Abstract
Patients with severe coagulation factor VIII deficiency require frequent infusions of human factor VIII (hFVIII) concentrates to treat life-threatening hemorrhages. Because these patients are immunologically hFVIII-naive, a significant treatment complication is the development of inhibitors or circulating alloantibodies against hFVIII, which bind the replaced glycoprotein, increase its plasma clearance, and inhibit its activity, preventing subsequent treatments from having a therapeutic effect. A genetic approach toward the induction of immunologic unresponsiveness to hFVIII has the conceptual advantage of a long-term, stable elimination of undesired immune responses against hFVIII. Here, we report that in a factor VIII (FVIII)-deficient mouse model for severe hemophilia A, genetic modification of donor bone marrow cells with a retroviral vector encoding hFVIII, and transplant to hemophiliac mouse recipients, results in the induction of immune tolerance to FVIII in 50% of treated animals after immunization with hFVIII, despite the fact that hFVIII protein or activity is undetectable. In tolerized animals, the titers of anti-hFVIII binding antibodies and of hFVIII inhibitor antibodies were significantly reduced, and there was evidence for hFVIII unresponsiveness in CD4+ T cells. Importantly, the plasma clearance of hFVIII was significantly decreased in tolerized animals and was not significantly different from that seen in a FVIII-naive hemophiliac mouse. This model system will prove useful for the evaluation of genetic therapies for hFVIII immunomodulation and bring genetic therapies for hFVIII tolerance closer to clinical application for patients with hemophilia A.
Keywords: Factor VIII-deficient mice, immune tolerance, gene therapy, transplantation
Hemophilia A is an X-linked, recessive bleeding disorder, which affects 1 in 10,000 males, resulting in defective or deficient human clotting factor VIII (hFVIII) molecules, which, in its severe form (50% of cases), is a life-threatening, crippling hemorrhagic disease (1). Factor VIII (FVIII) is a large glycoprotein cofactor, which accelerates the activation of coagulation factor X, catalyzed by factor IX. Its major site of synthesis and secretion into the plasma is the liver (2), and it consists of a series of homologous domains (3), circulating in the plasma as a metal ion-linked heterodimer, noncovalently associated with von Willebrand factor (vWf; ref. 4).
Severe hemophilia A is commonly treated by replacement therapy, consisting of frequent i.v. infusions of hFVIII concentrates. A serious treatment complication in severe hemophilia A is the development of “inhibitor” antibodies (5) directed against FVIII. These antibodies bind hFVIII, indirectly reduce its function by increasing its plasma clearance rate (i.e., reducing bioavailability), and/or directly inhibit its function as an enzymatic cofactor. These inhibitory antibodies are oligoclonal IgG that bind to at least six different hFVIII epitopes, most commonly located within the A2 (heavy chain) and C2 (light chain) domains (6, 7). The reported incidence of inhibitors in patients with severe hemophilia A is ≈20%, ranging from 5% to 52% (8, 9). Patients with mild or moderate hemophilia A are at a much lower risk of inhibitor development. Of severe hemophiliacs with inhibitors, ≈25% are low responders, with low titer inhibitors [3–5 Bethesda units], and 75% are high responders, with higher titers (>10 Bethesda units), which increase with further exposure to hFVIII (anamnestic response). To date, ≈75% of high responder patients treated with high-dose tolerance regimens have been “cured” of inhibitors long term, but this approach requires very frequent injections of FVIII concentrate and is thus extremely costly, in some cases exceeding 1 million dollars (10).
The murine FVIII gene and protein are highly homologous to their human counterparts. Recently, mouse models for severe hemophilia A were described. Two lines of FVIII-knockout mice were generated by Neo gene disruptions in exon 16 or 17 of the murine FVIII gene. These mice completely lack plasma FVIII activity and do not survive tail biopsies without cautery (11). Whereas both lines of mice are devoid of FVIII light chain antigen in the plasma (12), it is not known whether FVIII heavy chain antigen is present. Thus, it is not known whether these mice are immunologically FVIII-naive for all FVIII epitopes. However, these mice do mount a FVIII inhibitor antibody response after repeated i.v. injection of hFVIII, in the absence of adjuvant (J. Qian and L. Hoyer, personal communication).
It is well known that, in adult rodents, hematopoietic chimerism created via allogeneic bone marrow (BM) transplant into conditioned recipients is associated with donor-specific allograft transplantation tolerance (reviewed in ref. 13). Similarly, the induction of donor-specific immune tolerance to transgene proteins encoded in hematopoietic donor cells derived from transgenic animals has been reported (14). This central form of tolerance is thought to derive from the expression of donor antigens in BM-derived antigen-presenting cells (e.g., dendritic cells, macrophages, and B cells), during immune reconstitution, resulting in the deletion or anergic inactivation of T cell clones bearing “self”-reactive T cell antigen receptor (reviewed in ref. 15). The methods developed for retroviral vector-mediated gene transfer into hematopoietic progenitors in the mouse are now very efficient, allowing routine achievement of >30% gene transfer in circulating white blood cells (16, 17). Thus, several laboratories recently have applied gene transfer to central tolerance induction, using murine hematopoietic precursors as tolerogenic vehicles to induce vector-specific tolerance to murine class I H-2Kb (18, 19), to a lymphocytic choriomeningitis virus glycoprotein associated with experimental autoimmune diabetes (20), to HLA-A2.1 (21), and to the bacteriophage λ peptide antigen 12–26 fused to IgG (22). These protein antigens range in size from 2 to 64 kDa. Herein, we report the successful genetic induction of immune tolerance to the complex (>170 kDa), hFVIII glycoprotein in nonimmune FVIII-deficient mice.
MATERIALS AND METHODS
FVIII-Deficient Mice.
Eight- to 16-wk-old affected male, exon 17 FVIII knockout mice (11, 12) were used as allogeneic BM transplant donors and recipients. This colony was derived by serial breeding of a 129SV founder knockout mouse three times with inbred C57BL/6 mice, followed by inbreeding. All animal procedures were carried out in accordance with institutional and National Institutes of Health guidelines.
Retroviral Vectors and Producer Cells.
The Moloney-based retroviral vectors used were GCsamF8EN (23), encoding human B domain-deleted hFVIII plus neomycin phosphotransferase as a selectable marker, and LNL6 (24), encoding only the latter. Ecotropic producer clones were derived by transduction of the packaging line GP+E86 (25), G418 selection, and limiting dilution cloning. The titers of the vectors were 3–5 × 106 G418-resistant colony-forming units/ml on NIH 3T3 cells.
Mouse Bone Marrow Transplant/Transductions.
Gene transfer into total mouse BM, and BM transplants were carried out as described (16). Recipients were transplanted with 1–2 × 106 transduced BM cells, given i.v. Immediately before transplant, they were conditioned with 900 rad whole body irradiation from a 137Cs source.
Humoral Immune Responses.
At 16 wk post-BM transplant, recipient mice were given a primary i.p. immunization of 10 μg of hFVIII, in the form of clinical grade, full-length hFVIII (Recombinate, Baxter Health Care, Mundelein, IL) emulsified with Hunter’s TiterMax adjuvant (Sigma), given in 0.5–1.0 ml. The hFVIII preparation also contained 2% by mass of hvWf. At 20 wk posttransplant, recipients received a boost of 1 μg of hFVIII without adjuvant, delivered i.m. in 0.1 ml to the hind limbs, and at 26 wk, they received a second boost of 1 μg of hFVIII, delivered i.v. in 0.2 ml. Before and after immunizations, blood samples were collected by periorbital bleeding and serum was analyzed for anti-hFVIII and anti-hvWf binding antibody titers by ELISA using 96-well Immulon 4 plates (Dynatech). Plates were coated overnight at 4°C with 500 ng/ml purified B domain-deleted hFVIII (Genetics Institute, Cambridge, MA) or with purified hvWf (ref. 26; a gift of E. Saenko and L. W. Hoyer, American Red Cross, Rockville, MD) in 0.05 M sodium carbonate/bicarbonate buffer (pH 9.6), blocked for 2 h at room temperature with ELISA blocker (PBS/10% horse serum/1 mM CaCl2), incubated overnight at 4°C with duplicate 2-fold serial dilutions of test sera, incubated for 2 h at 4°C with 1:10,000 sheep–anti-mouse IgG-horseradish peroxidase (Amersham) for 2 h at 4°C, and finally developed with O-phenylenediamine dihydrochloride (Sigma). Plates were read in an Emax precision microplate reader (Molecular Devices) at 490 nm. Titers were taken as the dilution of test serum, relative to the dilution of preimmune serum, needed to reduce the signal to <0.02 A490.
Plasma FVIII Assays.
hFVIII expression was assayed in preimmune, citrated recipient plasma, collected as for sera collection and frozen at −70°C in aliquots, by two assays. The first was a chromogenic activity assay (COATEST FVIII; Chromogenix, Molndal, Sweden), which measures the FVIII-dependent generation of FXa from FX. The second expression assay was an ELISA specific for hFVIII (27), by which hFVIII clearance also was determined in experimental animals. The sensitivity of the activity assay was ≈1 ng/ml FVIII using reconstituted, normal pooled human plasma as a standard. The sensitivity of the hFVIII ELISA assay, in which plasma was diluted 5-fold, was ≈5 ng/ml (plasma equivalent), using purified B domain-deleted hFVIII as a standard.
FVIII Inhibitor Assays.
hFVIII inhibitor titers were measured after the second boost in Bethesda units (28) in test sera heated to 56°C for 30 min to inactivate any residual thrombin. hFVIII function was measured by activated partial thromboplastin time, with activated partial thromboplastin time-FSL activating reagent (Sigma), by using a BBL Fibrosystem Fibrometer (Fisher Scientific) in a total volume of 0.3 ml.
Cell-Mediated Immune Responses Against FVIII.
Recipient mice were sacrificed, and T cell responses to hFVIII were evaluated by proliferation assays in 96-well plates, as described (29). The T cell source consisted of CD4+ splenocytes positively enriched by one round of selection with CD4 immunomagnetic beads (Dynal, Great Neck, NY). The resulting populations contained ≈80% CD4+ cells by FACS (Becton Dickinson). The accessory cell fraction consisted of spleen cells from FVIII-naive, untreated exon 17 FVIII-“knockout” mice, depleted of T cells by negative selection with Thy 1.2 immunomagnetic beads (Dynal) and treated with mitomycin C 50μ/ml to prevent their proliferation. The 1 × 105 T cells and 5 × 105 accessory cells were mixed with various concentrations of purified B domain-deleted hFVIII in triplicate for 4½ days in RPMI medium 1640 plus 10% fetal bovine serum at 37°C. Following a 24-hr incubation at 37°C in the presence of 1 μCi [3H]thymidine (New England Nuclear), incorporation was measured via an automatic plate harvester (Tomtec, Orange, CT) and a scintillation counter.
Vector Nucleic Acid Analysis.
DNA and RNA were isolated by using commercial reagents, for genomic DNA (Nucleon II, Scotlab, Shelton, CT) and for total RNA (Trizol, Life Technologies, Gaithersburg, MD). Proviral vector DNAs were measured in 0.2 μg of BM genomic DNA by semiquantitative PCR, including 0.5 μCi (1 Ci = 37 GBq) of [α-32P]dCTP (Amersham) in a 100 μl reaction, and using GeneAmp PCR reagents (Perkin-Elmer). The sequences of the GCsamF8EN vector primers, which amplify a 557-bp fragment, are: F82F, 5′-GACTTTCGGAACAGAGGCATGACCGCC-3′; and F82R, 5′-GGCCCCAGGAGTCCCAAATGTTCATTT-3′. An endogenous mouse β-globin DNA fragment was coamplified as described (30). The PCR conditions used were 25 cycles with an annealing temperature of 59°C. All PCR reaction products were resolved by 4% polyacrylamide gel electrophoresis. They were then visualized and quantified on a BAS-1500 phosphorimager (Fuji). The percent gene transfer values were estimated by normalizing vector band intensities to endogenous band intensities for the same genomic DNA sample and comparing the resulting ratio to that obtained with standards (genomic DNA derived from predetermined mixes of vector-negative NIH 3T3 cells with vector-positive NIH 3T3/GCsamF8EN cells).
For RNA analysis by semiquantitative reverse transcript–PCR (RT-PCR), RNA was reverse transcribed using the Reverse Transcription System (Promega), and then 5% of this product was used in 100-μl PCR reactions with 0.5 μCi of [α-32P]dCTP for amplification of a hFVIII fragment (same as above) or, in a separate reaction, of a β2-microglobulin control fragment (31). These PCR reactions were carried out for 32 cycles with annealing temperatures of 60°C (FVIII) and 58°C (β2-microglobulin), and the reactions were mixed before gel analysis.
RESULTS
Humoral Immune Responses Against hFVIII in Hemophiliac Mice Transplanted with BM Transduced by a Retroviral hFVIII Vector.
Outbred, affected male, exon 17 FVIII-“knockout” mice were used as both donors and recipients for allogeneic BM transplants, coupled with retroviral vector transduction. Recipients were transplanted with BM transduced by either the GCsamF8EN vector (23), encoding a hFVIII cDNA and neomycin phosphotransferase, or the LNL6 vector (24), encoding only the latter. A total of 52 mice were transplanted, one-half with BM transduced with each vector, but ≈25% died from radiation-associated causes within 1 wk of transplant, leaving 37 animals for analysis. At 16 wk posttransplant, hFVIII expression was evaluated in the plasma of 19 GCsamF8EN recipients by a chromogenic activity assay (COATEST FVIII, Chromogenix, Molndal, Sweden) and by hFVIII-specific ELISA. Untreated exon 17 FVIII-“knockout” mice are known to be completely devoid of FVIII activity in the COATEST assay (11). By either assay, hFVIII was undetectable (data not shown), and thus, these animals expressed <1 ng/ml hFVIII in their plasma. This result was true despite the fact that vector DNA and RNA were detected easily by PCR (Fig. 4; see below). Mixing experiments with normal mouse and recipient plasmas in the activity assay failed to reveal the presence of any inhibitor substance in the recipient plasma. The step(s) of gene expression at which hFVIII secretion is blocked in hematopoietic cells is under investigation.
Figure 4.
Semiquantitative analysis of hFVIII retroviral vector (GCsamF8EN) DNA and RNA sequences in recipients at 26–35 wk posttransplant. Genomic DNA and total RNA were prepared in parallel from the same BM source. Representative data shown are from 8 of 13 analyzed GCsamF8EN BM transplant recipients. (A) Vector DNA was measured via coamplification of hFVIII vector sequences and mouse β-globin sequences by semiquantitative PCR, using NIH 3T3/GCsamF8EN DNA quantitative standards (Left). The estimated percent gene transfer for experimental animals (1–8) is shown at the top of each lane (Right). T, tolerant. NT, nontolerant. (B) Vector-specific hFVIII RNA was measured by semiquantitative RT-PCR using Raji/GCsamF8EN (Raji/FVIII) RNA and BM RNA from LNL6 recipients as positive and negative controls respectively. RT, reverse transcriptase. β2-m, β2-microglobulin. (−), PCR reactions without added reverse transcriptase; (+), reverse transcriptase added.
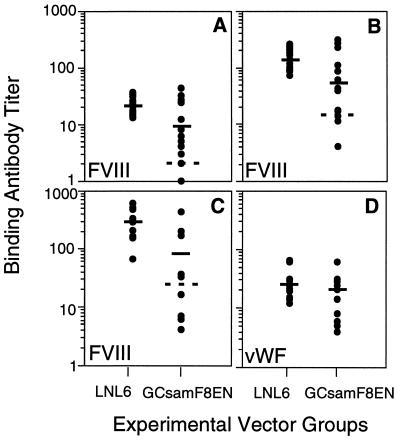
To test whether the BM transplant/gene transfer procedure with GCsamF8EN could confer immune tolerance to hFVIII, 18–19 animals in each vector group were immunized three times with clinical grade hFVIII beginning at 16 wk posttransplant, and anti-hFVIII binding antibody titers were determined by ELISA. The primary immunization consisted of a 10 μg hFVIII-equivalent dose of Recombinate in Titermax adjuvant (Sigma), given i.p. Four weeks later, the test (GCsamF8EN) sera had titers on average fourfold lower than the control (LNL6) group, and this difference was statistically significant (P < 0.01; Fig. 1A). Within the test group, a subgroup, consisting of low titer hFVIII responders (the 30% of the animals with the lowest titers), had an average titer 10-fold lower than the control group that was highly significant (P < 0.001). After a secondary immunization (without adjuvant, given i.m.), 17 sera from each vector group were analyzed and the titers were significantly higher in both groups but remained twofold lower in the test group (P < 0.01; Fig. 1B). Again, a subgroup of the GCsamF8EN recipients, now comprising 35% of the animals, had an average titer 17-fold lower than the control group (P < 0.001).
Figure 1.
Humoral immune responses against hFVIII and hvWf in hemophilia A mice after allogeneic transplant of BM transduced with GCsamF8EN or LNL6 retroviral vectors and immunization with clinical grade hFVIII. Anti-hFVIII (A–C) and anti-hvWf (D) binding antibody titers were measured by ELISA for the LNL6 control group (Left) and the GCsamF8EN vector group (Right), after each of three immunizations. Solid bars represent average titers; broken bars separate the GCsamF8EN group into two subgroups (high and low hFVIII responders). (A) Primary immunization. (B) First boost. (C) Second boost, anti-hFVIII response. (D) Second boost, anti-hvWf response. Data were pooled from two independent transplant experiments, and 2–4 independent measurements of antibody titers.
Two weeks after the final immunization (given i.v.) approximately one-third of each vector group had died, and >50% of these animals showed signs of severe i.p. hemorrhage. FVIII-deficient mice have an unusually high spontaneous mortality rate without handling (S. Connelly and M. Kaleko, personal communication; G.L.E. and R.A.M., unpublished data), and the rate may be in fact higher with the extensive handling needed in these experiments. Importantly, analysis of 10–12 sera from the survivors in each vector group again showed an overall threefold reduced average titer in the test group (P < 0.01) and a 28-fold reduced average titer in a low titer hFVIII responder subgroup, comprising fully 50% (5/10) of the test animals (P < 0.001; Fig. 1C). Overall, after all three immunizations, 30–50% of the test (GCsamF8EN) animals were at least partially unresponsive to hFVIII at the humoral level. At no time was a low titer test animal observed to convert to a high titer. Finally, as clinical grade hFVIII also contains hvWf, albeit at a much lower level than hFVIII, and because hvWf was not encoded in the test vector, anti-hvWf antibody titers were measured at the same time point as a specificity control. The FVIII-deficient mice are presumably wild type for a hvWf-like protein, but as the human and mouse counterparts are likely similar in structure but not identical, some cross-species immune response is expected. As expected, the average anti-hvWf titers in both groups were much lower than the anti-hFVIII titers at the same time point, but were measurable, and the slight differences between the two vector groups were not statistically significant (P < 0.4; Fig. 1D). Thus, the induction of immune tolerance in the test group was related specifically to hFVIII.
hFVIII Inhibitor Titers.
Antibodies that bind hFVIII may or may not be directly inhibitory to its cofactor function. To see whether GCsamF8EN BM transplant recipients had reduced inhibitor titers in addition to reduced hFVIII binding antibody titers, hFVIII inhibitor titers were measured by the Bethesda assay (activated partial thromboplastin time). This assay was done using the seven lowest anti-hFVIII-titer sera from the GCsamF8EN group and seven sera picked at random from the control group. On average, hFVIII inhibitor titers were reduced 60-fold in the low titer hFVIII responder subgroup of the GCsamF8EN group, compared with LNL6 controls, and in two sera were undetectable (Table 1). The inhibitor titers in three sera from the high titer hFVIII-responder GCsamF8EN subgroup were not statistically different from LNL6 controls (data not shown).
Table 1.
hFVIII inhibitor titers after hFVIII immunization of hemophilia A mice transplanted with retroviral vector-transduced bone marrow
| LNL6, n = 7 | GCsamF8EN (low titer antiFVIII responders), n = 7 |
|---|---|
| 400 ± 35 | 8 ± 1 |
| 255 ± 26 | 6 ± 2 |
| 210 ± 10 | 4 ± 1 |
| 125 ± 15 | 2 ± 1 |
| 120 ± 7 | 2 ± 1 |
| 115 ± 20 | 0 |
| 105 ± 10 | 0 |
Inhibitor titers were measured in heat-treated sera after the third hFVIII immunization (second boost), for 7/12 surviving recipients in the LNL6 group (all high titer anti-hFVIII binding antibody responders), and all of the low titer anti-hFVIII recipients (7/10 survivors) in the GCsamF8EN group. Preimmune sera, sera from the high titer anti-hFVIII recipients in the GCsamF8EN group (3/10 survivors), and high-responder inhibitor plasma from a severe hemophiliac, had average titers of 0, 125, and 75 BU, respectively. Data are presented as average ± SD for triplicate determinations. BU, Bethesda units.
Clearance of hFVIII.
The presence of high titer anti-hFVIII inhibitory antibodies is correlated strongly with a reduced plasma half-life of hFVIII (5). Conversely, low titer binding and inhibitor antibodies should correlate with a normal hFVIII half-life. To see whether GCsamF8EN BM transplant recipients had prolonged (i.e., normal) plasma hFVIII half-lives, a 12-hr plasma clearance study was carried out immediately after the final boost immunization (given i.v.). hFVIII was measured by human-specific FVIII ELISA. In the six GCsamF8EN recipients with the lowest anti-hFVIII binding antibody titers after the first boost immunization, hFVIII was recoverable in super-physiologic amounts and clearance was normal for a mouse, with a biphasic clearance curve characterized by an initial t1/2 of 1–2 hr, followed by a second phase with a t1/2 of 4–5 hr (Fig. 2B). This clearance curve was indistinguishable from either that seen in FVIII-naive, untreated exon 17 FVIII-knockout mice in previous experiments (Fig. 2B; G.L.E. and R.A.M., unpublished data) or from that reported previously in FVIII-naive, normal C57BL/6 mice (27). In contrast, in seven of nine control animals analyzed in the same way, hFVIII was undetectable 1 hr after injection (Fig. 2A).
Figure 2.
Clearance of hFVIII in the two vector groups immediately after the secondary FVIII immunization. At time zero, 1 μg of clinical grade hFVIII was injected i.v., and at the times indicated, plasma was collected and hFVIII was measured by ELISA as described in Materials and Methods. Data shown are average detectable plasma concentrations of hFVIII ± SD for the LNL6 control animals (A; n = 9), for the GCsamF8EN low titer anti-hFVIII antibody responders (B, filled symbols, n = 6), and the GCsamF8EN high titer responders (B, open symbols, n = 3). The broken lines represent hFVIII clearance in FVIII-naive, untreated exon 17 FVIII-deficient mice, determined in separate experiments.
Cell-Mediated Immune Responses Against hFVIII.
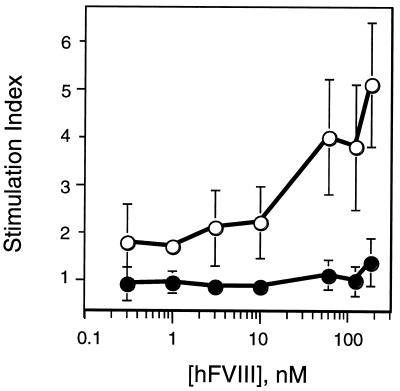
Robust humoral immune responses against protein antigens proceed with the help of CD4+ T cells, activated by peptide antigen presented via the class II major histocompatibility complex pathway. To see whether the two vector groups could be distinguished with respect to T cell responses to hFVIII, at 35 wk post-BM transplant, T cell proliferation assays were carried out with CD4+ T splenocytes. The test animals were four surviving low titer hFVIII responders from the GCsamF8EN group, which had exhibited reduced anti-hFVIII binding antibody titers, reduced or undetectable inhibitors, and a normal hFVIII plasma clearance profile, and six control animals, all immunized in vivo. Although CD4+ splenocytes enriched from the test group proliferated poorly in the presence of hFVIII in vitro, the proliferation of control CD4+ cells was stimulated up to 5-fold by high concentrations of hFVIII (Fig. 3). This result was true despite the fact that the background proliferation values for both groups were relatively high and variable (see Fig. 3 legend) under the conditions used (see Materials and Methods). Because of the limited number of surviving experimental animals in the test group, alternative sera or pulsing times were not investigated extensively.
Figure 3.
hFVIII-dependent proliferation of mouse CD4+ splenocytes derived from the two vector groups 35 wk posttransplant. Incorporation of [3H]thymidine into CD4+ splenocyte DNA was measured for the LNL6 group (open symbols) and for the GCsamF8EN group, low titer anti-FVIII antibody responders (filled symbols), after culture for 4½ days in the presence of purified B domain-deleted hFVIII. Animals were from two independent BM transplant experiments. The stimulation index represents the incorporation of 3H with the indicated concentration of FVIII divided by control incorporation with no FVIII for the same T cell source. The control values (average ± SD) were: LNL6, 14,232 ± 7,115 cpm and GCsamF8EN, 26,699 ± 13,265 cpm. Accessory cells alone gave <100 cpm. Data are presented as average stimulation index ± SD for n = 6 (LNL6) and n = 4 (GCsamF8EN).
hFVIII Gene Transfer Efficiency and Expression.
To investigate the mechanisms accounting for the observed differences in hFVIII humoral immune responses between the two GCsamF8EN subgroups, retroviral vector gene transfer efficiencies were quantified by semiquantitative DNA PCR, and the hFVIII RNA status was evaluated by RT-PCR. DNA and RNA was isolated from the BM of 10 low responders and three high responders at 26–35 wk posttransplant. Overall, the BM gene transfer efficiency was high in both subgroups, estimated at >30% on average (Fig. 4A and data not shown). Southern blots of BM- and spleen-genomic DNA with a Neo probe verified that these mice contained the FVIII-knockout allele and also showed a vector-specific band for those samples with >30% gene transfer by PCR (data not shown). There was no significant difference in gene transfer between the two subgroups. The gene transfer efficiency for the GCsamF8EN vector varied between 53% to 1% (on average) in the two independent BM transplant experiments, but, even with the lower level of gene transfer, 30–50% of the recipients were hFVIII-tolerant (data not shown). Similar variations in gene transfer efficiencies were observed in 13 LNL6 control animals (data not shown). Analysis of hFVIII RNA status by RT-PCR showed that all of the 13 GCsamF8EN recipients tested, tolerant or nontolerant to hFVIII, were positive for a low level of vector-derived hFVIII RNA in the BM (Fig. 4B and data not shown).
DISCUSSION
The development of inhibitor antibodies directed against hFVIII is a serious complication of severe hemophilia A patients, who are immunologically FVIII-naive. These antibodies effectively render subsequent factor hFVIII infusions useless because they rapidly bind hFVIII, reduce its bioavailability, and/or directly inhibit its function. The most effective current treatment for long-term removal of hFVIII inhibitor antibodies is high dose i.v. hFVIII immune tolerance induction (10), but this approach has disadvantages: It requires the patient to undergo very frequent injections, and the cost of this procedure is so high as to be prohibitive. The concept of using genetic therapy for hFVIII inhibitors has the potential advantage of an effective long-term resolution of the inhibitor problem, with potentially a single treatment and thus potentially the advantage of reduced overall cost. Experiments carried out in mice in the last 5 years have brought this concept closer to clinical implementation, in that they have demonstrated conclusively that genetic induction of immune tolerance to cell-surface or secreted protein antigens encoded in retroviral vectors is possible, using BM-derived hematopoietic cells (18–22) or peripheral, mature B cells (14, 22) as tolerogenic vehicles. Here, we present the preliminary application of the hematopoietic cell approach to the inhibitor problem in hemophilia A, in a mouse model for hemophilia A, which like severe hemophiliacs is immunologically FVIII-naive.
We found that we could specifically induce immune tolerance to hFVIII in 30–50% of FVIII-deficient mice by transplant of FVIII-deficient BM transduced with a retroviral vector expressing hFVIII. This success rate is similar to that reported previously in experiments involving the genetic induction of tolerance to murine class I H-2Kb (37%; ref. 19) but is lower than that reported for HLA-A2.1 or the 12–26 peptide-IgG fusion (both 100%; refs. 21, 22). This rate may result from inefficient hFVIII peptide presentation in BM-derived antigen-presenting cells (e.g., thymic dendritic cells) related to poor expression of the hFVIII protein. The increased structural complexity of hFVIII compared with the protein studied in previous reports also may be a factor. In the 30–50% of animals unresponsive to hFVIII in our study, the tolerance phenotype was characterized by partial hFVIII unresponsiveness at the humoral level (Fig. 1 and Table 1), which was correlated with a normal hFVIII half-life (Fig. 2). The low level of anti-hFVIII binding antibodies that could be detected in the low titer hFVIII responder group after the first boost immunization (titer <15; Fig. 1B), apparently had no significant effect on hFVIII in the plasma during the subsequent plasma clearance study immediately after the second boost immunization (Fig. 2). The same animals had inhibitors titers <10 Bethesda units 2 wk later. Thus, these animals had a phenotype analogous to that of hemophilia A patients with undetectable or low levels of inhibitors (i.e., low responder inhibitors) and normal hFVIII bioavailability. Anti-hFVIII titers did rise in this subgroup with hFVIII exposure over time, but never rose above ≈15, presumably because the anti-hFVIII immune response was lacking T cell help, as evidenced by the lack of proliferation of CD4+ splenocytes in vitro in the presence of hFVIII (Fig. 3).
The mechanisms accounting for the 30–50% success rate in this study were not revealed. hFVIII-tolerant and hFVIII-nontolerant BM transplant recipients within the GCsamF8EN group did not differ significantly with respect to BM gene transfer or with respect to BM hFVIII RNA expression at 8 mo posttransplant (Fig. 4B and data not shown). Among both subgroups the level of BM gene transfer varied between 1% and 100%, and all animals expressed low levels of vector-derived hFVIII RNA in their BM. Although we were unable to detect differences in BM hFVIII RNA expression at 8 mo post-BM transplant, differences may have been evident in the first 4 mo post-BM transplant, during which time clonal fluctuation in the hematopoietic system post-BM transplant previously has been identified (32). The critical event for genetic induction of tolerance to hFVIII in this model system may in fact be expression in a particular type of BM-derived antigen-presenting cells (e.g., thymic dendritic cells or nonthymic B cells) at a particular time during immune reconstitution. This might result from hFVIII expression in a particular type of hematopoietic progenitor cell. Further experiments are required to specifically identify these events.
This study confirms that the genetic induction of tolerance, via gene transfer coupled with BM chimerism in mice, is possible even for very complex proteins such as hFVIII, which bear potentially very large numbers of immunogenic epitopes. Previous, analogous studies (18–22) have reported success with protein antigens less than one-half as large as hFVIII. Moreover, it confirms the reports of others that only very low levels of tolerogen (tolerance-inducing protein) gene transfer and expression are necessary for genetic induction of immune tolerance in mice and that there is often no correlation between tolerogen expression at a biochemical level and immune tolerance (19, 21, 22). It has been estimated that a mere 10–100 major histocompatibility complex class I– or major histocompatibility complex class II–peptide complexes per antigen-presenting cell are required for T cell recognition leading to T cell activation (33), and it is likely that the threshold level of major histocompatibility–tolerogen complexes for tolerance induction is similar. This level of tolerogen expression is obviously far below the limit of detection for current protein or nucleic acid detection methods.
The potential uses of a genetic therapy for hFVIII inhibitors in humans are 2-fold. First, it might be used as a stand-alone therapy for patients with inhibitors, or as a prophylactic measure for young or older patients without inhibitors, to prevent inhibitor formation in the context of conventional hFVIII replacement therapy. Second, it might be used in conjunction with a second genetic therapy designed to deliver hFVIII to the circulation, so as to prevent the development of an anti-hFVIII antibody response. Based on our results in the mouse model, autologous BM transplant of severe hemophilia A inhibitor patients, with hFVIII gene modification, may be an attractive approach for a future clinical trial using different conditioning methods. The morbidity and risks associated with lethal total body irradiation as conditioning for BM transplants in humans at present renders the current approach an unacceptable method, except in cases of concomitant malignant disease, in which the potential benefit of BM transplant outweighs the risks. Studies in animals suggest that total body irradiation may not be necessary for successful hematopoietic cell transfer. Mice conditioned with sublethal whole body irradiation (34), sublethal irradiation and hematopoietic growth factors (35), or nonmyeloablative thymic irradiation plus antibody treatment (36, 37) and dogs receiving no conditioning (38) can develop long-term bone marrow chimerism. Another potential obstacle to widespread future clinical application of the current approach is a preexisting inhibitor response in the recipients. It is not known how effective the current approach will be in this context, which contrasts with the FVIII-naive context reported on here. If the current approach is not successful in hemophiliacs with preexisting inhibitors, this might limit the patient population to hFVIII-naive infants in families with a history of hemophilia A. Future experiments in our mouse model, and necessarily in larger animal models, perhaps hemophilic dogs, will address these and other potential obstacles, as well as the alternative approach of using gene-modified antigen-presenting cells as peripheral tolerogenic delivery vehicles.
Acknowledgments
We wish to acknowledge Elias Zambidis for suggesting these experiments, Haig Kazazian, Jr. and Ann Lawler for providing FVIII-knockout mice, David Bodine for help with mouse BM transplant/transductions, Jay Lozier for help with hemophilia issues and ELISAs, Zhili Zheng for technical assistance, and Genetics Institute, Leon Hoyer, and Evgueni Saenko for the gift of purified proteins.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: hFVIII, human clotting factor VIII; hvWf, human von Willebrand factor; BM, bone marrow.
References
- 1.Antonarakis S E, Youssoufian H, Kazazian H H., Jr Mol Biol Med. 1987;4:81–94. [PubMed] [Google Scholar]
- 2.Wion K L, Kelly D, Summerfield J A, Tuddenham E G D, Lawn R M. Nature (London) 1985;317:726–728. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]
- 3.Vehar G A, Keyt B, Eaton D, Rodriguez H, O’Brien D P, Rotblat F, Oppermann H, Keck R, Lawn R M, Capon D J. Nature (London) 1984;312:337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 4.Weiss H J, Sussman I I, Hoyer L W. J Clin Invest. 1977;60:390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brettler D. Baillieres Clin Haematol. 1996;9:319–329. doi: 10.1016/s0950-3536(96)80066-7. [DOI] [PubMed] [Google Scholar]
- 6.Scandella D, Kessler C, Esmon P, Hurst D, Courter S, Gomperts E, Felch M, Prescott R the Recombinate and Kogenate Study Groups. In: Inhibitors to Coagulation Factors. Aledort L M, Hoyer L W, Lusher J M, Reisner H M, White G C, II, editors. New York: Plenum; 1995. pp. 47–75. [Google Scholar]
- 7.Prescott R, Nakai H, Saenko E, Scharrer I, Nilsson I M, Humphries J E, Hurst D, Bray G, Scandella D the Recombinate and Kogenate Study Groups. Blood. 1997;89:3663–3671. [PubMed] [Google Scholar]
- 8.Ciavarella N, Schiavoni M. Lancet. 1992;339:1301. [PubMed] [Google Scholar]
- 9.Ehrenforth S, Kreuz W, Scharrer I, Linde R, Funk M, Gungor T, Krackhardt B, Kornhuber B. Lancet. 1992;339:594–598. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 10.Kasper C K. Prog Hemost Thromb. 1989;9:57–86. [PubMed] [Google Scholar]
- 11.Bi L, Lawler A M, Antonarakis SE, High K A, Gearhart J D, Kazazian H H., Jr Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 12.Bi L, Sarkar R, Naas T, Lawler A M, Pain J, Shumaker S L, Bedian V, Kazazian H H., Jr Blood. 1996;88:3446–3450. [PubMed] [Google Scholar]
- 13.Charlton B, Auchincloss H, Jr, Fathman C G. Annu Rev Immunol. 1994;12:707–734. doi: 10.1146/annurev.iy.12.040194.003423. [DOI] [PubMed] [Google Scholar]
- 14.Zambidis E T, Barth R K, Scott D W. J Immunol. 1997;158:2174–2182. [PubMed] [Google Scholar]
- 15.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 16.Bodine D M, McDonagh K T, Seidel N E, Nienhuis A W. Exp Hematol (Charlottesville, Va) 1991;19:206–212. [PubMed] [Google Scholar]
- 17.Harrison D E, Lerner C P. Blood. 1991;78:1237–1240. [PubMed] [Google Scholar]
- 18.Sykes M, Sachs D H, Nienhuis A W, Pearson D A, Moulton A D, Bodine D M. Transplantation. 1993;55:197–202. doi: 10.1097/00007890-199301000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Fraser C C, Sykes M, Lee R S, Sachs D H, LeGuern C. J Immunol. 1995;154:1587–1595. [PubMed] [Google Scholar]
- 20.Ally B A, Hawley T S, McKall-Faienza K J, Kundig T M, Oehen S U, Pircher H, Hawley R G, Ohashi P S. J Immunol. 1995;155:5404–5408. [PubMed] [Google Scholar]
- 21.Schumacher I K, Newberg M H, Jackson J D, Hammel J M, Rubocki R J, Engelhard V J, Fox I J. Transplantation. 1996;62:831–836. doi: 10.1097/00007890-199609270-00022. [DOI] [PubMed] [Google Scholar]
- 22.Zambidis Z T, Kurup A, Scott D W. Mol Med. 1997;3:212–224. [PMC free article] [PubMed] [Google Scholar]
- 23.Chuah M K L, VandenDriessche T, Morgan R A. Hum Gene Ther. 1995;6:1363–1377. doi: 10.1089/hum.1995.6.11-1363. [DOI] [PubMed] [Google Scholar]
- 24.Bender M A, Palmer T D, Gelinas R E, Miller A D. J Virol. 1987;61:1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markowitz D, Hesdorffer C, Ward M, Goff S, Bank A. Ann NY Acad Sci. 1990;612:407–414. doi: 10.1111/j.1749-6632.1990.tb24328.x. [DOI] [PubMed] [Google Scholar]
- 26.Saenko E L, Scandella D. J Biol Chem. 1995;270:13826–13833. doi: 10.1074/jbc.270.23.13826. [DOI] [PubMed] [Google Scholar]
- 27.Connelly S, Smith T A G, Dhir G, Gardner J M, Mehaffey M G, Zaret K S, McClelland A, Kaleko M. Hum Gene Ther. 1995;6:185–193. doi: 10.1089/hum.1995.6.2-185. [DOI] [PubMed] [Google Scholar]
- 28.Kaspar C K, Aledort L M, Counts R B, Edson J R, Fratontoni J, Green D, Hampton J W, Hilgartner M W, Lazerson J, Levine P H, et al. Thromb Diath Haemorrh. 1975;34:869–872. [PubMed] [Google Scholar]
- 29.Kruisbeek A M, Shevach E. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1996. , Unit 3.12, pp. 1–21. [Google Scholar]
- 30.Sorrentino B P, Brandt S J, Bodine D M, Gottesman M M, Pastan I, Cline A, Nienhuis A W. Science. 1992;257:99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- 31.Orlic D, Anderson S, Bieseker L G, Sorrentino B P, Bodine D M. Proc Natl Acad Sci USA. 1995;92:4601–4605. doi: 10.1073/pnas.92.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan C T, Lemischka I R. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 33.Porgador A, Yewdell J W, Deng Y, Bennink J R, Germain R N. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 34.Abouel-Ezz A Y, Boggs S S, Johnson P C, Li H, Patrene K D, Itskowitz M S, Kaufman C L, Ildstad S T. Transplant Immunol. 1995;3:98–106. doi: 10.1016/0966-3274(95)80036-0. [DOI] [PubMed] [Google Scholar]
- 35.Mardiney M, Malech H L. Blood. 1996;87:4049–4056. [PubMed] [Google Scholar]
- 36.Sharabi Y, Aksentijevich T, Sundt T M, III, Sachs D H, Sykes M. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sykes M, Szot G L, Swenson K A, Pearson D A. Nat Med. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 38.Felsburg P J, Somburg R L, Hartnett B J, Suter S F, Henthorn P S, Moore P F, Weinberg K I, Ochs H D. Blood. 1997;90:3214–3221. [PubMed] [Google Scholar]