Abstract
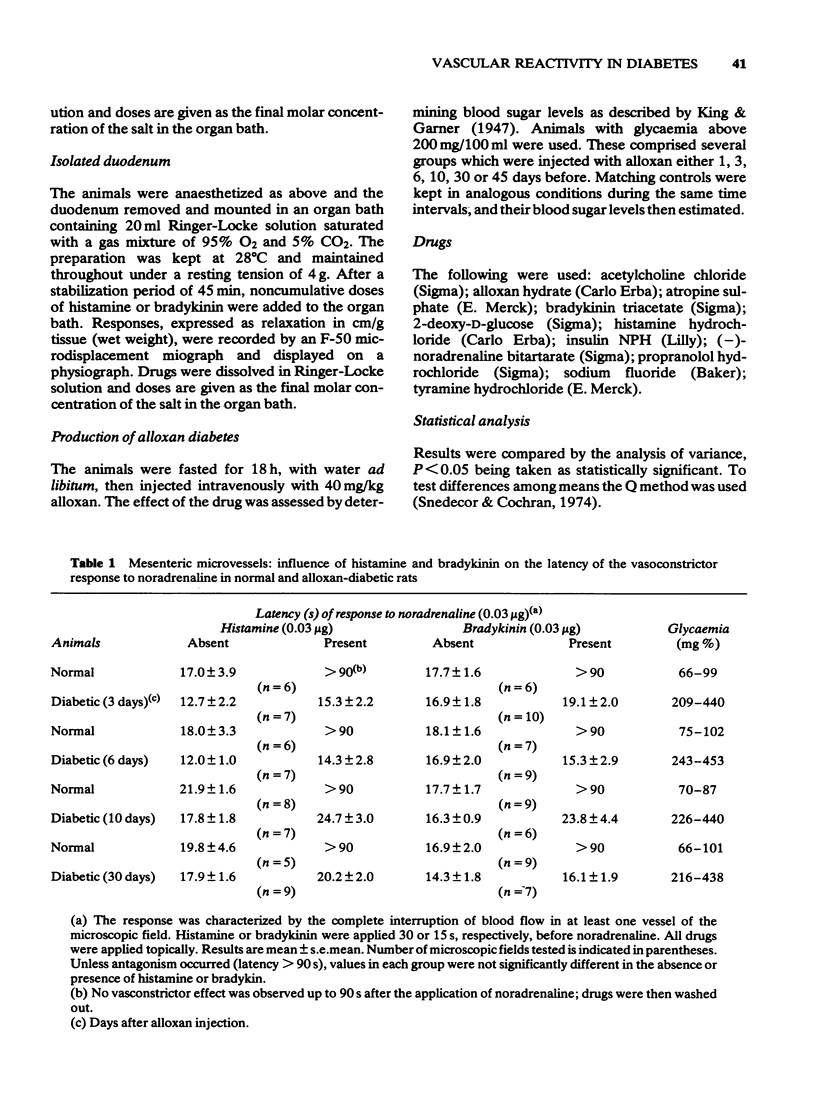
1 Noradrenaline (NA) evoked a vasoconstrictor response in rat mesenteric microvessels in situ, the latency and nature of which was analogous in normal and alloxan-diabetic animals.
2 Histamine and bradykinin (Bk) were capable of antagonizing the response to NA in normal but not in diabetic animals. In contrast, acetylcholine (ACh) was equally effective as an antagonist to NA in both groups of animals.
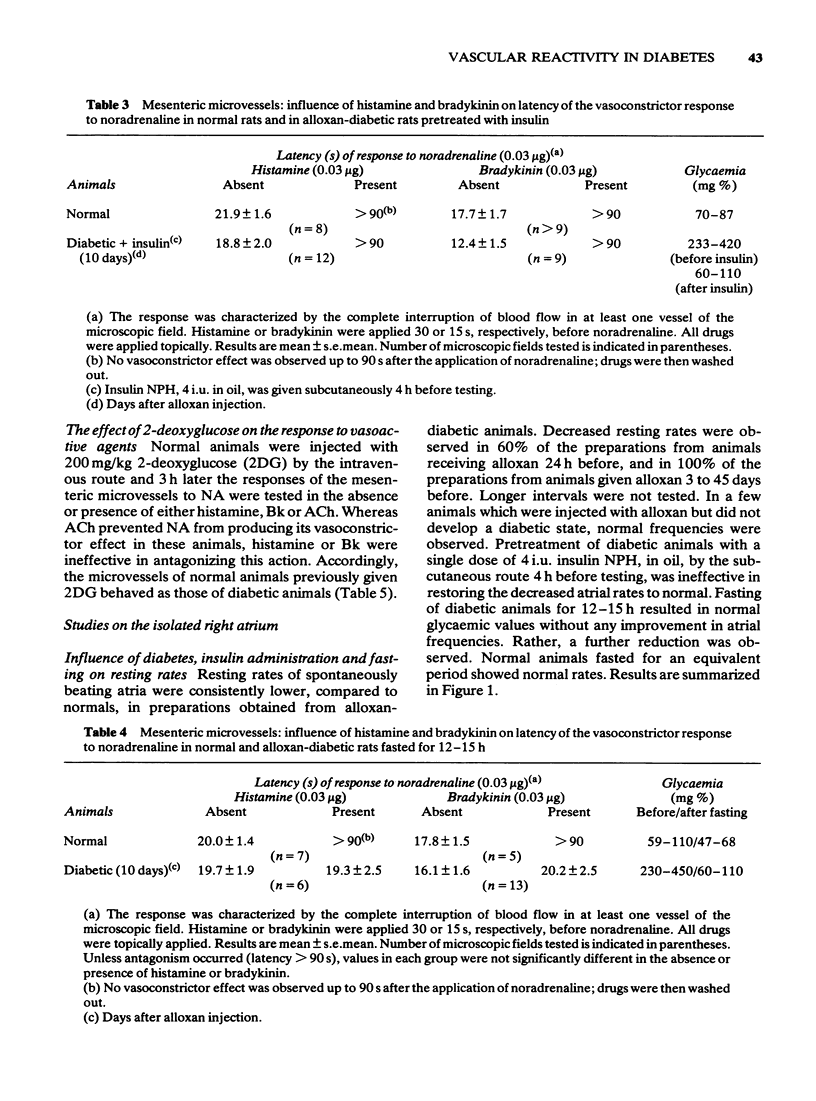
3 The altered responses to histamine and Bk were not associated with hyperglycaemia since fasting rendered the diabetic animals normoglycaemic and yet did not restore the reactivity of microvessels. Previous administration of insulin to diabetic animals corrected the impaired responses to histamine and Bk.
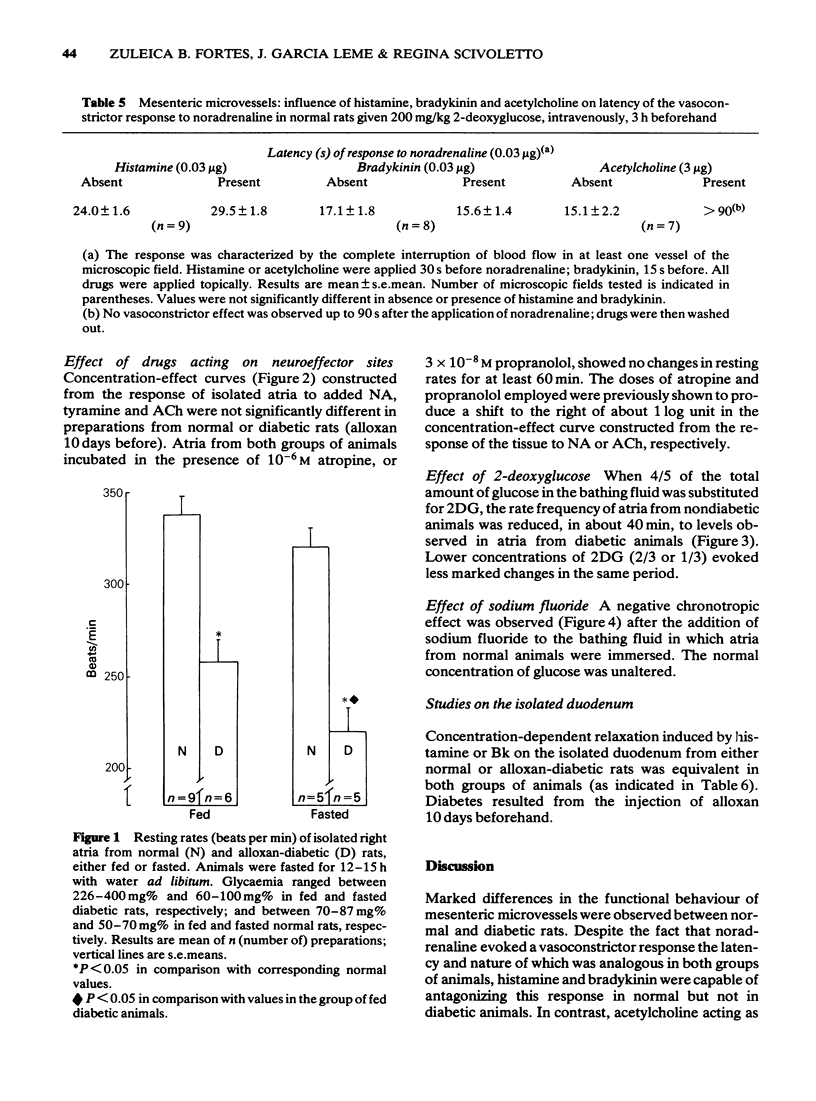
4 A similar condition of impaired responses to histamine and Bk was produced in normal animals by the intravenous injection of 2-deoxyglucose although ACh remained fully active.
5 Apparently, the functional changes observed in the response to histamine or Bk, as antagonists of the vasoconstrictor reaction to NA, were not associated with a defective response of all smooth muscle. First, because ACh remained active in diabetic animals, and, second, because extravascular smooth muscles obtained from either normal or diabetic rats were equally relaxed by histamine or Bk in vitro.
6 It is suggested that histamine and Bk antagonized the vasoconstrictor response of microvessels to NA through an action on lining endothelial cells resulting in increased vascular permeability and hyperosmolarity of extracellular fluids.
7 The process depended on the availability of insulin, and, therefore, might be affected by intracellular glucopaenia as occurring in diabetes.
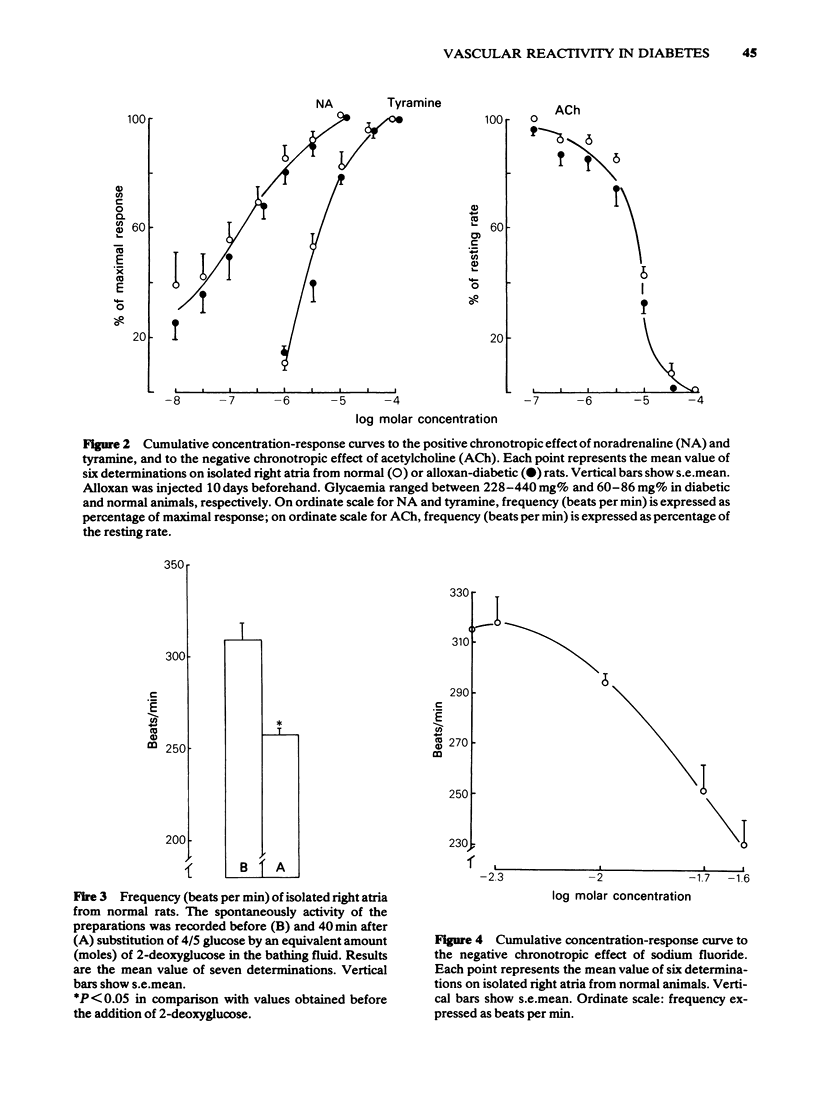
8 Intracellular glucopaenia markedly affected other structures. Reduced atria rates were observed in diabetes, despite the fact that the isolated preparation responded normally to NA, ACh or tyramine. Partial substitution of glucose in the bathing fluid by 2-deoxyglucose or addition of NaF to the organ bath evoked similar changes in atria from normal animals.
9 ACh which has little effect on vascular permeability must exert its vasodilator effects through mechanisms which are different from those influenced by the biochemical changes occurring in diabetes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GOTH A. Inhibition of anaphylactoid edema in the rat by 2-deoxyglucose. Am J Physiol. 1959 Nov;197:1056–1058. doi: 10.1152/ajplegacy.1959.197.5.1056. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C., Rona G. Cytoplasmic contractile apparatus in aortic endothelial cells of hypertensive rats. Lab Invest. 1975 Feb;32(2):227–234. [PubMed] [Google Scholar]
- Garcia-Leme J., Böhm G. M., Migliorini R. H., de Souza M. Z. Possible participation of insulin in the control of vascular permeability. Eur J Pharmacol. 1974 Dec;29(2):298–306. doi: 10.1016/0014-2999(74)90030-2. [DOI] [PubMed] [Google Scholar]
- Haddy F. J., Scott J. B. Metabolic factors in peripheral circulatory regulation. Fed Proc. 1975 Oct;34(11):2006–2011. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- King E. J., Garner R. J. The Colorimetric Determination of Glucose. J Clin Pathol. 1947 Nov;1(1):30–33. doi: 10.1136/jcp.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG C., THOMSON A. R. Studies involving enzymic phosphorylation. 5. The activation of rat-brain hexokinase by erythrocyte lysates and muscle extracts. Biochem J. 1955 Nov;61(3):465–472. doi: 10.1042/bj0610465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme J. G., Hamamura L., Migliorini R. H., Leite M. P. Influence of diabetes upon the inflammatory response of the rat. A pharmacological analysis. Eur J Pharmacol. 1973 Jul;23(1):74–81. doi: 10.1016/0014-2999(73)90246-x. [DOI] [PubMed] [Google Scholar]
- Llorach M. A., Böhm G. M., Leme J. G. Decreased vascular reactions to permeability factors in experimental diabetes. Br J Exp Pathol. 1976 Dec;57(6):747–754. [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. A., Shepherd J. T. Hyperosmolarity: effects on nerves and smooth muscle of cutaneous veins. Am J Physiol. 1976 Jul;231(1):141–147. doi: 10.1152/ajplegacy.1976.231.1.141. [DOI] [PubMed] [Google Scholar]
- Mellander S., Lundvall J. Role of tissue hyperosmolality in exercise hyperemia. Circ Res. 1971 Jan;28(Suppl):39–45. [PubMed] [Google Scholar]
- Osterby R., Gundersen H. J., Christensen N. J. The acute effect of insulin on capillary endothelial cells. Diabetes. 1978 Jul;27(7):745–749. doi: 10.2337/diab.27.7.745. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Apparent reversal of insulin resistance in cardiac muscle in alloxan-diabetes by 2-bromostearate. Nature. 1969 Feb 22;221(5182):777–777. doi: 10.1038/221777a0. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]


