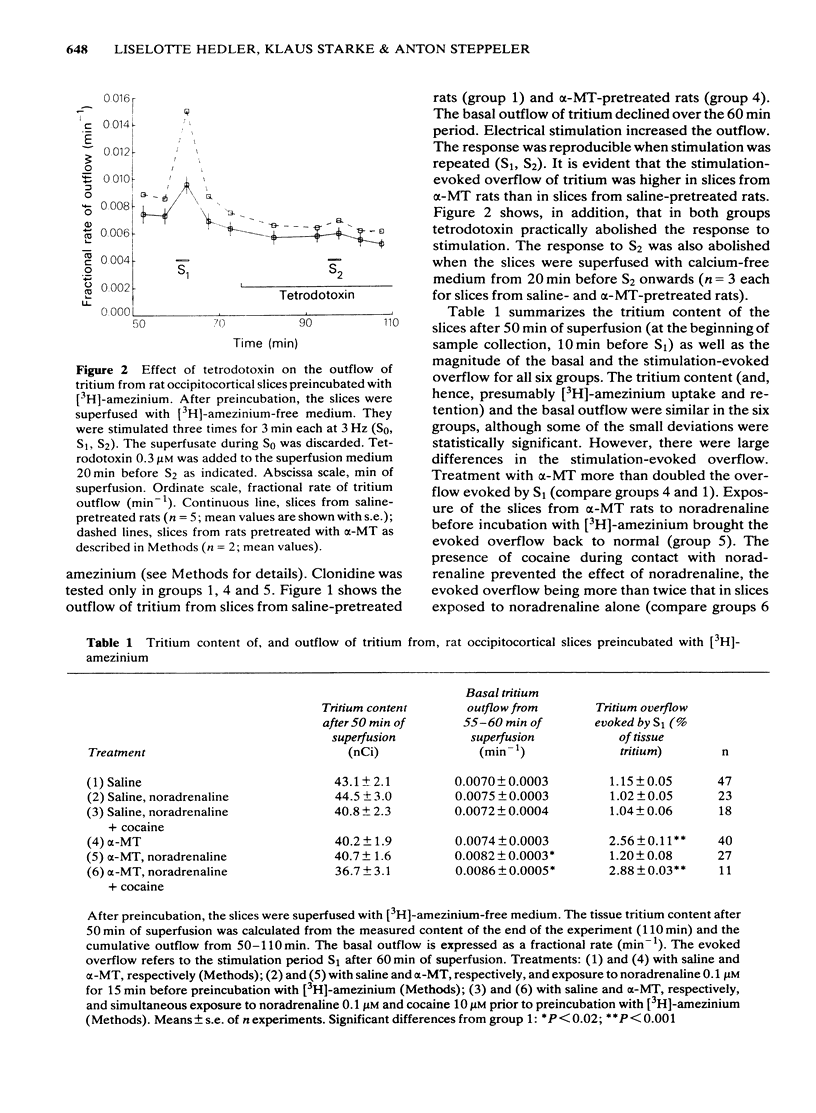
3 The stimulation-evoked overflow of tritium, expressed as a percentage of the tritium content of the tissue, was 1.15% in slices from saline-pretreated rats, and was similar in slices from saline-pretreated rats after exposure to noradrenaline or noradrenaline plus cocaine. It was 2.56% in slices from α-MT-treated rats, 1.20% from α-MT-treated rats after exposure to noradrenaline, and 2.88% from α-MT-treated rats after exposure to noradrenaline plus cocaine.
4 Yohimbine 0.1 and 1 μM increased the stimulation-evoked overflow of tritium in slices from all groups of saline-pretreated rats and in those slices from α-MT rats that had been in contact with exogenous noradrenaline. Yohimbine did not change the evoked overflow in slices from α-MT rats that had not been exposed to noradrenaline, or had been exposed to noradrenaline plus cocaine.
5 Clonidine 0.01-1 μM decreased the stimulation-evoked overflow of tritium moderately in slices from saline-pretreated rats, markedly in slices from α-MT-treated rats, and moderately again when the latter slices had been exposed to noradrenaline.
6 It is concluded that the action potential-evoked release of [3H]-amezinium as well as the modulation of this release by yohimbine and clonidine depend on the presence or absence of α-adrenergic autoinhibition caused by the co-secretion of noradrenaline. When there is co-secretion of noradrenaline, the evoked release of [3H]-amezinium is relatively small, yohimbine increases the release, and clonidine can cause only moderate inhibition. When there is no or very little co-secretion of noradrenaline, the evoked release of [3H]-amezinium is at least doubled, yohimbine causes no further increase and clonidine produces strong inhibition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auch-Schwelk W., Starke K., Steppeler A. Experimental conditions required for the enhancement by alpha-adrenoceptor antagonists of noradrenaline release in the rabbit ear artery. Br J Pharmacol. 1983 Mar;78(3):543–551. doi: 10.1111/j.1476-5381.1983.tb08814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacq Z. M. Les contröles de la libération des médiateurs aux terminaisons des nerf adrénergiques. J Physiol (Paris) 1976;72(4):371–473. [PubMed] [Google Scholar]
- Borowski E., Starke K., Ehrl H., Endo T. A comparison of pre- and postsynaptic effects of alpha-adrenolytic drugs in the pulmonary artery of the rabbit. Neuroscience. 1977;2(2):285–296. doi: 10.1016/0306-4522(77)90095-1. [DOI] [PubMed] [Google Scholar]
- Cubeddu L., Weiner N. Nerve stimulation-meditated overflow of norepinephrine and dopamine-beta-hydroxylase. III. Effects of norepinephrine depletion on the alpha presynaptic regulation of release. J Pharmacol Exp Ther. 1975 Jan;192(1):1–14. [PubMed] [Google Scholar]
- Dixon W. R., Mosimann W. F., Weiner N. The role of presynatpic feedback mechanisms in regulation of norepinephrine release by nerve stimulation. J Pharmacol Exp Ther. 1979 May;209(2):196–204. [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z. Influence of reserpine-induced depletion of noradrenaline on the negative feed-back mechanism for transmitter release during nerve stimulation. Br J Pharmacol. 1973 Oct;49(2):214–225. doi: 10.1111/j.1476-5381.1973.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B., Jonsson G. Release of ( 3 H)noradrenaline and ( 3 H)dopamine from field stimulated cerebral cortex slices. Effect of tyrosine hydroxylase and dopamine- -hydroxylase inhibition. J Neurochem. 1971 Dec;18(12):2491–2500. doi: 10.1111/j.1471-4159.1971.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Göthert M., Huth H., Schlicker E. Characterization of the receptor subtype involved in alpha-adrenoceptor-mediated modulation of serotonin release from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1981 Nov;317(3):199–203. doi: 10.1007/BF00503816. [DOI] [PubMed] [Google Scholar]
- Hedler L., Stamm G., Weitzell R., Starke K. Functional characterization of central alpha-adrenoceptors by yohimbine diastereomers. Eur J Pharmacol. 1981 Mar 5;70(1):43–52. doi: 10.1016/0014-2999(81)90430-1. [DOI] [PubMed] [Google Scholar]
- Kalsner S. The role of calcium in the effects of noradrenaline and phenoxybenzamine on adrenergic transmitter release from atria: no support for negative feedback of release. Br J Pharmacol. 1981 Jun;73(2):363–371. doi: 10.1111/j.1476-5381.1981.tb10430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Lenke D., Gries J., Kretzschmar R. Pharmacology of amezinium, a novel antihypotensive drug. III. Studies on the mechanism of action. Arzneimittelforschung. 1981;31(9A):1558–1565. [PubMed] [Google Scholar]
- Reichenbacher D., Reimann W., Starke K. alpha-Adrenoceptor-mediated inhibition of noradrenaline release in rabbit brain cortex slices. Receptor properties and role of the biophase concentration of noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):71–77. doi: 10.1007/BF00491481. [DOI] [PubMed] [Google Scholar]
- Reimann W., Steinhauer H. B., Hedler L., Starke K., Hertting G. Effect of prostaglandins D2, E2 and F2alpha on catecholamine release from slices of rat and rabbit brain. Eur J Pharmacol. 1981 Feb 19;69(4):421–427. doi: 10.1016/0014-2999(81)90445-3. [DOI] [PubMed] [Google Scholar]
- Starke K. Alpha sympathomimetic inhibition of adrenergic and cholinergic transmission in the rabbit heart. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):18–45. doi: 10.1007/BF00501004. [DOI] [PubMed] [Google Scholar]
- Starke K., Altmann K. P. Inhibition of adrenergic neurotransmission by clonidine: an action on prejunctional -receptors. Neuropharmacology. 1973 Apr;12(4):339–347. doi: 10.1016/0028-3908(73)90093-2. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H., Gayk W., Merker R. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Alpha-sympathomimetic inhibition of Neurogenic vasoconstriction. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):133–150. doi: 10.1007/BF00501149. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Starke K., Wagner J., Schümann H. J. Adrenergic neuron blockade by clonidine: comparison with guanethidine and local anesthetics. Arch Int Pharmacodyn Ther. 1972 Feb;195(2):291–308. [PubMed] [Google Scholar]
- Steppeler A., Döring C., Hedler L., Starke K. Effect of amezinium on the release and catabolism of 3H-monoamines in brain slices. Biochem Pharmacol. 1982 Jul 15;31(14):2395–2402. doi: 10.1016/0006-2952(82)90535-4. [DOI] [PubMed] [Google Scholar]
- Steppeler A., Pfändler R., Hedler L., Starke K. An analysis of the effects of amezinium on postganglionic sympathetic neurones. Naunyn Schmiedebergs Arch Pharmacol. 1980 Oct;314(1):1–11. doi: 10.1007/BF00498425. [DOI] [PubMed] [Google Scholar]
- Steppeler A., Starke K. Fate of [3H]amezinium in sympathetically innervated rabbit tissues. Biochem Pharmacol. 1982 Mar 15;31(6):1075–1080. doi: 10.1016/0006-2952(82)90345-8. [DOI] [PubMed] [Google Scholar]
- Steppeler A., Starke K. Selective inhibition by amezinium of intraneuronal monoamine oxidase. Naunyn Schmiedebergs Arch Pharmacol. 1980 Oct;314(1):13–16. doi: 10.1007/BF00498426. [DOI] [PubMed] [Google Scholar]
- Traut M., Brode E., Hoffmann H. D. Pharmacology of amezinium, a novel antihypotensive drug. IV. Biochemical investigations of the mechanism of action. Arzneimittelforschung. 1981;31(9A):1566–1574. [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Somogyi G. T., Hadházy P., Knoll J. Effect of duration and frequency of stimulation on the presynaptic inhibition by alpha-adrenoceptor stimulation of the adrenergic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(1):79–91. doi: 10.1007/BF00505357. [DOI] [PubMed] [Google Scholar]
- Wemer J., Frankhuyzen A. L., Mulder A. H. Pharmacological characterization of presynaptic alpha-adrenoceptors in the nucleus tractus solitarii and the cerebral cortex of the rat. Neuropharmacology. 1982 Jun;21(6):499–506. doi: 10.1016/0028-3908(82)90039-9. [DOI] [PubMed] [Google Scholar]
- Westfall T. C. Local regulation of adrenergic neurotransmission. Physiol Rev. 1977 Oct;57(4):659–728. doi: 10.1152/physrev.1977.57.4.659. [DOI] [PubMed] [Google Scholar]


