Abstract
1 Three drugs have been tested for activity against antigen-induced histamine release from passively sensitized human lung fragments after increasing periods of pre-incubation before challenge.
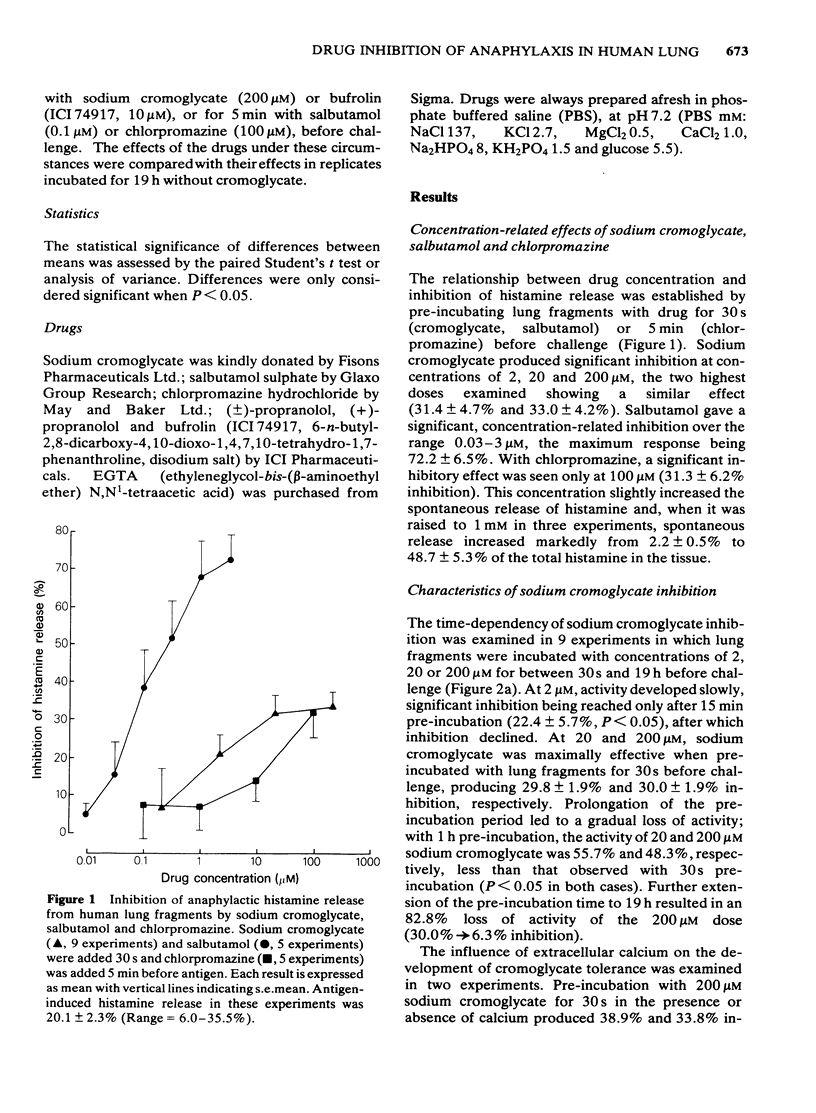
2 After 30 s pre-incubation, sodium cromoglycate inhibited histamine release in the concentration range 0.2-200 μM, producing a maximum inhibition of 33.0%. As the pretreatment period was extended, tolerance developed in a dose-related manner, resulting in a 48.3% and 82.8% loss of activity of the 200 μM dose after 60 min and 19 h pre-incubation, respectively. Tolerance was independent of extracellular calcium and was poorly reversible. Lung tissue desensitized to cromoglycate was cross-tolerant to the related drug, bufrolin, but not to salbutamol or chlorpromazine.
3 In acute studies, salbutamol (0.03-3.0 μM) produced dose-related inhibition of histamine release, with a maximum inhibition of 72.2%. The effect was blocked stereoselectively by 1 μM propranolol, suggesting that it occurred through an interaction with lung β-adrenoceptors. Increasing the pre-incubation time with salbutamol from 30 s to 19 h did not produce tolerance. Inhibition produced by incubation with salbutamol for 19 h was totally prevented when propranolol was added at the beginning of the pre-incubation period, indicating that it resulted from stimulation of β-receptors and not from a non-specific or toxic effect. However, studies of reversibility of effect through washing or late addition of propranolol did indicate some change in the nature of salbutamol inhibition with time.
4 Chlorpromazine was a weak inhibitor of immunological histamine release. A 100 μM concentration was ineffective after 30 s pre-incubation but its activity increased with time. Pre-incubation of lung fragments with this concentration for 1 h or longer, or with a 1 mM dose for a shorter period, provoked histamine release in the absence of antigen. Effects of chlorpromazine were not reversed by washing.
5 The different characteristics shown by sodium cromoglycate, salbutamol and chlorpromazine indicate that these drugs inhibit histamine release by interfering with the secretory mechanisms in different ways.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Schild H. O. Inhibition by sympathomimetic amines of histamine release by antigen in passively sensitized human lung. Nature. 1969 Dec 6;224(5223):1028–1029. doi: 10.1038/2241028a0. [DOI] [PubMed] [Google Scholar]
- Butchers P. R., Fullarton J. R., Skidmore I. F., Thompson L. E., Vardey C. J., Wheeldon A. A comparison of the anti-anaphylactic activities of salbutamol and disodium cromoglycate in the rat, the rat mast cell and in human lung tissue. Br J Pharmacol. 1979 Sep;67(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Butchers P. R., Skidmore I. F., Vardey C. J., Wheeldon A. Characterization of the receptor mediating the antianaphylactic effects of beta-adrenoceptor agonists in human lung tissue in vitro. Br J Pharmacol. 1980;71(2):663–667. doi: 10.1111/j.1476-5381.1980.tb10987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Lewis R. A., Hein A., Austen K. F. Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol. 1980 May;85(2):299–312. doi: 10.1083/jcb.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. K., Gradidge C. F. Inhibition of histamine release from human lung in vitro by antihistamines and related drugs. Br J Pharmacol. 1980 Aug;69(4):663–667. doi: 10.1111/j.1476-5381.1980.tb07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. K., Gradidge C. F. The activity of sodium cromoglycate analogues in human lung in vitro: a comparison with rat passive cutaneous anaphylaxis and clinical efficacy. Br J Pharmacol. 1980 Oct;70(2):307–311. doi: 10.1111/j.1476-5381.1980.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Conolly M. E. Tachyphylaxis to beta-adrenoceptor agonists in human bronchial smooth muscle: studies in vitro. Br J Clin Pharmacol. 1980 Nov;10(5):417–423. doi: 10.1111/j.1365-2125.1980.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk-Holmberg M. Drug-induced changes in the release and uptake of biogenic amines. A study on mast cells. Acta Physiol Scand Suppl. 1972;376:1–36. doi: 10.1111/j.1748-1716.1972.tb05299.x. [DOI] [PubMed] [Google Scholar]
- Grönneberg R., Strandberg K. Effect of terbutaline on cutaneous responses in man to rechallenge with allergen and compound 48/80. Allergy. 1981 Jan;36(1):33–38. doi: 10.1111/j.1398-9995.1981.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Cotton C. U., Waldo G. L., Lutton J. K., Perkins J. P. Catecholamine-induced alteration in sedimentation behavior of membrane bound beta-adrenergic receptors. Science. 1980 Oct;210(4468):441–443. doi: 10.1126/science.6254143. [DOI] [PubMed] [Google Scholar]
- Harvey J. E., Tattersfield A. E. Airway response to salbutamol: effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax. 1982 Apr;37(4):280–287. doi: 10.1136/thx.37.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Baldwin C. J., Tattersfield A. E. beta-adrenergic agonist resistance in normal human airways. Lancet. 1977 Aug 20;2(8034):375–377. doi: 10.1016/s0140-6736(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Howe R., Shanks R. G. Optical isomers of propranolol. Nature. 1966 Jun 25;210(5043):1336–1338. doi: 10.1038/2101336a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka T. The Robert A. Cooke memorial lecture. Analysis of triggering events in mast cells for immunoglobulin E-mediated histamine release. J Allergy Clin Immunol. 1981 Feb;67(2):90–96. doi: 10.1016/0091-6749(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Moran N. C. Inhibition of the release of histamine from rat mast cells: the effect of cold and adrenergic drugs on release of histamine by compound 48-80 and antigen. J Pharmacol Exp Ther. 1970 Dec;175(3):632–640. [PubMed] [Google Scholar]
- Kusner E. J., Dubnick B., Herzig D. J. The inhibition by disodium cromoglycate in vitro of anaphylactically induced histamine release from rat peritoneal mast cells. J Pharmacol Exp Ther. 1973 Jan;184(1):41–46. [PubMed] [Google Scholar]
- MOTA I., da SILVA W. The anti-anaphylactic and histamine-releasing properties of the antihistamines. Their effect on the mast cells. Br J Pharmacol Chemother. 1960 Sep;15:396–404. doi: 10.1111/j.1476-5381.1960.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. W., Thomson D. S., Evans D. P. The mechansim of tachyphylaxis to ICI 74,917 and disodium cromoglycate. Int Arch Allergy Appl Immunol. 1976;51(3):274–283. doi: 10.1159/000231602. [DOI] [PubMed] [Google Scholar]
- Morr H. Effect of disodium cromoglycate (Intal) on antigen-induced histamine release from passively sensitized human lung in vitro. Lung. 1978;155(1):33–38. doi: 10.1007/BF02730678. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr T. S. Recent developments concerning the mast cell and the mode of action of disodium cromoglycate. Acta Allergol. 1975;30 Suppl 12:13–32. doi: 10.1111/j.1398-9995.1975.tb02305.x. [DOI] [PubMed] [Google Scholar]
- Paterson J. W., Woolcock A. J., Shenfield G. M. Bronchodilator drugs. Am Rev Respir Dis. 1979 Nov;120(5):1149–1188. doi: 10.1164/arrd.1979.120.5.1149. [DOI] [PubMed] [Google Scholar]
- Rosen A., Tham S. Y. The binding of chlorpromazine to some fractions of homogenized rat brain. J Pharm Pharmacol. 1980 May;32(5):357–359. doi: 10.1111/j.2042-7158.1980.tb12936.x. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sharpe T. J., Ross J. W., Spicer B. A. The effect of sodium 5,6-dimethyl-2-nitroindanedione on anaphylactic reactions in vitro. Agents Actions. 1978 Apr;8(3):199–202. doi: 10.1007/BF01966603. [DOI] [PubMed] [Google Scholar]
- Sheard P., Blair A. M. Disodium cromoglycate. Activity in three in vitro models of the immediate hypersensitivity reaction in lung. Int Arch Allergy Appl Immunol. 1970;38(2):217–224. [PubMed] [Google Scholar]
- Sung C. P., Saunders H. L., Krell R. D., Chakrin L. W. Studies on the mechanism of tachyphylaxis to disodium cromoglycate. Int Arch Allergy Appl Immunol. 1977;55(1-6):374–384. doi: 10.1159/000231948. [DOI] [PubMed] [Google Scholar]
- Sung C. P., Saunders H. L., Lenhardt E., Chakrin L. W. Further studies on the tachyphylaxis to DSCG. The effects of concentration and temperature. Int Arch Allergy Appl Immunol. 1977;55(1-6):385–394. doi: 10.1159/000231949. [DOI] [PubMed] [Google Scholar]
- Taylor W. A., Francis D. H., Sheldon D., Roitt I. M. Anti-allergic actions of disodium cromoglycate and other drugs known to inhibit cyclic 3',5'-nucleotide phosphodiesterase. Int Arch Allergy Appl Immunol. 1974;47(2):175–193. doi: 10.1159/000231212. [DOI] [PubMed] [Google Scholar]
- Taylor W. A., Sheldon D. Effect of lung tissue on the inhibitory actions of isoprenaline, theophylline, disodium cromoglycate and PGE1 on antigen-induced mast cell degranulation. Int Arch Allergy Appl Immunol. 1977;54(4):322–328. doi: 10.1159/000231844. [DOI] [PubMed] [Google Scholar]
- Thomson D. S., Evans D. P. Inhibition of immediate hypersensitivity reactions by disodium cromoglycate. Clin Exp Immunol. 1973 Apr;13(4):537–544. [PMC free article] [PubMed] [Google Scholar]


