Abstract
Transglutaminase 2 (TGase 2) is one of a family of enzymes that catalyze protein modification through the incorporation of polyamines into substrates or the formation of protein crosslinks. However, the physiological roles of TGase 2 are largely unknown. To elucidate the functions of TGase 2, we have searched for its interacting proteins. Here we show that TGase 2 interacts with E7 oncoprotein of human papillomavirus type 18 (HPV18) in vitro and in vivo. TGase 2 incorporates polyamines into a conserved glutamine residue in the zinc-binding domain of HPV18 E7 protein. This modification mediates the inhibition of E7’s Rb binding ability. In contrast, TGase 2 does not affect HPV16 E7, due to absence of a glutamine residue at this polyamination site. Using E7 mutants, we demonstrate that TGase 2-dependent inhibition of HPV E7 function correlates with the presence of the polyamination site. Our results indicate that TGase 2 is an important cellular interfering factor and define a novel host–virus interaction, suggesting that the inability of TGase 2 to inactivate HPV16 E7 could explain the high prevalence of HPV16 in cervical cancer.
Keywords: host–virus interaction/human papillomavirus E7 oncoprotein/polyamination/transglutaminase 2
Introduction
Transglutaminases (TGases) are a family of calcium-dependent enzymes that undertake the post-translational modification of proteins by catalyzing the acyl transfer reaction between the γ-carboxamide group of a peptide-bound glutamine residue and the ε-amino group of a lysine residue in a polypeptide chain or the primary amine group of a polyamine (Folk, 1980). Eight distinct TGases, referred to as TGase 1–7 and coagulation factor XIIIa, that share a common catalytic mechanism and protein structure (Liu et al., 2002) have been identified previously in human (Grenard et al., 2001). The transamidation activity of TGases, resulting in protein crosslinking or polyamine incorporation, plays essential roles in blood clot formation, wound healing, semen coagulation, apoptosis and terminal differentiation of epidermal keratinocyte (Lorand and Graham, 2003), thereby providing protective structures for tissues or organs. TGase 2 is a unique enzyme among the TGase family with respect to its tissue distribution and enzymatic properties. TGase 2 is widely expressed throughout all human tissues and is localized in cytosol, plasma membrane, nucleus and extracellular space (Fesus and Piacentini, 2002). More over, TGase 2 serves both as a crosslinking enzyme and as a GTP binding/hydrolyzing enzyme that mediates intracellular signaling by the α1-adrenergic receptor (Feng et al., 1996). However, the identification of specific roles of TGase 2 is complicated by the expression of multiple TGases in human tissues, and by the lack of substrates with high specificity for TGase 2 (Grootjans et al., 1995). Furthermore, recent studies revealed that TGase 2-deficient mice showed no developmental abnormalities and displayed only minor apparent phenotypic changes (De Laurenzi and Melino, 2001; Nanda et al., 2001).
In contrast to other TGases, TGase 2 can translocate into the nucleus in response to the elevation of intracellular calcium levels (Lesort et al., 1998) by interacting with importin-α3 (Peng et al., 1999). The nuclear TGase 2 modifies core histones (Ballestar et al., 1996), and polyaminates retinoblastoma (Rb) protein to protect it from caspase-mediated degradation (Boehm et al., 2002). These studies suggest that the modification of nuclear proteins by TGase 2 may account for the physiological roles distinct from those of other TGases in the regulation of gene expression or cell cycle. To elucidate the functions of TGase 2 in the nucleus, we analyzed the nuclear proteins that interact with the C-terminus of TGase 2. Alignment of different TGases shows that the amino acid sequences comprising the C-terminal barrel domains are the most divergent (Liu et al., 2002). Compared with other TGases, TGase 2 has a unique C-terminal region, which is critical for nuclear localization (Peng et al., 1999), GTP binding (Liu et al., 2002), and the recognition and stimulation of PLCδ1 (Baek et al., 2001).
Human papillomaviruses (HPVs) are species-specific DNA viruses that infect only epidermal and mucosal keratinocytes. Of more than 120 HPV types, high-risk types, such as type 16, 18, 31 and 45, are known to be responsible for the development of cervical cancer (Clifford et al., 2003). Among the high-risk-type HPVs, HPV16 is the most prevalent type in cervical cancer. HPV induces cervical cancer through the expression of two viral oncoproteins, E6 and E7. While the E6 binds and degrades p53 by the ubiquitin-dependent proteolysis pathway, the E7 binds and inactivates Rb by causing the release of active E2F (Munger and Howley, 2002). In this report, we show that HPV E7 is a TGase 2-interacting protein. Our results demonstrate that TGase 2 inhibits the function of HPV18 E7, but not that of HPV16 E7, by incorporating polyamines. The specificity of this enzymatic activity is ascribed to sequence variation at a polyamination site. These data suggest a potential role of TGase 2 as a cellular interfering factor.
Results
Identification of HPV E7 as a TGase 2-interacting protein
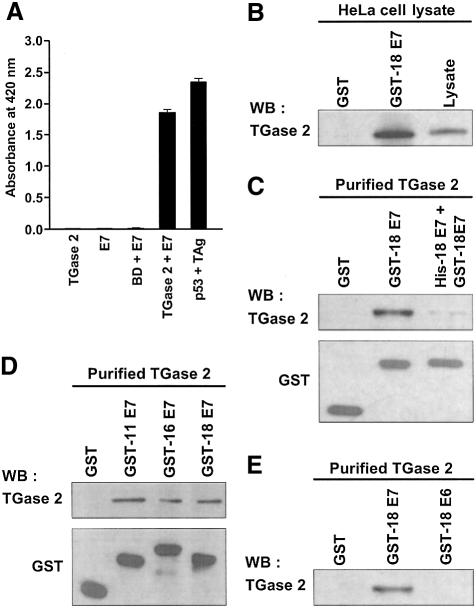
In an attempt to identify the proteins that interact with TGase 2, we performed a yeast two-hybrid assay. HeLa cell cDNA library was screened using the C-terminal barrel 1 and 2 domains of human TGase 2 (amino acids 472–687) as bait. BLAST search of the partial sequences from four clones revealed a 100% match to HPV18 E7. In the liquid culture β-galactosidase assays, only co-transformants carrying both TGase 2 and HPV18 E7 showed significant β-galactosidase activity (Figure 1A). To verify the specificity of yeast two-hybrid results, we performed in vitro pull-down experiments. When added to HeLa cell lysates, glutathione S-transferase (GST)–HPV18 E7 fusion protein was efficiently associated with endogenous TGase 2 (Figure 1B). We have demonstrated using purified TGase 2 that the association of HPV18 E7 with TGase 2 is a consequence of direct interaction between the two proteins by a competitive inhibition test. The interaction could be effectively inhibited by allowing GST–HPV18 E7 to compete with His-tagged HPV18 E7 (Figure 1C).
Fig. 1. TGase 2 interacts with HPV E7. (A) Interaction of TGase 2 with HPV18 E7 in the yeast two-hybrid assay. SFY526 yeast cells were transformed with the indicated plasmids. Using ONPG as a β-galactosidase substrate, liquid culture reporter assay was performed to measure the color development at 420 nm. p53 and SV40 TAg were used as positive controls for the yeast two-hybrid assay. BD, Gal4 DNA binding domain. The graph shows the mean values and SD based on three independent experiments. (B) Pull-down assay with GST–HPV18 E7. HeLa cell lysates were incubated with GST or GST–HPV18 E7 pre-bound to glutathione–Sepharose beads. Precipitated protein complexes were analyzed by western blotting using anti-TGase 2 antibody. HeLa cell lysate was used as a positive control for the presence of TGase 2. WB, western blot. (C) His-tagged HPV18 E7 was used as a competitor for GST–HPV18 E7 to verify the specific interaction between TGase 2 and HPV18 E7. (D) GST–E7s of high-risk HPV (type 16 and 18) and low-risk HPV (type 11) were used to test HPV-type specificity. (E) GST–HPV18 E6 was used to test the interaction with TGase 2. Protein complexes of purified TGase 2 and GST–HPV E7s were precipitated with glutathione–Sepharose beads. TGase 2 was detected by probing with anti-TGase 2 antibody. GST or GST fusion proteins were detected by probing with anti-GST antibody (C–E).
Since E7 proteins of different HPV types show high sequence similarity in their three conserved regions, known as CR1, CR2 and zinc-binding domains (Munger and Halpern, 1997), we tested whether the E7 of HPV types other than HPV18 also interact with TGase 2. Figure 1D illustrates that purified TGase 2 bound E7s of HPV11 (low-risk HPV) as well as HPV16 (high-risk HPV). However, TGase 2 did not bind another oncoprotein of HPV, GST–HPV18 E6 (Figure 1E). These results indicate that TGase 2 specifically interacts with E7s of HPV18 as well as other HPV types.
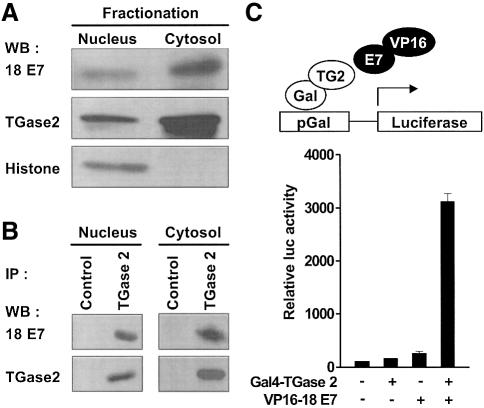
To investigate whether TGase 2 interacts with HPV18 E7 in vivo, co-immunoprecipitation assays were carried out with HeLa cells. TGase 2 has been reported to localize in the nucleus as well as in the cytosol of HeLa cells (Lesort et al., 1998). Therefore, we fractionated HeLa cell lysate into cytosolic and nuclear fractions and performed immunoblotting using antibodies specific for either HPV18 E7 or TGase 2. As reported previously (Fujikawa et al., 1994), both TGase 2 and HPV18 E7 were detected in the cytosol and the nucleus of the cell lysates (Figure 2A). Using anti-TGase 2 antibody, HPV18 E7 co-precipitated with TGase 2 both in the cytosolic and in the nuclear fractions (Figure 2B). To further demonstrate the physiological interaction between TGase 2 and HPV18 E7, we carried out mammalian cell two-hybrid assays. The co-transfection of Gal4–TGase 2 and VP16–HPV18 E7 into NIH 3T3 cells markedly increased luciferase activity (Figure 2C). However, an absence of one of the hybrid constructs failed to activate a reporter gene. These results suggest that the interaction of TGase 2 with HPV18 E7 does have a significant physiological role.
Fig. 2. TGase 2 interacts with HPV18 E7 in cells. (A) HeLa cells were fractionated into cytosolic and nuclear fractions. Fractionated extracts were subjected to 6–15% SDS–PAGE and the indicated proteins were detected by probing with anti-HPV18 E7, TGase 2 and histone H1 antibodies. (B) Endogenous interacting complexes were immuno precipitated from the fractionated HeLa cell extracts using anti-TGase 2 antibody and an isotype-matched control antibody (Control). The precipitates were analyzed by western blot using antibodies to HPV18 E7 and TGase 2. IP, immunoprecipitation. (C) Mammalian two-hybrid assay. HPV18 E7 and TGase 2 were respectively fused to the trans activation domain of VP16 protein (VP16) and Gal4 DNA binding domain (Gal) that can bind to Gal4-responsive elements (pGal) upstream of a luciferase reporter gene (upper). NIH 3T3 cells were transiently transfected with the indicated plasmids plus pCMV-β-Gal. Luciferase activity is normalized by β-galactosidase activity, and expressed relative to the activity presented by the transfection of reporter construct only, which is set to 100%. The figure represents the mean values and SD of three independent experiments.
TGase 2 modifies E7 protein of HPV18 but not HPV16 by polyamination
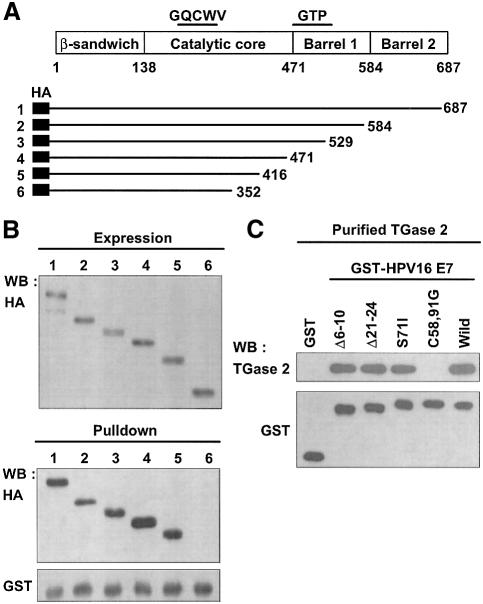
To assess the functional consequences of the interaction between TGase 2 and HPV18 E7, we have identified the binding region on TGase 2. A series of C-terminal deletion constructs of TGase 2 was generated based on the domain structure of TGase 2 (Figure 3A; Liu et al., 2002). TGase 2 deletion constructs were expressed in the yeast cells (Figure 3B, upper panel), and pull-down experiments were performed with GST–HPV18 E7. All TGase 2 deletion mutants interacted with HPV18 E7, but TGase 2 that lacks the sequence between 353 and 687 failed to bind HPV18 E7 (Figure 3B, lower panel). These results indicate that the C-terminal region in catalytic core domain of TGase 2 is essential for its binding to HPV18 E7. To elucidate the TGase 2 binding region of E7, we used CR1 (Δ6–10), CR2 (Δ21–24) deletion constructs and S71I, C58,91G point mutants of HPV16 E7 (Park et al., 2000). The interactions between TGase 2 and the mutant HPV16 E7s were comparable to that of the native HPV E7, except C58,91G. The C58,91G mutant that disrupts the formation of the zinc-binding motif did not bind TGase 2 (Figure 3C). This finding leads us to conclude that the zinc-binding domain of E7 is necessary for HPV E7 to bind TGase 2.
Fig. 3. Mapping the interaction domain. (A) Schematic presentation of TGase 2 and its deletion mutants. All deletion mutants were tagged with HA epitope at the N-terminal region, and deleted sites are indicated. GQCWV, active site of TGase 2; GTP, GTP binding region. (B) Mapping the HPV18 E7 binding domain of TGase 2. INVsc1 yeast cells were transformed with the deletion constructs. The expression of all deletion mutants was validated by western blot analysis using anti-HA antibody. Yeast extracts expressing each mutant were incubated with GST–HPV18 E7 bound to glutathione–Sepharose beads. The precipitated proteins were subjected to western blot using anti-HA antibody. GST fusion proteins were probed with anti-GST antibody. Cell extracts equivalent to the expression level for the each deletion mutant of TGase 2 were used for pull-down assay. (C) Mapping the TGase 2 binding domain of HPV16 E7. Protein complexes, comprising TGase 2 and GST or GST–HPV16 E7 proteins, were precipitated by glutathione–Sepharose beads, and were probed with anti-TGase 2 antibody. Δ6–10 and Δ21–24, deletion mutants of HPV16 E7. S71I and C58,91G, point mutation of HPV16 E7.
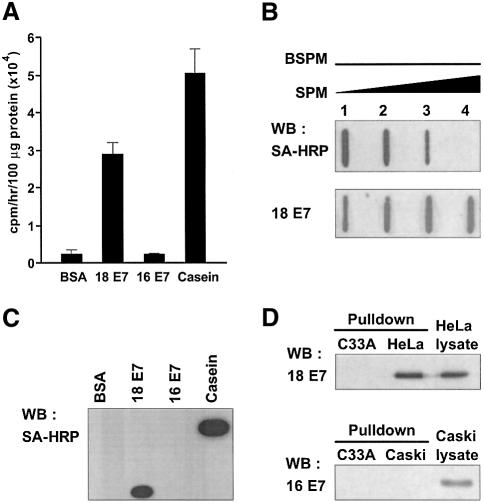
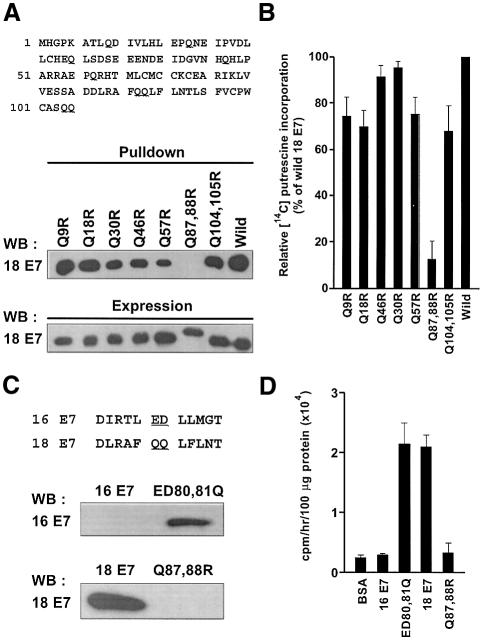
Since the catalytic core domain of TGase 2 is essential for binding HPV E7, there exists the possibility that HPV E7 could be a substrate for TGase 2. To test this hypothesis, we performed an in vitro assay in which TGase 2 catalyzes incorporation of a polyamine (14C putrescine) into a protein. TGase 2 was able to incorporate 14C putrescine into HPV18 E7, although not into HPV16 E7 (Figure 4A). To further verify the specificity of TGase 2 to recognize the E7s of different HPV types, the E7s were incubated with biotinylated spermine along with TGase 2, and the polyaminated E7s were subjected to immunoblotting and visualized with horseradish peroxidase (HRP)-conjugated streptavidin. TGase 2 was capable of incorporating biotinylated spermine into HPV18 E7, but it failed to do so into HPV16 E7 (Figure 4C). This is consistent with the results of the 14C putrescine incorporation experiment. Polyamination of HPV18 E7 by TGase 2 was further confirmed by competition experiment, in which biotinylated spermine was allowed to compete with free spermine. The incorporation of biotinylated spermine into HPV18 E7 decreased with increasing concentration of free spermine (Figure 4B). In addition, we explored the possibility that TGase 2 may produce crosslinked product of HPV E7 in the experimental conditions under which the catalytic function of TGase 2 was tested. Formation of E7 dimer, trimer or oligomers was not detectable in the immunoblotting analysis (data not shown), suggesting that the E7 had not crosslinked under the conditions tested.
Fig. 4. TGase 2 catalyzes polyamination of HPV18 E7, but not HPV16 E7. (A) One hundred micrograms of His-tagged HPV18 E7 and HPV16 E7 were incubated with TGase 2 in the presence of 14C putrescine. Radioactivity bound to each protein was measured by a liquid scintillation counter. BSA and casein were employed as negative and a positive controls respectively. The figure shows the mean values and SD of three independent experiments. (B) HPV18 E7 was incubated with TGase 2 in the presence of 0.1 mM of biotinylated spermine and 0, 0.1, 1 and 10 mM of spermine (lane 1–4). The incorporation of biotinylated spermine was analyzed by dot-blotting using HRP-conjugated streptavidin (SA-HRP). HPV18 E7 was probed with anti-HPV18 E7 antibody. (C) The reaction mixtures were subjected to 15% SDS–PAGE and polyaminated HPV E7 was probed with SA-HRP. (D) HeLa and Caski cells were treated with 1 mM of biotinylated pentylamine for 1 h in the presence of A23187. Biotinylated pentylamine-incorporated E7s were precipitated by streptavidin-conjugated magnetic beads. Proteins bound to beads were analyzed by western blot using antibodies to HPV18 E7 or HPV16 E7. C33A cells were used as a negative control.
Next, we examined the catalytic specificity of TGase 2 using HeLa and Caski cells, which contain the integrated HPV18 and HPV16 genomes, respectively. The cells were grown in a medium containing biotinylated pentylamine and treated with A23187 calcium ionophore to activate TGase 2. After cell extracts were incubated with streptavidin magnetic beads, the precipitates were analyzed by western blot method using antibodies specific for either HPV18 E7 or HPV16 E7. Polyamine-incorporated E7 was detectable in HeLa cells but not in Caski and C33A cells (HPV-negative cervical cancer cell) (Figure 4D). Thus, these results indicate that TGase 2 specifically modifies HPV18 E7 by polyamination.
TGase 2 mediates site-specific polyamination of HPV18 E7 protein
Although TGases display broad substrate specificity for amine donors, they tend to be more specific for substrates that are amine acceptors (Lorand and Graham, 2003). Since the glutamine residues of a substrate act as amine acceptors in TGase catalyzed transamidation, our results suggest that the glutamine residue(s) in HPV E7 functions as an amine acceptor and contributes to HPV type-specific polyamine incorporation. To identify the polyaminated glutamine residue(s), seven HPV18 E7 mutants were generated by site-directed mutagenesis replacing all glutamine residues with arginine residues in HPV18 E7 (Figure 5A, upper panel; Pastor et al., 1999). Wild-type and mutant HPV18 E7s expressed in bacteria were purified using Ni-affinity column chromatography (Figure 5A, lower panel). When polyaminated HPV18 E7 mutants were analyzed by pull-down assay using streptavidin-conjugated magnetic beads, only Q87,88R failed to precipitate (Figure 5A, middle panel). Similar results were obtained in the 14C putrescine incorporation assay using E7 mutants as amine acceptor substrates. TGase 2 catalyzed incorporation of 14C putrescine into all the E7 mutants except Q87,88R (Figure 5B), revealing that the glutamine 87 or 88 residues are the only sites where polyamination takes place.
Fig. 5. Identification of the polyamination site(s) in HPV E7. (A) All glutamine residues in HPV18 E7 were replaced by arginines (upper). E7s were expressed in bacteria as His-tagged proteins (lower). Purified E7s were incubated with TGase 2 in the presence of biotinylated spermine. Biotinylated spermine-incorporated proteins were precipitated with streptavidin-conjugated magnetic beads. Proteins bound to beads were analyzed by western blot using anti-HPV18 E7 antibody (middle). (B) 14C putrescine incorporated into E7 by TGase 2 is presented in relation to the radioactivity of wild-type E7. (C) HPV16 E7 mutant was generated by replacing E80,D81 with Q80,Q81, which are corresponded to Q87,Q88 of HPV18 E7 (upper panel). Biotinylated spermine-incorporated proteins were precipitated with streptavidin- conjugated magnetic beads. Proteins bound to beads were analyzed by western blot using anti-HPV16 E7 antibody (lower panel). (D) 14C putrescine incorporation into wild-type and mutant E7 of HPV16. The amount of 14C putrescine incorporated is presented as the mean values and SD of three independent experiments.
The amino acid sequence alignments of E7s of HPV frequently detected in cervical cancer show that glutamine residues corresponding to Q87 or Q88 of HPV18 E7 are absent only in HPV16 (see Figure 9). To test whether a sequence variation has produced the HPV type-specific polyamination, we generated a mutant of HPV16 E7 in which E80 and D81 were replaced by Q80 and Q81, respectively. The new HPV16 E7 mutant thereby acquired the same essential sequence corresponding to Q87 and Q88 of HPV18 E7 (Figure 5C, upper panel). The polyamination of ED80,81Q was analyzed by pull-down assay. These substitutions in HPV16 E7 resulted in polyamination by TGase 2 (Figure 5C, middle panel). 14C putrescine incorporation assay confirmed that the extent of polyamination in ED80,81Q was comparable to that in HPV18 E7 (Figure 5D), demonstrating that glutamine residues within this site is indispensable for TGase 2-mediated polyamination. We thus conclude that TGase 2 cannot modify HPV16 E7 because of the sequence variation at a polyamination site.
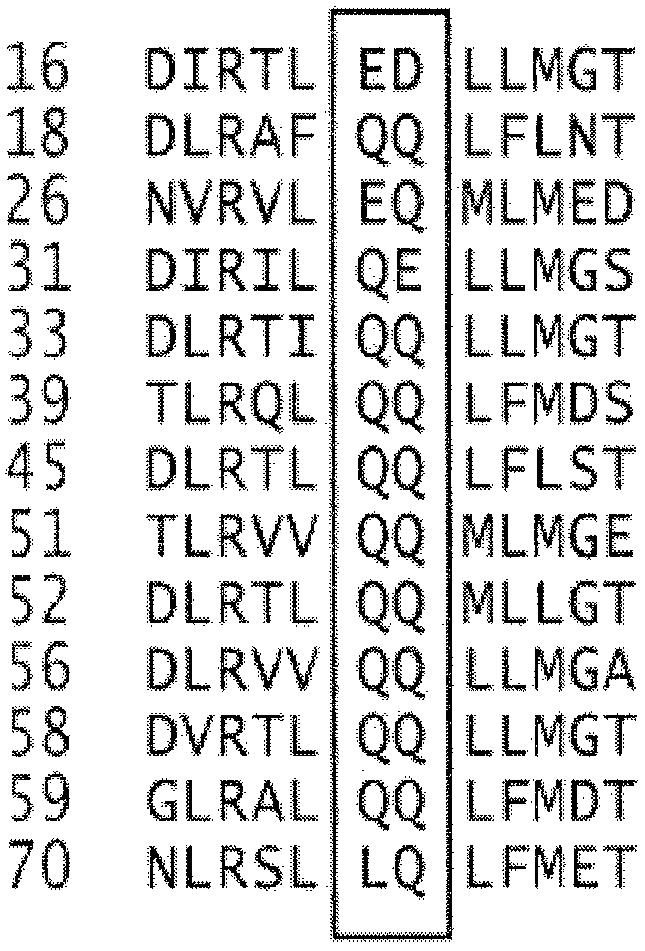
Fig. 9. Sequence alignments of E7 of high-risk HPVs. The box encloses conserved glutamine residues that could be potential targets for TGase 2-catalyzed polyamination.
Polyaminated HPV E7 loses its ability to bind Rb protein
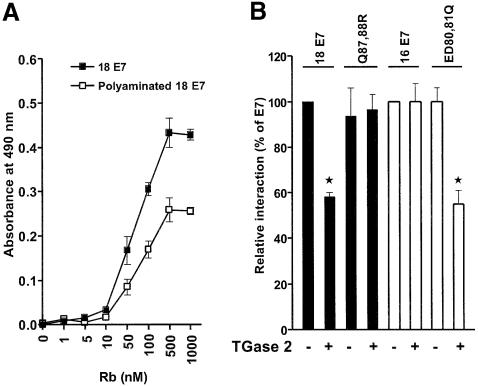
Disruption of Rb function by the binding of E7 is crucial for the tumorigenicity of HPV. The affinity of HPV E7 binding to Rb is largely dependent on the structure of CR2 domain in E7. It is also reported that the zinc-binding domain is required for the high affinity of HPV E7 to bind Rb and the subsequent E2F–Rb dissociation process (Huang et al., 1993). As polyamines are fully charged at physiological pH, the incorporation of polyamine into E7 might influence Rb–E7 interaction by conferring positive charges at the polyamination site, which lie on the loop region of zinc-binding domain (Zwerschke et al., 1999). To test whether polyamination of zinc-binding domain affects the interaction between E7 and Rb, we developed an enzyme-linked immunosorbent assay (ELISA) (Hammad et al., 1997). GST–Rb fusion protein (pocket domain of Rb, amino acids 372–787; Lee et al., 1998) was added to a microtiter plate coated with E7 or polyaminated E7 (Pol-E7). Bound GST–Rb was quantitated using anti-GST monoclonal antibody. As shown in Figure 6A, binding of GST–Rb to E7 or Pol-E7 was saturated at ∼500 nM of Rb, and maximal Rb binding to Pol-E7 was ∼55% of that to E7. These experiments demonstrate that polyamination of HPV18 E7 reduced its binding to Rb. The effect of polyamination in the loop region on the interaction with Rb was further investigated using the E7 mutant of HPV18 as well as that of HPV16. The binding of Q87,88R and HPV16 E7 to Rb was not affected by the presence of TGase 2. However, the binding of HPV18 E7 or ED80,81Q to Rb was significantly lower when TGase 2 was present in the system (Figure 6B). These results are consistent with those of the site-specific polyamination experiments (Figure 5), and indicate that polyaminated E7, indeed, loses its ability to bind Rb protein.
Fig. 6. Polyamination of HPV E7 by TGase 2 interferes with its binding to Rb. (A) Polyaminated HPV18 E7 binds Rb with reduced affinity. Microtiter wells coated with HPV18 E7 or polyaminated HPV18 E7 were incubated with increasing concentrations of GST–Rb. Bound GST–Rb was detected using anti-GST antibody and HRP- conjugated goat anti-mouse IgG antibody. (B) Effect of mutation at the site of polyamination in HPV16 E7 and HPV18 E7 on Rb-binding. The purified E7s were incubated with spermine in the presence or absence of TGase 2, and a solid-phase ELISA was performed with 500 nM of GST–Rb. Bound GST–Rb were quantified as described earlier. Q87,88R and ED80,81Q denote mutants of HPV18 E7 and HPV16 E7 at the site of polyamination, respectively. Rb binding of E7s is expressed as a relative value to that of wild type in the absence of TGase 2, which is set to 100%. The figure shows the mean values and SD of three independent experiments. *P < 0.01.
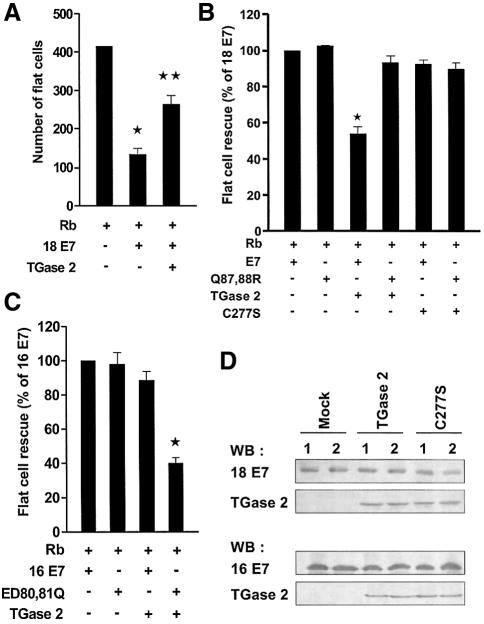
We next analyzed the effect of polyamination on HPV E7-mediated flat cell formation using Saos2 cells (Brehm et al., 1999), which is Rb-null and also showed no detectable TGase 2 expression (Figure 7D). The expression of functional Rb in Saos2 cells leads to a growth arrest at the G1 phase with a dramatic morphological change. Co-expression of Rb and HPV18 E7 in Saos2 cells rescues Rb-induced flat cell formation. When TGase 2 was co-transfected in addition to Rb and HPV18 E7, the rescue activity of HPV18 E7 was markedly reduced (Figure 7A). However, the expression of TGase 2 did not affect the rescue activity of Q87,88R, and the expression of C277S (an active site mutant of TGase 2) did not have a noticeable effect on the flat cell rescue activity of either HPV18 E7 or Q87,88R (Figure 7B). In contrast, the expression of TGase 2 appeared to suppress the rescue activity of ED80,81Q, but not that of HPV16 E7 (Figure 7C). In addition, western blot analysis confirmed the expression of similar amounts of wild-type and mutant E7 proteins in transfected Saos cells, and co-expression of either TGase 2 or C277S with HPV E7 did not influence the expression level of HPV E7 (Figure 7D). As we observed in the polyamination reactions, TGase 2 exerted specific contrasting effects on the flat cell rescue activity of Q87,88R and ED80,81Q. Taken together, these results indicate that TGase 2 interferes with Rb–HPV E7 interaction via polyamination of HPV E7.
Fig. 7. HPV E7 mutants lacking the site for polyamination avoids the suppressive effect of TGase 2 in flat cell assay. (A) TGase 2 expression suppresses HPV18 E7-induced flat cell rescue. Saos2 cells were transfected with indicated plasmids and pBABE vector. After puromycin selection, flat cells stained with crystal violet were counted under a light microscope. *P < 0.01, **P < 0.05. (B) Effect of polyamination of HPV18 E7 on flat cell rescue activity. The number of flat cell reduced by HPV18 E7 is arbitrarily set to 100%. The relative rescue activities by HPV18 E7 and Q87,88R were compared in the presence of TGase 2 or C277S. *P < 0.01. (C) Flat cell rescue activity of HPV16 E7. The relative rescue activities by HPV16 E7 and ED80,81Q were compared in the presence or absence of TGase 2. *P < 0.05. The figure shows the mean values and SD of three independent experiments. (D) Western blot analysis of HPV E7 and TGase 2 in co-transfected Saos 2 cell. Lane 1, wild-type HPV18 E7 (top) or 16 E7 (bottom); lane 2, Q87,88R mutant (top) or ED80,81Q mutant (bottom).
Differential effect of TGase 2 on functional activity of E7 due to the presence of a polyamination site
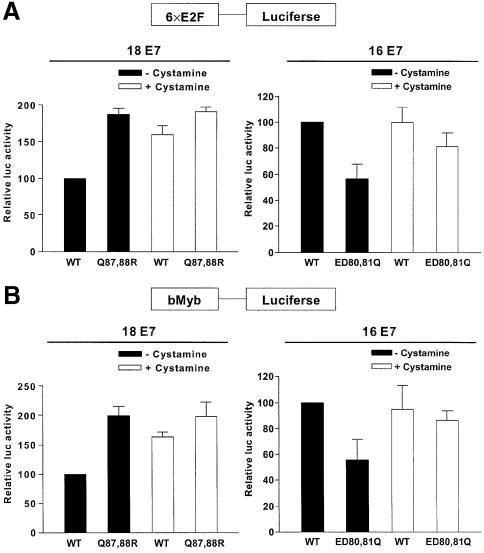
Of more than 40 different types of HPV capable of infecting the cervical epithelia, HPV16 has been detected in ∼50% of cervical cancers (Clifford et al., 2003). Our new findings in this area raise the possibility that the absence of a site that can be polyaminated in HPV16 E7 may be attributable to the high-risk potential of HPV16 by avoiding the catalytic activity of TGase 2. To test whether TGase 2 modulates the function of E7 type-specifically, we examined the effect of polyamination by endogenous TGase 2 on HPV E7-mediated E2F activation. HaCaT cells, immortalized epidermal keratinocytes, were transfected with E2F or bMyb promoter-dependent luciferase reporter constructs (Lam et al., 1994), together with wild-type or mutant E7s. HaCaT cells, which contain functional Rb, showed high TGase 2 expression level and activity (data not shown). Expression of Q87,88R mutant showed higher promoter activities in both E2F (Figure 8A, right) and bMyb (Figure 8B, right) reporters than those of wild-type HPV18 E7. To validate the role of TGase 2 in the observed differential activation of E2F promoter, we analyzed the effects of cystamine, an inhibitor of TGase 2. Treatment with cystamine significantly enhanced the reporter activity of wild-type HPV18 E7, but did not affect the reporter activity of Q87,88R. In contrast, introduction of polyamination sites to HPV16 E7 (ED80,81Q) lowered luciferase activity, which was abrogated by cystamine. However, E2F activation by wild-type HPV16 E7 was not affect by cystamine (Figure 8A and B, left). These data further confirmed our results that TGase 2 specifically modifies HPV E7 by polyamination, and TGase 2–HPV E7 interaction may contribute as a factor in understanding the HPV epidemiology of cervical cancer.
Fig. 8. Effects of cystamine on HPV E7-mediated E2F release. E2F responsive element (6× E2F) (A) and bMyb promoter (B) upstream of a luciferase gene were used as reporter constructs. HaCaT cells were transiently transfected with the indicated plasmids (–cystamine), and then treated with 0.5 mM cystamine for 24 h in fresh medium (+cystamine). Luciferase activity is expressed relative to that of the wild-type E7 construct. The figure shows the mean values and SD of three independent experiments.
Discussion
The intriguing ability of TGase 2 to generate crosslinked proteins, which may prevent the leakage of cell constituents in apoptotic cells, and the expression pattern of TGase 2 suggest that this enzyme serves as a final mediator for apoptotic signaling pathways. However, mice with null mutations in the TGase 2 gene show no change in apoptosis (De Laurenzi and Melino, 2001). These findings indicate that other TGases may compensate the TGase 2 function. Thus, the precise physiological function(s) of TGase 2 remains obscure.
The nuclear localization of TGase 2, unlike other TGases, suggests that the identification of nuclear target proteins for TGase 2 could provide a crucial understanding on how TGase 2 mediates specific biological processes by means of irreversible protein modification. Using yeast two-hybrid screens, we identified HPV E7 protein as a major TGase 2 binding partner in the nucleus. Our results show that TGase 2 can modify HPV18 E7 protein by incorporating polyamines into the glutamine residue in the loop region of the zinc-binding domain. In addition, the ability of the E7 protein to bind and inactivate Rb was abolished by TGase 2-mediated polyamination. However, TGase 2 failed to modify E7 protein of HPV16 because it lacked a glutamine residue(s) at the polyamination site. These results provide the first example of a cellular factor that inhibits HPV E7 functions.
TGase 2 as a cellular interfering factor of HPV-induced carcinogenesis
The E7s of high-risk HPVs are oncoproteins that can induce cellular transformation and immortalization by promoting the G1–S phase cell cycle transition via specific protein interactions with cell cycle regulators (Munger et al., 2001). Our results here provide evidence for a stable interaction between the catalytic core domain of TGase 2 and the zinc-binding domain of E7 that results in polyamination of E7. Analyses of E7 mutants show that a loss of E7 activity by TGase 2 is correlated with the presence of glutamine residue at a polyamination site, indicating that E7 is subjected to cellular interference by the post-translational modification (Figure 8). The presence of glutamine residue at polyamination sites in E7s from all high-risk HPVs, except HPV16, further supports the notion that these sites represent conserved targets for TGase 2 (Figure 9).
The position Q87 or Q88 in E7 was identified as a site that can be polyaminated by TGase 2. This region is also responsible in E7 for the interaction with TATA box binding protein (Massimi et al., 1997), histone deacetylase complex (Brehm et al., 1999), M2 pyruvate kinase (Zwerschke et al., 1999) and acid α-glucosidase (Zwerschke et al., 2000). These interactions of E7 with regulatory proteins for transcription and glycolysis might provide HPV E7 with the capacity to stimulate the proliferation of differentiated keratinocytes. These reports and the present results suggest that TGase 2-catalyzed polyamination can antagonize the oncogenic potential of HPV E7 protein by inhibiting its interaction with cellular proteins.
The role of TGase 2 in HPV epidemiology
The results of this study have established that the catalytic activity of TGase 2 can incorporate polyamine into E7, and thereby inhibit its binding to Rb. Nevertheless, a question remains as to whether the polyamination is a physiological mechanism by which the host cell interferes with the function of E7. To answer this question would require evidence that polyaminated E7 is present in HPV-infected mucosal epithelium of human subjects. However, information on the levels of polyaminated E7 in HPV-infected cells is currently limited due to the poor immunoreactivity of E7 (Jeon et al., 2002b) and the lack of appropriate antibodies. In fact, expression of E7 is tightly regulated by E2 in HPV-infected cells. In high-grade squamous intraepithelial lesions, integration of HPV into human genome abrogates the transcriptional control by disrupting E2 gene, and results in up-regulation of E7 expression (Romanczuk and Howley, 1992). It is conceivable, therefore, that the high levels of E7 in epithelium infected by high-risk HPVs other than HPV16 may overcome the TGase 2-mediated modifcation, which could lead to cancer progression.
Epidemiological studies on the prevalence of HPV types in cervical cancer and the general population tend to support our conclusion that the polyamination of E7 by TGase 2 can interfere with HPV-induced cervical carcinogenesis. More than 40 HPV types commonly infect the female genital tract, but only a few types, which are classified as high-risk groups, have been detected in cervical cancer. In a random series of women without cervical lesion, HPV16 accounts for ∼20% of the HPV present, followed by HPV18 (Woodman et al., 2001). In contrast, HPV16 is found in 50–70% of cervical cancer cases in most countries (Clifford et al., 2003). Although a significant difference in Rb-binding activity of E7 between high-risk and low-risk HPVs has been reported (Heck et al., 1992), viral factors of HPV16 that account for the highest prevalence among high-risk HPVs have not been conclusively identified. Indeed, in vitro transformation assay indicated that the transforming activity of HPV16 E7 showed no significant difference than that of HPV18 E7 (Sang et al., 1992). These epidemiological and molecular data suggest the presence of host factors that modulate the oncogenic potential of HPV. Our results show that HPV16 is the only type among high-risk HPVs that was not modified by TGase 2, due to lack of a glutamine residue(s) at the site where polyamination takes place in E7. Thus, our findings offer a possible explanation for the observed difference in the prevalence of HPV16 between cervical cancer and the general population.
Is polyamination a common modification to modulate protein function(s)?
The present results may provide an insight into the physiological significance of polyamination of proteins in general. Although most of the substrate proteins identified so far are known to be crosslinked by TGase 2, several observations suggest that polyamination is an important mechanism for modulation of the target protein’s activity. In neuroblastoma cells, activated TGase 2 polyaminated tau protein, and the modified tau protein becomes less susceptible to calpain without altering the microtubule-binding capacity (Tucholski et al., 1999). Incorporation of polyamine into phospholipase A2 has been reported to increase its enzyme activity (Cordella-Miele et al., 1993). Recent studies show that TGase 2 catalyzes the polyamination of Rb that protects cells from apoptotic insults (Boehm et al., 2002). In this system, polyaminated Rb is resistant to caspase-7 cleavage. Thus, these results indicate that TGase 2 can polyaminated either HPV E7 or Rb (or alternatively both), which results in interfering with HPV E7–Rb interaction by way of charge repulsion. Together, these findings suggest that polyamination is an important post-translational modification for various physiological processes, though the mechanisms of how intracellular TGase 2 is regulated has yet to be resolved.
It is worth noting that TGase 2 can function as a host-cell defense system against infection of viruses other than HPV. Indeed, in hepatitis C virus (HCV)-infected cells, TGase 2 was found to crosslink the HCV core proteins to form a dimer, which reduced the original RNA binding activity (Lu et al., 2001). In case of HIV-infected cells, the activity of TGase 2 is increased (Amendola et al., 1996). Although the physiological consequence of these findings has yet to be determined, it is tempting to speculate that TGase 2 may be involved in the post-translational modification of foreign proteins, including viral proteins.
In summary, the results of this study provide evidence that TGase 2 may play a role in modulating the oncogenic function of HPV E7 through the site-specific polyamination. Our findings suggest that the inability of TGase 2 to modify E7 of HPV16 contribute to its high cancer risk. The interaction between TGase 2 and HPV E7 reveals for the first time the existence of the cellular interfering factor.
Materials and methods
Yeast two-hybrid screen
MATCHMAKER Gal4 Two-Hybrid System 2 was used according to manufacturer’s instructions (Clontech). The C-terminal domain of TGase 2 (amino acids 472–687) was transformed into HF7c yeast cells with HeLa cell library. Positive clones were selected on triple dropout plates (SD-Leu, Trp, His) and further confirmed by β-galactosidase assay. After the isolation of positive clones, plasmid DNAs was rescued and re-transformed into SFY526 yeast cells to validate protein–protein interactions. Liquid culture β-galactosidase assay using ONPG (Sigma) was performed to quantitate the level of interactions between bait and isolated clones.
Mammalian two-hybrid screen
Mammalian MATCHMAKER two-hybrid system was used according to manufacturer’s instructions (Clontech). Each expression construct was transiently transfected into NIH 3T3 cells using LipofectAMINE (Invitrogen). Cell extracts were prepared 24 h after transfection, and luciferase activity was assayed according to manufacturer’s instructions (Promega). pCMV-β-galactosidase was co-transfected as an internal control to normalize luciferase activity.
Production of recombinant proteins
Recombinant E7s (wild type or mutant) of HPV11, -16 and -18, and E6 of HPV18 were expressed in BL21 Escherichia coli cells as His-tagged (pET-15b, Novagen) or GST fusion proteins (pGEX-4T-1; Amersham Biosciences). Proteins were purified with a Ni-affinity column or a glutathione–Sepharose column chromatography according to manufacturer’s instructions.
Deletion constructs of TGase 2
The deletion constructs of HA-tagged TGase 2 were generated by PCR and cloned into pYES2.0 plasmids (Invitrogen). Deletion mutants were transformed and expressed in INVsc1 yeast cell (Invitrogen). Yeast lysates were prepared with glass bead lysis method according to manufacturer’s instructions (Invitrogen). The expression of each construct was confirmed by western blot analysis using anti-HA antibody (Roche Applied Biosciences).
GST pull-down assay
GST (0.5–1 µg) and GST fusion proteins pre-bound to glutathione–Sepharose beads were incubated with 1–5 µg of purified TGase 2, 50 µg of HeLa cell extract or 50 µg of yeast extracts expressing deletion mutants of TGase 2 at room temperature for 30 min. The beads were washed four times with binding buffer [50 mM Tris pH 7.5, 150 mM NaCl, 10 µM ZnCl2, 1% Nonidet P-40, 2 µg/ml phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, 2 µg/ml pepstatin A, 2 µg/ml trypsin inhibitor], and bound proteins were eluted and analyzed by western blot using anti-TGase 2 or anti-HA antibodies. GST and GST fusion proteins were detected by probing with anti-GST antibody (Santa Cruz Biotechnology).
Co-immunoprecipitation
HeLa cells were fractionated into cytosolic and nuclear fractions as described previously (Zhang et al., 1998). Each fraction was dialyzed against the binding buffer and precleared with protein A–agarose (Pierce) for 3 h at 4°C. Immunoprecipitation was performed by incubating with anti-TGase 2 antibody overnight at 4°C, followed by protein A–agarose for 3 h at 4°C. Beads were washed four times with the binding buffer at 4°C, and bound proteins were eluted and analyzed by western blot using anti-HPV18 E7 antibody.
Purification of human TGase 2
TGase 2 was purified from human red blood cells as described previously (Jeon et al., 2002a). Human RBCs were lysed by osmotic pressure in distilled water. TGase 2 was purified from the crude extract by sequential column chromatography of a DEAE-cellulose column followed by a heparin column. Purity of TGase 2 was evaluated by 12% SDS–PAGE with Coomassie staining.
Site-directed mutagenesis
Amino acid substitution in E7s of HPV16, HPV18 and TGase 2 was performed using QuickChange mutagenesis kit according to manufacturer’s instructions (Stratagene). Wild-type cDNAs of HPV16 E7, HPV18 E7 and TGase 2 cloned in pET-15b, pcDNA3 or pSG5 (Stratagene) were used as templates for each mutagenesis reaction. The mutation in each construct was confirmed by DNA sequencing.
14C putrescine incorporation assay
Polyamination was determined by measuring the incorporation of 14C putrescine into E7 as described previously (Jeon et al., 2003). One hundred micrograms of wild-type or mutant E7 of HPV16 and HPV18 was incubated with TGase 2 and 500 nCi of 14C putrescine (NEN) in reaction buffer [50 mM Tris–acetate pH 7.5, 10 mM CaCl2, 5 mM dithiothreitol (DTT), 150 mM NaCl, 0.5% Triton X-100, 2 µg/ml PMSF, 2 µg/ml leupeptin, 2 µg/ml pepstatin A, 2 µg/ml trypsin inhibitor]. The reaction was stopped after 1 h at 37°C by the adding ice-cold 7.5% trichloroacetic acid (TCA). The reaction mixture was then allowed to be precipitated for more than 1 h at 4°C. Precipitated proteins were filtered (Wattman GF/A) and washed three times with 5% TCA solution. Radioactivity bound to E7 was measured by liquid scintillation counter. Bovine serum albumin (BSA) was a negative control and N,N′-dimethylcasein was used as a positive control.
Biotinylated polyamine incorporation
To evaluate the level of polyamine incorporation into E7, transamidation reaction was performed using 0.5 mM biotinylated spermine in 50 µl of the reaction buffer (50 mM Tris–acetate pH 7.5, 10 mM CaCl2, 5 mM DTT, 150 mM NaCl, 0.5% Triton X-100, 0.5 mM EDTA pH 8.0) as described previously (Jeon et al., 2003). The reaction mixtures were subjected to 15% SDS–PAGE and transferred to nitrocellulose membrane (Amersham Biosciences). After blocking and probing with HRP-conjugated streptavidin, the blots were developed by ECL protocol (Pierce). The above reaction mixture was dialyzed against PBS and the biotinylated spermine-incorporated E7 was isolated using streptavidin-conjugated magnetic beads (Dynal). HPV16 E7 or HPV18 E7 protein was evaluated by modified western blot analysis using antibodies to HPV16 E7 and HPV18 E7, respectively (Jeon et al., 2002b).
In situ substrate assay
In situ substrate assay was performed as described previously (Jeon et al., 2003). Briefly, HeLa, Caski and C33A cells (2 × 106 each) were treated with 1 mM biotinylated pentylamine (Pierce) for 1 h at 37°C in the presence of A23187 (Calbiochem). Cell lysate was dialyzed and biotinylated pentylamine-incorporated proteins were isolated using streptavidin-conjugated magnetic beads and probed by western blot using antibodies to HPV16 E7 and HPV18 E7, respectively. The amount of cell lysates equivalent to an equal expression level of TGase 2 was used in the experiment.
Solid-phase ELISA
Affinity of Rb to bind E7 or polyaminated E7 was measured by solid-phase ELISA as described previously (Hammad et al., 1997). In brief, 96-well microtiter plates were coated with 100 µl/well of E7 or polyaminated E7 (3 µg/ml) and overcoated with BSA. Various concentrations of GST–Rb protein (amino acids 372–787) were incubated for 2 h at room temperature (Lee et al., 1998). Bound GST–Rb was detected by incubating with anti-GST antibody (Santa Cruz Biotechnology), followed by incubation with HRP-conjugated goat anti-mouse IgG antibody (Pierce). A chromogenic substrate o-phenylenediamine dihydrochloride (Sigma) developed color, which was evaluated by measuring absorbance at 490 nm on microplate spectrophotometer (Molecular Devices).
Flat cell assay
The interference effect on Rb-binding of HPV E7 by TGase 2 was assessed by the flat cell assay as described previously (Brehm et al., 1999). cDNAs of TGase 2, HPV16 E7 and HPV18 E7 (wild type or mutant) were cloned into pSG5 (Stratagene) and pcDNA3 (Invitrogen), respectively. Saos2 cells were transfected with cDNAs and pBABE vector using LipofectAMINE (Invitrogen). Forty-eight hours after transfection, cells were split and subjected to puromycin selection (1 µg/ml) for 6–10 days. Cells were then fixed and stained with crystal violet. Flat cells were counted under a light microscope (40× magnification).
Luciferase assay
HaCaT cells were transfected in triplicate with E2F- and bMyb-luciferase reporter plasmids plus E7 expression constructs. Empty pcDNA3 vector was used to adjust the equal DNA amount. Cells were extracted 24 h after transfection, and luciferase activity was assayed using a commercial kit according to manufacturer’s instructions (Promega). Luciferase level was normalized by total protein amount. For inhibiting the activity of TGase 2, HaCaT cells were incubated with 0.5 mM of cystamine (Sigma).
Production of monoclonal antibodies
Monoclonal antibodies specific for HPV16 E7, HPV18 E7 and TGase 2 were generated by immunizing Balb/c mice with the His-tagged HPV16 E7, HPV18 E7 and TGase 2. After immunizing Balb/c mice with 10 µg of these proteins at 1-month intervals for a period of 3 months, splenocytes were isolated and fused with SP2 myeloma cells in the presence of polyethylene glycol 1500 according to manufacturer’s instructions (Roche). Hybridoma cells were grown in HAT selection media in 96-well plates. About 2 weeks later, hybridoma clones were selected by repeated limiting dilution and ELISA.
Acknowledgments
Acknowledgements
We thank Dr Y.D.Kim for critical comments on the manuscript. We are grateful to Drs T.Kouzarides (pRb and pBABE), K.Helin (E2F reporter construct), R.J.Watson (bMyb reporter construct) and S.J.Um (cDNAs of wild-type and mutant HPV E7) for providing constructs. This work was supported by Korea Science and Engineering Foundation (KOSEF) through the Center for Aging and Apoptosis Research at Seoul National University (R11-2002-001-04003-0) and Seoul National University Hospital Research Fund (to I.-G.K.). J.-H.J., S.-Y.C., C.-W.K., D.-M.S. and J.-C.K. were supported by graduate program of BK21 project from Ministry of Education & Human Resources Development.
References
- Amendola A., Gougeon,M.L., Poccia,F., Bondurand,A., Fesus,L. and Piacentini,M. (1996) Induction of ‘tissue’ transglutaminase in HIV-pathogenesis: evidence for high rate of apoptosis of CD4+ T lymphocytes and accessory cells in lymphoid tissues. Proc. Natl Acad. Sci. USA, 93, 11057–11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K.J., Kang,S., Damron,D. and Im,M. (2001) Phospholipase Cdelta1 is a guanine nucleotide exchanging factor for transglutaminase II (Galpha h) and promotes alpha 1B-adrenoreceptor-mediated GTP binding and intracellular calcium release. J. Biol. Chem., 276, 5591–5597. [DOI] [PubMed] [Google Scholar]
- Ballestar E., Abad,C. and Franco,L. (1996) Core histones are glutaminyl substrates for tissue transglutaminase. J. Biol. Chem., 271, 18817–18824. [DOI] [PubMed] [Google Scholar]
- Boehm J.E., Singh,U., Combs,C., Antonyak,M.A. and Cerione,R.A. (2002) Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J. Biol. Chem., 277, 20127–20130. [DOI] [PubMed] [Google Scholar]
- Brehm A., Nielsen,S.J., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1999) The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J., 18, 2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford G.M., Smith,J.S., Plummer,M., Munoz,N. and Franceschi,S. (2003) Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer, 88, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordella-Miele E., Miele,L., Beninati,S. and Mukherjee,A.B. (1993) Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J. Biochem., 113, 164–173. [DOI] [PubMed] [Google Scholar]
- De Laurenzi V. and Melino,G. (2001) Gene disruption of tissue transglutaminase. Mol. Cell. Biol., 21, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.F., Rhee,S.G. and Im,M.J. (1996) Evidence that phospholipase delta1 is the effector in the Gh (transglutaminase II)-mediated signaling. J. Biol. Chem., 271, 16451–16454. [DOI] [PubMed] [Google Scholar]
- Fesus L. and Piacentini,M. (2002) Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem. Sci., 27, 534–539. [DOI] [PubMed] [Google Scholar]
- Folk J.E. (1980) Transglutaminases. Annu. Rev. Biochem., 49, 517–531. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Furuse,M., Uwabe,K., Maki,H. and Yoshie,O. (1994) Nuclear localization and transforming activity of human papillomavirus type 16 E7-beta-galactosidase fusion protein: characterization of the nuclear localization sequence. Virology, 204, 789–793. [DOI] [PubMed] [Google Scholar]
- Grenard P., Bates,M.K. and Aeschlimann,D. (2001) Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J. Biol. Chem., 276, 33066–33078. [DOI] [PubMed] [Google Scholar]
- Grootjans J.J., Groenen,P.J. and de Jong,W.W. (1995) Substrate requirements for transglutaminases. Influence of the amino acid residue preceding the amine donor lysine in a native protein. J. Biol. Chem., 270, 22855–22858. [DOI] [PubMed] [Google Scholar]
- Hammad S.M., Ranganathan,S., Loukinova,E., Twal,W.O. and Argraves,W.S. (1997) Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. J. Biol. Chem., 272, 18644–18649. [DOI] [PubMed] [Google Scholar]
- Heck D.V., Yee,C.L., Howley,P.M. and Munger,K. (1992) Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl Acad. Sci. USA, 89, 4442–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.S., Patrick,D.R., Edwards,G., Goodhart,P.J., Huber,H.E., Miles,L., Garsky,V.M., Oliff,A. and Heimbrook,D.C. (1993) Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol. Cell. Biol., 13, 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J.H., Cho,S.Y., Kim,C.W., Shin,D.M., Kweon,J.C., Choi,K.H., Park,S.C. and Kim,I.G. (2002a) GTP is required to stabilize and display transamidation activity of transglutaminase 2. Biochem. Biophys. Res. Commun., 294, 818–822. [DOI] [PubMed] [Google Scholar]
- Jeon J.H., Cho,S.Y., Kim,C.W., Shin,D.M., Kwon,J.C., Choi,K.H. and Kim,I.G. (2002b) Improved immunodetection of human papillomavirus E7. Exp. Mol. Med., 34, 481–484. [DOI] [PubMed] [Google Scholar]
- Jeon J.H., Kim,C.W., Shin,D.M., Kim,K.I., Cho,S.Y., Kwon,J.C., Choi,K.H., Kang,H.S. and Kim,I.G. (2003) Differential incorporation of biotinylated polyamines by transglutaminase 2. FEBS Lett., 534, 180–184. [DOI] [PubMed] [Google Scholar]
- Lam E.W., Morris,J.D., Davies,R., Crook,T., Watson,R.J. and Vousden,K.H. (1994) HPV16 E7 oncoprotein deregulates B-myb expression: correlation with targeting of p107/E2F complexes. EMBO J., 13, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.O., Russo,A.A. and Pavletich,N.P. (1998) Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature, 391, 859–865. [DOI] [PubMed] [Google Scholar]
- Lesort M., Attanavanich,K., Zhang,J. and Johnson,G.V. (1998) Distinct nuclear localization and activity of tissue transglutaminase. J. Biol. Chem., 273, 11991–11994. [DOI] [PubMed] [Google Scholar]
- Liu S., Cerione,R.A. and Clardy,J. (2002) Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl Acad. Sci. USA, 99, 2743–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L. and Graham,R.M. (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol., 4, 140–156. [DOI] [PubMed] [Google Scholar]
- Lu W., Strohecker,A. and Ou,J.H. (2001) Post-translational modification of the hepatitis C virus core protein by tissue transglutaminase. J. Biol. Chem., 276, 47993–47999. [DOI] [PubMed] [Google Scholar]
- Massimi P., Pim,D. and Banks,L. (1997) Human papillomavirus type 16 E7 binds to the conserved carboxy-terminal region of the TATA box binding protein and this contributes to E7 transforming activity. J. Gen. Virol., 78, 2607–2613. [DOI] [PubMed] [Google Scholar]
- Munger K. and Halpern,A.L. (1997) HPV16 E7: primary structure and biological properties. In Human Papillomaviruses 1997 Compendium, Part III, pp. 17–36. [Online]. The human papillomaviruses compendium on line: http://hpv-web.lanl.gov [Google Scholar]
- Munger K. and Howley,P.M. (2002) Human papillomavirus immortalization and transformation functions. Virus Res., 89, 213–228. [DOI] [PubMed] [Google Scholar]
- Munger K., Basile,J.R., Duensing,S., Eichten,A., Gonzalez,S.L., Grace,M. and Zacny,V.L. (2001) Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene, 20, 7888–7898. [DOI] [PubMed] [Google Scholar]
- Nanda N., Iismaa,S.E., Owens,W.A., Husain,A., Mackay,F. and Graham,R.M. (2001) Targeted inactivation of Gh/tissue transglutaminase II. J. Biol. Chem., 276, 20673–20678. [DOI] [PubMed] [Google Scholar]
- Park J.S., Kim,E.J., Kwon,H.J., Hwang,E.S., Namkoong,S.E. and Um,S.J. (2000) Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J. Biol. Chem., 275, 6764–6769. [DOI] [PubMed] [Google Scholar]
- Pastor M.T., Diez,A., Perez-Paya,E. and Abad,C. (1999) Addressing substrate glutamine requirements for tissue transglutaminase using substance P analogues. FEBS Lett., 451, 231–234. [DOI] [PubMed] [Google Scholar]
- Peng X., Zhang,Y., Zhang,H., Graner,S., Williams,J.F., Levitt,M.L. and Lokshin,A. (1999) Interaction of tissue transglutaminase with nuclear transport protein importin-alpha3. FEBS Lett., 446, 35–39. [DOI] [PubMed] [Google Scholar]
- Romanczuk H. and Howley,P.M. (1992) Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl Acad. Sci. USA, 89, 3159–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang B.C. and Barbosa,M.S. (1992) Single amino acid substitutions in ‘low-risk’ human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the ‘high-risk’ HPV E7 oncoproteins. Proc. Natl Acad. Sci. USA, 89, 8063–8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J., Kuret,J. and Johnson,G.V. (1999) Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J. Neurochem., 73, 1871–1880. [PubMed] [Google Scholar]
- Woodman C.B., Collins,S., Winter,H., Bailey,A., Ellis,J., Prior,P., Yates,M., Rollason,T.P. and Young,L.S. (2001) Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet, 357, 1831–1836. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kang,D.E., Xia,W., Okochi,M., Mori,H., Selkoe,D.J. and Koo,E.H. (1998) Subcellular distribution and turnover of presenilins in transfected cells. J. Biol. Chem., 273, 12436–12442. [DOI] [PubMed] [Google Scholar]
- Zwerschke W., Mazurek,S., Massimi,P., Banks,L., Eigenbrodt,E. and Jansen-Durr,P. (1999) Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc. Natl Acad. Sci. USA., 96, 1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerschke W., Mannhardt,B., Massimi,P., Nauenburg,S., Pim,D., Nickel,W., Banks,L., Reuser,A.J. and Jansen-Durr,P. (2000) Allosteric activation of acid alpha-glucosidase by the human papillomavirus E7 protein. J. Biol. Chem., 275, 9534–9541. [DOI] [PubMed] [Google Scholar]