Abstract
Cdc7 kinase is essential for initiation of DNA replication. Cdc7–/– mouse embryonic stem (ES) cells are non-viable but their growth can be rescued by an ectopically expressed transgene (Cdc7–/–tg). Here we report that, despite the normal growth capability of Cdc7–/–tg ES cells, the mice with the identical genetic background exhibit growth retardation. Concomi tantly, Cdc7–/–tg embryonic fibroblasts (MEFs) display delayed S phase entry and slow S phase progression. Furthermore, spermatogenesis of Cdc7–/–tg mice is disrupted prior to pachytene stage of meiotic prophase I. The impairment in spermatogenesis correlates with the extremely low level of Cdc7 protein in testes, and is rescued by introducing an additional allele of transgene, which results in increase of Cdc7 expression. The increased level of Cdc7 also recovers the growth of Cdc7–/–tg MEFs and mice, indicating that the developmental abnormalities of Cdc7–/–tg mice are due to insufficiency of Cdc7 protein. Our results indicate the requirement of a critical level of a cell-cycle regulator for mouse development and provide genetic evidence that Cdc7 plays essential roles in meiotic processes in mammals.
Keywords: Cdc7 kinase/cellular proliferation/growth retardation/hypomorphic mutation/spermatogenesis
Introduction
Cell-cycle regulators involved in DNA replication or cell division generally play essential roles in cell viability, and their roles in mammalian ontogeny, including in the development of specific tissues and organs, cannot be analyzed through the studies of conventional gene targeting. In particular, effects of reduced expression of these essential regulators on mouse development have not been addressed.
Cdc7, originally discovered in budding yeast, is required for initiation of DNA replication (Hollingsworth and Sclafani, 1990; Sclafani and Jackson, 1994; Stillman, 1996; Dutta and Bell, 1997; Johnston et al., 1999; Bell and Dutta, 2002). Cdc7 homologs have been identified from fission yeast, Xenopus, mouse, human and other eukaryotes, suggesting that DNA replication in higher eukaryotes may be regulated by a conserved mechanism involving Cdc7 (Masai et al., 1995; Jiang and Hunter, 1997; Sato et al., 1997; Kim et al., 1998). In higher eukaryotes, Cdc7 plays essential roles in cell-cycle progression from late G1 to S phase in cooperation with Cdk2–cyclin E. The kinase activity of Cdc7 is regulated by association with its regulatory subunit, Dbf4/ASK (Johnston and Thomas, 1982a,b; Jackson et al., 1993; Brown and Kelly, 1999; Jiang et al., 1999; Kumagai et al., 1999; Takeda et al., 1999). The levels of Dbf4/ASK increase sharply at the G1/S boundary and peak in S phase, while those of Cdc7 remain constant during the cell cycle (Chapman and Johnston, 1989; Brown and Kelly, 1999; Jiang et al., 1999; Kumagai et al., 1999; Takeda et al., 1999).
Characterization of cdc7ts revealed potential roles of Saccharomyces cerevisiae Cdc7 in meiosis. Sporulation is arrested in cdc7ts diploid cells, presumably due to impaired synaptonemal complex formation (Sclafani et al., 1988). In adult mice, Cdc7 transcript and protein are expressed at a high level in testis (Kim et al., 1998), suggesting possible involvement of Cdc7 in germ cell development in mammals. However, the roles of Cdc7 in mammalian development are unknown.
We previously reported that Cdc7-deficient mice die between embryonic day (E) 3.5 and E6.5, indicating that Cdc7 functions are essential for early embryogenesis and cell proliferation (Kim et al., 2002). We also established a conditional Cdc7-deficient (muCdc7–/–tg) embryonic stem (ES) cell line lacking both alleles of the Cdc7 gene in the presence of a single copy of transgene carrying a loxP-flanked Cdc7 cDNA. muCdc7–/–tg ES cells showed morphology and growth properties indistinguishable from those of wild-type ES cells. Upon removal of the transgene by Cre-recombinase, DNA synthesis rapidly ceases within S phase and checkpoint responses are triggered, leading to recombinational repair and eventually to p53-dependent cell death (Kim et al., 2002).
In this study, to further investigate the functions of Cdc7 during mammalian development, we generated mice harboring a genetically modified Cdc7 allele using muCdc7–/–tg ES cells. Despite the normal growth capability of the muCdc7–/–tg ES cells, the mutant mice (Cdc7–/–tg mice) exhibited growth retardation. This paralleled with reduced growth rate of Cdc7–/–tg mouse embryonic fibroblasts (MEFs). Most strikingly, testicular development was severely impaired and spermatogenesis was abrogated in Cdc7–/–tg mice. We found that Cdc7–/–tg MEFs and testes expressed Cdc7 at levels considerably lower than those of wild-type counterparts, and showed that all these defects were corrected by introducing an additional allele of transgene in vivo, which resulted in increase of Cdc7 expression. Our results demonstrate the requirement of a critical level of Cdc7 for mammalian development and its crucial roles in spermatogenesis.
Results
Cdc7–/–tg mice exhibit growth retardation
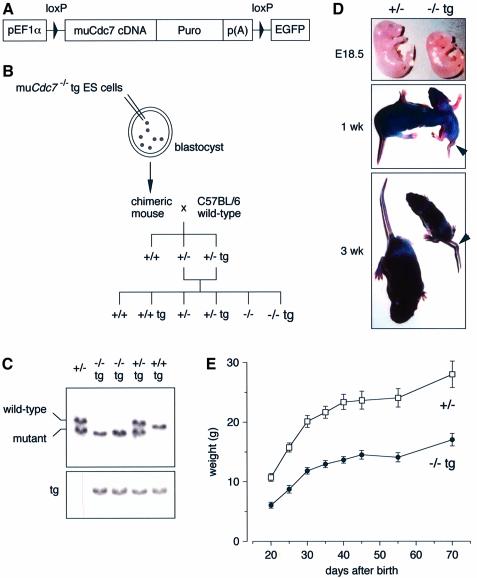
To elucidate the functions of Cdc7 during mammalian development, we attempted to generate mice harboring a genetically modified Cdc7 allele using muCdc7–/–tg ES cells (Figure 1A–C). Two independent ES cell clones (DKO20 and DKO28) transmitted the mutation into the germline. The Cdc7+/–tg mice (derived from DKO28 ES cells) were indistinguishable from the wild-type mice in size and appearance. The crosses of Cdc7+/–tg mice with Cdc7+/– mice generated Cdc7–/–tg mice, but at a considerably reduced frequency; at birth, only 1.6% were Cdc7–/–tg instead of the expected Mendelian ratio of 12.5% (Table I). Of the Cdc7–/–tg newborns, <25% (only 0.38% of total offspring) survived to adulthood. Indeed, Cdc7–/–tg embryos at E18.5 were substantially smaller and thinner than their littermates (Figure 1D), and >75% of Cdc7–/–tg newborns died within 3 days post partum. This indicates that transgene-encoded Cdc7 rescued early embryonic lethality, but that Cdc7–/–tg mice were susceptible to prenatal or neonatal lethality. Cdc7–/–tg mice that survived to weaning age had a normal life span but weighed only 50–55% of their normal littermates (Figure 1E). The sizes of the major organs, including heart, liver, kidney, lung, thymus, spleen and brain, of Cdc7–/–tg mice were proportional to body weight, and histological examination of these organs revealed no discernable defects (data not shown). In contrast, the testes and ovaries were extremely small and exhibited severe developmental defects (see below). In addition, Cdc7–/–tg mice exhibited a tail flexion anomalies (Figure 1D), as has been observed in curly-tail (ct) mutant mice (Gruneberg, 1954). The identical phenotypes were observed with DKO20 ES-derived mice.
Fig. 1. Cdc7–/–tg mice exhibit growth retardation. (A) The schematic drawing of the transgene used to create conditional Cdc7-deficient (Cdc7–/–tg) mice, consisting of the human elongation factor 1α promoter (pEF1α), loxP-flanked murine Cdc7 cDNA, puromycin resistance cassette (Puro) and polyadenylation signal (pA). (B) The strategy for generating Cdc7–/–tg mice. (C) Southern blot hybridization. Tail DNA was digested with BglII and hybridized with a 3′ flanking probe (Kim et al., 2002). The positions of the DNA fragments derived from wild-type and mutant alleles hybridized to the probe are indicated. Tg indicates the band derived from transgene. (D) Gross appearance of embryos (E18.5; top), 1- (middle) and 3-week-old (bottom) mice, with Cdc7+/– (left) or Cdc7–/–tg (right) genotype. Note the flexion in the tail of the Cdc7–/–tg mice (arrowheads). (E) Representative growth curve of Cdc7+/– (n = 6) and Cdc7–/–tg (n = 4) male mice. Mice were weighed at the indicated days after birth.
Table I. Genotypes of offspring from crosses between Cdc7+/–tg and Cdc7+/– mice.
| Stage | Genotype |
Total | |||||
|---|---|---|---|---|---|---|---|
| +/+ | +/+ tg | +/– | +/– tg | –/– | –/– tg | ||
| E13.5 | 15 (1) | 14 (0.93) | 27 (1.8) | 28 (1.87) | 0 (0) | 16 (1.07) | 100 |
| 3 weeks old | 354 (1) | 332 (0.94) | 696 (1.97) | 724 (2.04) | 0 (0) | 8 (0.02) | 2114 |
| Expected Medelian ratio | 1 | 1 | 2 | 2 | 1 | 1 | |
The numbers and the genotypes of viable embryos (E13.5) and mice (3 weeks old) are given. The values in parentheses indicate the ratio with respect to the number of wild-type (+/+) mice.
Cdc7–/–tg MEFs display decreased proliferative capability
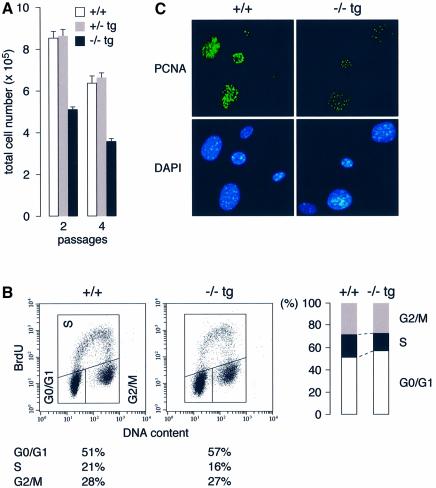
To investigate whether the growth retardation of Cdc7–/–tg mice reflected a defect in cellular proliferation, we examined the growth properties of Cdc7–/–tg MEFs. The increase in cell number of Cdc7–/–tg MEFs was considerably slower than in wild-type or Cdc7+/–tg MEFs (Figure 2A). FACS analysis of asynchronously growing Cdc7–/–tg MEFs revealed an increased G0/G1 phase population with concomitant decrease in S phase population compared with wild-type MEFs (Figure 2B). [3H]thymidine incorporation in Cdc7–/–tg MEFs was about half that in the wild-type MEFs (see Figure 9C). To detect actively proliferating cells, we performed immunofluorescence study using anti-proliferating cell nuclear antigen (PCNA) antibody (Figure 2C). While an extensive and strong nuclear PCNA staining was observed in the wild-type MEFs, the intensity and numbers of nuclear foci in the Cdc7–/–tg MEFs were significantly low. These results indicate the numbers of replication forks and rate of DNA replication are significantly reduced in Cdc7–/–tg cells, causing slow progression of S phase.
Fig. 2. Cdc7–/–tg MEFs display decreased proliferative capacity with reduced fraction of S phase cells. (A) Passage 2 and 4 MEFs were plated in triplicate at 3 × 105/60 mm dish. Cell numbers, which represent the average of three independent experiments, were determined after 2 days. (B) Cell cycle distribution of asynchronously growing MEFs. The decrease in S phase population of Cdc7–/–tg MEFs was accompanied by a moderate increase in the G0/G1 phase population. The data represent three separate experiments with different clones of MEFs. (C) Passage 3 MEFs were fixed in methanol/acetone and stained for PCNA. The intensity and numbers of nuclear foci of PCNA staining in the Cdc7–/–tg MEFs (right) were significantly reduced compared with wild-type MEFs (left).
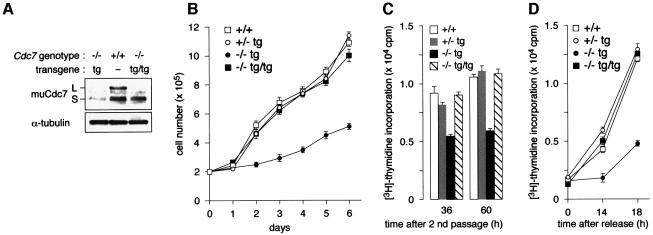
Fig. 9. An additional allele of transgene rescues proliferation defects of Cdc7–/–tg MEFs. (A) Expression of Cdc7 protein in MEFs derived from wild-type (+/+), Cdc7–/–tg and Cdc7–/–tg/tg embryos. The level of Cdc7 in Cdc7–/–tg/tg MEFs is restored close to that in wild-type MEFs. (B) MEFs at passage 2 were plated in duplicate at 2 × 105/60 mm plate, and cell numbers were determined daily for 6 days. The cell number at each time point is the mean value of three independent experiments. Cdc7–/–tg/tg MEFs grow at a rate comparable to that of the wild type MEFs. In (C) and (D), [3H]thymidine incorporation was measured in asynchronously growing MEFs [passage 2, (C)] or in MEFs released from serum starvation [passage 3, (D)], respectively. The levels of [3H]thymidine incorporation in Cdc7–/–tg/tg MEFs are restored to those in the control MEFs in both experiments. The values represent averages of three independent experiments.
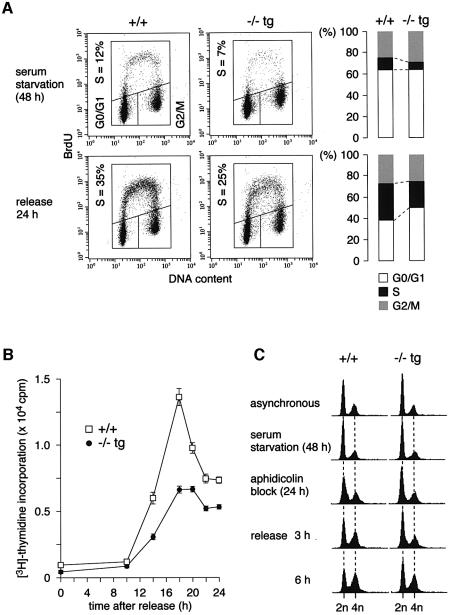
To further investigate the nature of the proliferative characteristics observed in the Cdc7–/–tg MEFs, we examined cell cycle entry from quiescence. Following serum starvation and release, DNA synthesis of primary MEFs was measured by examining the incorporation of BrdU and [3H]thymidine. FACS analysis demonstrated that, at 24 h after serum addition, the proportion of BrdU-positive cells was lower in the Cdc7–/–tg MEFs (25%) than in the wild-type (35%) population (Figure 3A). The level of [3H]thymidine incorporation of the Cdc7–/–tg MEFs was about half that of the wild-type MEFs at most of the time points analyzed (Figure 3B). Delayed S phase entry was also observed in the Cdc7–/–tg MEFs released from aphidicolin block at G1/S boundary (Figure 3C). The cells were sequentially arrested in G0 and early S phase by serum starvation and aphidicolin treatment, respectively. Although both Cdc7–/–tg and wild-type MEFs were arrested with similar DNA content following serum starvation, more G0/G1 phase population was detected in Cdc7–/–tg MEFs (69%) than in the wild-type MEFs (58%) after aphidicolin block. Furthermore, the increase of S phase population after release from the aphidicolin block was slower in Cdc7–/–tg MEFs; at 3 and 6 h after release, S phase populations of Cdc7–/–tg MEFs were 7 and 12%, respectively, whereas those of wild-type MEFs were 20 and 27%. Taken together, these results suggest that the decreased proliferative capability of Cdc7–/–tg MEFs is attributable to delayed S phase entry and slowed S phase progression.
Fig. 3. Cdc7–/–tg MEFs exhibit delayed S phase entry and slowed S phase progression. (A) Cell cycle distribution of Cdc7–/–tg MEFs following serum starvation and release. The proportion of BrdU-positive cells was lower in the Cdc7–/–tg MEFs (25%) than in the wild-type MEFs (35%). The gate settings for cells in G0/G1, S and G2/M phases and percentage of cells in S phase are indicated. Consistent data were obtained from two independent experiments. (B) Incorporation of [3H]thymidine during 2 h pulses at indicated time points following release from serum starvation. The values represent average of three independent experiments. (C) FACS analysis for DNA content. The cells were sequentially arrested in G0 and early S phase by serum starvation and aphidicolin treatment, respectively. At the time of aphidicolin block, Cdc7–/–tg MEFs contain more G0/G1 cells, and at 3 and 6 h after release from the aphidicolin block, they contain less S phase cells than the wild-type (+/+) MEFs.
Cdc7–/–tg mice show testicular atrophy
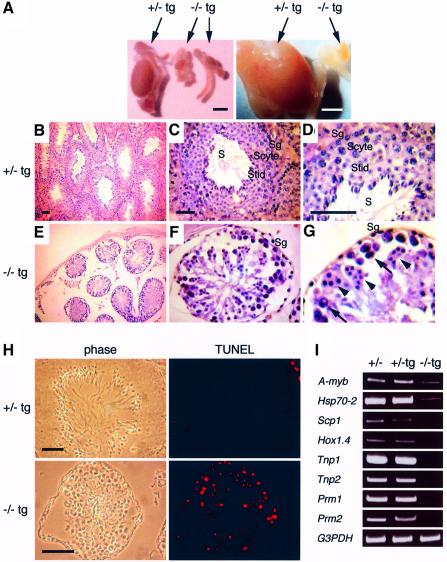
Male Cdc7–/–tg mice were infertile. Cdc7–/–tg testes were extremely small compared with those of the littermates (Figure 4A). Histological analysis of Cdc7+/–tg testes showed seminiferous tubules containing spermatogonial stem cells at the periphery of the tubule and more differentiated cells towards the lumen, into which the mature spermatozoa are finally released (Figure 4B–D). In contrast, Cdc7–/–tg testes showed considerably reduced number of seminiferous tubules that contained less spermatogonia and degenerated primary spermatocytes, together with abnormal multi-nucleated cells (Figure 4E–G). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assays revealed that there were large numbers of apoptotic cells in Cdc7–/–tg seminiferous tubules (Figure 4H). Remarkably, Cdc7–/–tg testes were completely devoid of post-meiotic cells such as spermatids or mature spermatozoa, indicating that spermatogenesis is severely impaired in Cdc7–/–tg mice. To further define the defect in spermatogenesis, we determined the expression of developmental markers by RT–PCR analysis (Figure 4I). A-myb is highly expressed in spermatogonia and spermatocytes at the stages of leptotene to pachytene (Mettus et al., 1994; Trauth et al., 1994). Expression of Hsp70-2 is first detected in leptotene and peaks in pachytene spermatocytes (Allen et al., 1988; Zakeri et al., 1988). The expression of these earliest spermatogenesis markers was markedly decreased in Cdc7–/–tg testes. Scp1, the expression of which is restricted in zygotene to diplotene spermatocytes (Meuwissen et al., 1992), was barely detectable in Cdc7–/–tg testes. Expression of Hox1.4, which initiates in pachytene spermatocytes and persists thereafter (Rubin et al., 1986), was not detected in Cdc7–/–tg testes. Likewise, transition protein-1 (Tnp1) and Tnp2, as well as protamine-1 (Prm1) and Prm2, which sequentially replace histones in post-meiotic spermatids (Kleene et al., 1984; Meistrich, 1989; Wolgemuth and Watrin, 1991), were not expressed in Cdc7–/–tg testes. Together with the histological observations, these results indicate that spermatogenesis in Cdc7–/–tg male is abrogated in the early stages of meiotic prophase I.
Fig. 4. Spermatogenesis is disrupted in Cdc7–/–tg mice. (A) Gross appearance of testes from Cdc7–/–tg mice. Cdc7–/–tg testis is considerably smaller than Cdc7+/–tg testis. Scale bars, 3 mm. (B–G) Histological analysis of Cdc7–/–tg testes. Staining with hematoxylin and eosin reveals considerably reduced number of spermatogonia (Sg), degenerated spermatocytes (arrows) and accumulation of abnormal multi-nucleated cells (arrowheads) in seminiferous tubules of the Cdc7–/–tg mice (E–G) in comparison with normal seminiferous tubules observed in Cdc7+/–tg mice (B–D). Spermatids (Stid) and spermatozoa (S) are completely absent in Cdc7–/–tg testes. Scyte, spermatocytes. Scale bars, 50 µm. (H) TUNEL assay demonstrates a massive increase of apoptotic cells (red fluorescence) in Cdc7–/–tg testes as compared with the Cdc7+/–tg testes. Scale bars, 30 µm. (I) Expression of developmental markers in testes of Cdc7–/–tg mice. RT–PCR analysis shows very low level of A-myb, Hsp70-2, barely detectable level of Scp1, and complete absence of Hox1.4, Tpn1, Tpn2, Prm1 and Prm2 in Cdc7–/–tg testes.
Spermatogenesis is abrogated prior to pachytene stage of meiotic prophase I in Cdc7–/–tg male mice
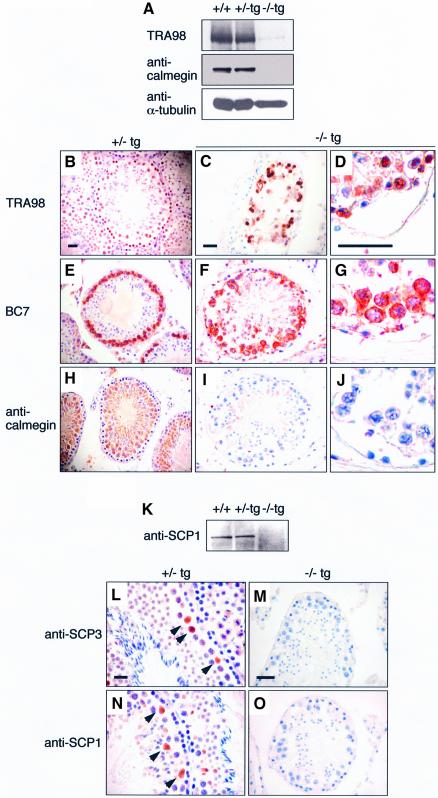
To explore more closely the substages of meiosis at which spermatogenesis is disrupted in Cdc7–/–tg testes, we examined the expression of stage-specific antigens of spermatogenic germ cells. Western blotting analysis using TRA98, an antibody the antigen of which is expressed in all types of spermatogonia, spermatocytes and round spermatids (Tanaka et al., 1997), detected a 110 kDa band in testicular extracts of all genotypes (Figure 5A). However, the expression level was markedly decreased in Cdc7–/–tg testes. Immunostaining with TRA98 also showed the reduced numbers of TRA98-positive cells in Cdc7–/–tg testes (Figure 5C and D) compared with Cdc7+/–tg testes (Figure 5B). Immunostaining with BC7 (Koshimizu et al., 1993) detected zygotene and early pachytene spermatocytes near the periphery of the Cdc7+/–tg seminiferous tubules (Figure 5E). In contrast, BC7-positive cells in Cdc7–/–tg testes were reduced in numbers and were more dispersed within the seminiferous tubule (Figure 5F and G). Calmegin, a 93 kDa protein expressed in cells ranging from early pachytene spermatocytes to elongated spermatids (Watanabe et al., 1992, 1994), was detected in wild-type and Cdc7+/–tg testes, but not in Cdc7–/–tg testes (Figure 5A). Immunostaining results also confirmed the absence of calmegin in Cdc7–/–tg testes (Figure 5I and J). These results indicate that spermatogenesis in the Cdc7–/–tg males was abrogated prior to pachytene stage of meiotic prophase I. Most of the TRA98- and BC7-positive spermatocytes in Cdc7–/–tg testes were also TUNEL-positive (data not shown), indicating they were undergoing apoptosis.
Fig. 5. Spermatogenesis is abrogated prior to pachytene stage in Cdc7–/–tg male mice. (A) Western blot analysis using TRA98 and anti-calmegin antibody. Antigen for TRA98 is expressed at low levels in Cdc7–/–tg testes, and calmegin is absent in Cdc7–/–tg testes. (B–J) Immunostaining with TRA98 (B–D), BC7 (E–G) and anti-calmegin antibody (H–J). In contrast to the Cdc7+/–tg testes (B, E and H), in which TRA98-, BC7- and anti- calmegin-positive cells are observed, the Cdc7–/–tg testes show small number of TRA98-positive (C and D) and BC7-positive (F and G) cells, and no anti-calmegin-positive (I and J) cells. Scale bars, 20 µm. (K) Western blot analysis using anti-SCP1 antibody. SCP1 is expressed in the wild-type and Cdc7+/–tg testes but not in Cdc7–/–tg testes. (L–O) Immunostaining with anti-SCP3 and anti-SCP1 antibodies. In Cdc7+/–tg testes (L and N), anti-SCP3-positive and anti-SCP1-positive spermatocytes were detected. In contrast, cells positive for either antibody were completely absent in Cdc7–/–tg testes (M and O). Scale bars, 20 µm. Hematoxylin was used for counter staining.
The absence of pachytene spermatocytes were further confirmed using antibodies against key regulatory proteins for the formation of synaptonemal complex (SC): SCP3/COR1 and SCP1/SYN1. During the zygotene and pachytene stages, SCP3 and SCP1 are localized along the chromosome synapsis (Dobson et al., 1994). Western blot analysis revealed that SCP1 was expressed in wild-type and Cdc7+/–tg testes, whereas its expression was not observed in the Cdc7–/–tg testis (Figure 5K). Immunostaining also confirmed the expression of SCP3 and SCP1 in Cdc7+/–tg testes (Figure 5L and N), but not in Cdc7–/–tg testes (Figure 5M and O). Based on these observations, we conclude that spermatogenesis in Cdc7–/–tg mice is disrupted prior to pachytene stage of meiotic prophase I, most likely at the early zygotene stage.
Oogenesis is impaired in Cdc7–/–tg female mice
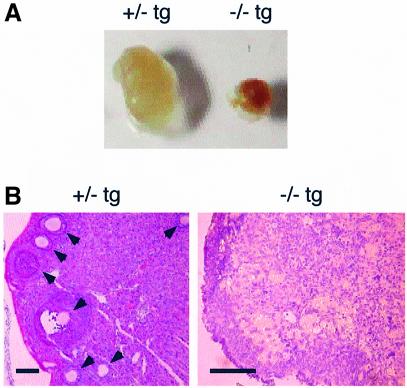
Cdc7–/–tg female mice were infertile. Ovaries of Cdc7–/–tg females were considerably small and grossly malformed (Figure 6A). Histological analysis showed that the ovaries of Cdc7–/–tg females contained no oocytes and follicles (Figure 6B). In contrast, the ovaries of Cdc7+/–tg females contained oocytes at various stages of maturation, forming large follicles.
Fig. 6. Abnormal oogenesis in Cdc7–/–tg females. (A) Gross appearance of each ovary from Cdc7+/–tg and Cdc7–/–tg females. The ovary from the Cdc7–/–tg female was considerably smaller than that from Cdc7+/–tg female. (B) Ovaries from adult Cdc7+/–tg and Cdc7–/–tg females were stained with hematoxylin and eosin. Arrows indicate the oocytes at various stages of maturation, forming large follicles in Cdc7+/–tg female (left). No oocytes and follicles are present in Cdc7–/–tg ovary (right). Scale bars, 100 µm.
A limited amount of Cdc7 protein was expressed in Cdc7–/–tg MEFs and testes
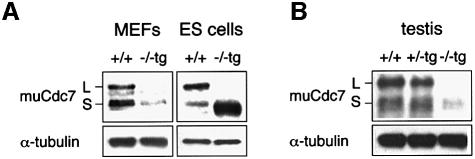
To understand the molecular basis underlying growth defects and testicular hypoplasia in Cdc7–/–tg mice, we examined the expression of transgene-encoded Cdc7 (Figure 7). Murine Cdc7 protein is expressed in two catalytically active forms of different sizes in ES cells, MEFs and various tissues, resulting from the alternative splicing (Kim et al., 1998). Both forms are identical in terms of kinase activity in vitro (J.M.Kim and H.Masai, unpublished data). cDNA used to generate muCdc7–/–tg ES cells encodes the shorter form and can perfectly support normal growth of Cdc7-deficient ES cells (Kim et al., 2002). In muCdc7–/–tg ES cells, the expression level of Cdc7 was ∼3- to 5-fold higher than that in the wild-type ES cells. In contrast, the expression level of Cdc7 in Cdc7–/–tg MEFs was five times lower than that in wild-type MEFs (Figure 7A). Thus, the transgene-encoded Cdc7 may not be present at a level sufficient to support normal growth of Cdc7–/–tg MEFs. The expression of Cdc7 in Cdc7–/–tg testes was even more severely decreased (Figure 7B). This closely correlates with developmental aberration of Cdc7–/–tg testes. It would thus appear that decreased Cdc7 expression in testes leads to impaired testicular development and profound defect in spermatogenesis.
Fig. 7. Diminished expression of Cdc7 in Cdc7–/–tg MEFs and testes. The levels of Cdc7 protein in MEFs [(A), left], ES cells [(A), right] and testes (B) were determined by western blot analysis. Cdc7–/–tg MEFs express Cdc7 protein at levels five times lower than that in wild-type MEFs, whereas the level of Cdc7 in Cdc7–/–tg ES cells is ∼3- to 5-fold higher than that of the wild-type ES cells. The Cdc7 protein level in Cdc7–/–tg testes is 10 times lower than those in the wild-type and Cdc7+/–tg testes. α-tubulin is shown as a loading control. L and S denote two alternative spliced forms of murine Cdc7 protein, which are identical in functions. The transgene encodes the form S.
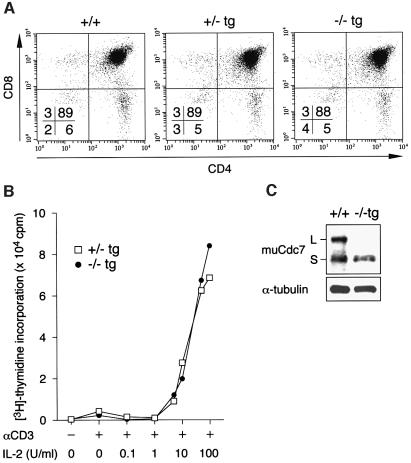
Thymic and peripheral lymphocytes develop normally in Cdc7–/–tg mice
In contrast to the impaired testicular development, no developmental defect was found in thymi of Cdc7–/–tg mice. Histological examination of Cdc7–/–tg thymi showed normal cortex–medulla compartmentalization (data not shown), and there were no significant differences in the CD4 and CD8 levels and distribution of subsets (Figure 8A). Moreover, proliferative response of Cdc7–/–tg thymocytes was unperturbed; rather, they showed modestly increased [3H]thymidine incorporation in response to stimulation with anti-CD3 plus varying doses of interleukin (IL)-2 (Figure 8B). Consistently, the expression level of transgene-encoded Cdc7 was only modestly decreased in thymi of Cdc7–/–tg mice (Figure 8C). Peripheral CD4 T and B cells of Cdc7–/–tg mice also expressed amounts of Cdc7 comparable to the wild-type level and retained the proliferative capacity in response to various mitogenic stimulations (data not shown).
Fig. 8. Thymic development was normal in Cdc7–/–tg mice. (A) Flow-cytometric analysis of thymocytes from Cdc7–/–tg mice. Expression of CD4 and CD8 in thymocytes of Cdc7–/–tg mice and their control littermates was analyzed. Proportions of cells in the quadrants are shown. Staining and analysis were performed on at least three animals of each genotype with similar results. (B) Thymocytes prepared from Cdc7+/–tg and Cdc7–/–tg mice were stimulated using anti-CD3ε antibody and IL-2 of various doses. Mitogenic activation of Cdc7–/–tg thymocytes is unperturbed. Data shown are averages of three independent experiments. (C) The levels of Cdc7 protein in thymi from wild-type (+/+) and Cdc7–/–tg mice were determined by western blot analysis with anti-murine Cdc7 antibody. Cdc7–/–tg thymi express Cdc7 protein at the level ∼65% that of wild-type thymi. α-tubulin is shown as a loading control. L and S denote two alternative spliced forms of murine Cdc7 protein.
Proliferative capacity was recovered in Cdc7–/–tg/tg MEFs
We next examined whether increase in Cdc7 protein level can rescue the developmental abnormalities of Cdc7–/–tg mice. To achieve this, we generated Cdc7–/–tg/tg mice harboring the homozygous Cdc7 transgene by extensive intercrossing between Cdc7+/–tg mice. The level of Cdc7 expression in Cdc7–/–tg/tg MEFs was recovered to approximately two-thirds that of the wild-type MEFs (Figure 9A). Accordingly, the proliferation rate and level of DNA synthesis of Cdc7–/–tg/tg MEFs were restored (Figure 9B and C). Cdc7–/–tg/tg MEFs also showed a level of [3H]thymidine incorporation similar to that observed in the wild-type MEFs, following release from serum starvation (Figure 9D). These results are in a sharp contrast to the reduced proliferative activities of Cdc7–/–tg MEFs, and indicate that increase of Cdc7 expression can indeed restore normal growth in Cdc7–/–tg MEFs.
Spermatogenesis is restored in Cdc7–/–tg/tg mice
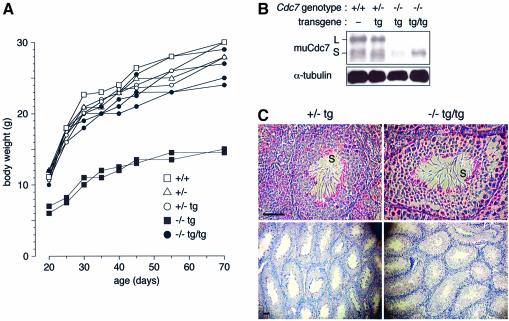
Cdc7–/–tg/tg mice gained normal body weight (Figure 10A). Notably, these mice were fertile and yielded progeny, with significant recovery of Cdc7 protein level in testes (Figure 10B). Histological analysis revealed the presence of considerable numbers of spermatids and mature spermatozoa in Cdc7–/–tg/tg testes (Figure 10C), indicating that increased Cdc7 expression restored spermatogenesis and rescued infertility, although the size of testes was not completely restored by the presence of an additional copy of the transgene (data not shown).
Fig. 10. Cdc7–/–tg/tg mice exhibit normal body weight and restored spermatogenesis. (A) Growth curve of wild-type, Cdc7+/–, Cdc7+/–tg, Cdc7–/–tg and Cdc7–/–tg/tg mice. Mice were weighed at intervals and plotted against age in days. (B) The level of Cdc7 protein in testes from mice with the indicated genotypes. Significant recovery of the Cdc7 protein level was observed in Cdc7–/–tg/tg testes. L and S denote two alternative spliced forms of murine Cdc7 protein. (C) Histological analyses in Cdc7+/–tg (left) and Cdc7–/–tg/tg (right) testes. Cdc7–/–tg/tg testes contained considerable numbers of spermatozoa (S). Scale bars, 50 µm. See Figure 3F for comparison.
Discussion
Highly regulated cell-cycle progression is attained by concerted actions of multiple kinases. Cdc7 plays essential roles in initiation of DNA replication, and its targeted deletion leads to early embryonic lethality, precluding further analyses of its role in mouse development. In this study, we generated and analyzed mice with a hypomorphic allele of Cdc7 under a Cdc7-null background. Our results demonstrate that the levels of Cdc7 are critical for normal mouse development. The mutant mice were growth-retarded and suffered from severe defects in gametogenesis. MEFs derived from Cdc7 mutant mice showed decreased proliferative properties and exhibited a substantial delay in S phase entry after serum starvation–restimulation. Intriguingly, all these defects were corrected by increasing the expression level of Cdc7 in vivo.
Growth defects of Cdc7–/–tg mice
Cdc7–/–tg mice arose at a frequency considerably lower than the expected Mendelian ratio. The growth retardation in Cdc7–/–tg mice was observed as early as at E13.5, and expected frequencies of viable Cdc7–/–tg embryos were observed until E17.5 (data not shown), suggesting that most of the missing Cdc7–/–tg mice died during later stages of embryogenesis or just after birth. One notable abnormality of Cdc7–/–tg mice was tail flexion anomalies. All of Cdc7–/–tg newborns that died at or shortly after birth displayed severe tail flexion, whereas the Cdc7–/–tg mice that reached adulthood suffered from hunched posture but from less severe tail abnormality. Among a number of mutant mice displaying tail flexion anomalies, the ct mutant mouse (Gruneberg, 1954) is regarded as an animal model of neural tube defects (NTDs) in humans, of which spina bifida and anencephaly are the most prevalent forms (Embury et al., 1979). Since spinal NTD in ct mutant mice has been ascribed to decreased cellular proliferation in the hindgut endoderm and notochord (Copp et al., 1988a,b), it is conceivable that the suboptimal expression of Cdc7 is detrimental to normal neurulation during embryogenesis. Histological examination of Cdc7–/–tg adult brains revealed normal morphogenesis, and mutant mice did not display neurological abnormalities such as incomplete eye opening, impaired movements and the leg-clasping reflex (data not shown). These results suggest that no obvious defects are associated with adult neural tissues in Cdc7–/–tg mice, although there may be some defects in development of neural cells during embryogenesis. The tail flexion anomalies may also be caused by abnormal skeletal development (Connor et al., 1997; Ishijima et al., 1998; Regnier et al., 2002). The longitudinal growth of the skeleton arises from the continuous process of bone formation, in which osteoblast proliferation comprises a critical component. Thus, it is also possible that Cdc7 is involved in the control of osteoblast proliferation in embryos undergoing bone formation, and its insufficiency results in prenatal or neonatal lethality in some Cdc7–/–tg mice.
Essential roles of Cdc7 in mouse germ cell development
Spermatogenesis in mammals is characterized by a well-defined sequence of mitotic and meiotic divisions that lead to the production of mature spermatozoa (McCarrey, 1993). The mitotic divisions of spermatogonial stem cells lead to the development of type A and type B spermatogonia, the latter of which differentiate into preleptotene spermatocytes. After meiotic S phase, preleptotene spermatocytes enter meiotic prophase I as leptotene spermatocytes (de Rooij and van Dissel-Emiliani, 1997). Cdc7–/–tg mice showed testicular atrophy with reduced numbers of spermatogonia, degenerated primary spermatocytes, and accumulation of abnormal multi-nucleated cells. Immunohistochemical analyses revealed the presence of arrested, degenerating zygotene spermatocytes in Cdc7–/–tg testes. These observations raise the intriguing possibility that Cdc7 might be involved in early meiotic prophase I in addition to its expected roles in germ cell proliferation. We recently found that a temperature-sensitive mutation in hsk1, the fission yeast homolog of Cdc7 (Masai et al., 1995; Takeda et al., 2001), results in arrest of meiotic process at or prior to prophase of meiosis I (K.Ogino and H.Masai, submitted), suggesting that essential roles of Cdc7 kinase in meiosis may be conserved from yeasts to mammals. Similar testicular atrophy has been also observed in Cdk4-null mice (Rane et al., 1999; Tsutsui et al., 1999). Seminiferous tubules in Cdk4–/– mice show reduced numbers of spermatogonia, degeneration of spermatocytes and numerous apoptotic bodies. Three D-type cyclins (cyclin D1, D2 and D3) are differentially expressed at the various stages throughout spermatogenesis (Ravnik et al., 1995; Zhang et al., 1999; Beumer et al., 2000). Thus, it seems that Cdk4, in combination with D-type cyclins, is involved in germ cell proliferation and differentiation. In somatic cell-cycle progression, activation of Cdk4 during early- to mid-G1 phase is followed by activation of Cdk2 and Cdc7 at the G1/S transition. Therefore, Cdc7 may promote mitotic cell cycle in spermatogonia downstream of Cdk4. Cdc7 may be also required for meiotic S phase and, possibly, the following meiotic prophase I in mice, as was previously indicated for budding yeast Cdc7 kinase (Sclafani et al., 1988).
Cdc7–/–tg females were also infertile and we did not observe any oocytes and follicles in the ovary. In mammals, oocytes undergo meiosis from E12 to E18 in the fetal ovary, and by 5 days post partum all oocytes are arrested in the postpachytene dictyate stage ready for maturation (Hogan et al., 1994). In contrast to male spermatogenesis, where germ cells proliferate continuously and repeat the differentiation cycle throughout their lifetime, oogonia do not proliferate after birth. By analogy with the disrupted spermatogenesis observed in Cdc7–/–tg males, defects of oogenesis in Cdc7–/–tg females may manifest before the pachytene stage in utero. Therefore, the meiotic arrest and subsequent apoptotic cell death may result in the complete degeneration of germ cells in Cdc7–/–tg females.
Requirement of a critical level of Cdc7 protein for mammalian development
Diminished expression of Cdc7 in Cdc7–/–tg testes and MEFs correlated with their developmental and proliferative defects. We also generated another line of Cdc7–/–tg mice bearing a single copy of transgene by the microinjection method. These mice displayed similar growth retardation and disrupted spermatogenesis with decreased expression of Cdc7. Since the repression of transgene expression is not restricted to testis and is observed in at least two independent strains, this could be more or less general repression of the EF1α promoter in some of the differentiated cells. However, we cannot exclude the possibility that the transgenes of both mice are integrated into one of the loci that undergo silencing in differentiating testes, since overall gene expression profile is profoundly altered during spermatogenesis (Sassone-Corsi, 2002). In contrast, the development of thymic and peripheral lymphocytes of Cdc7–/–tg mice was largely normal, in which the expression level of Cdc7 was only modestly decreased. The increased level of Cdc7 protein in Cdc7–/–tg/tg mice, although still below the level of endogenous one, reversed the defects of testes and MEFs. We did not detect any abnormality in Cdc7 heterozygous mice, supporting the notion that the endogenous Cdc7 protein level of wild-type cells may be present in excess. Taken together, it appears that Cdc7 protein must be kept above a certain threshold level to achieve normal mouse development. Since mouse Cdc7 is expressed in two catalytically active forms, it is also conceivable that each isoform has some specific functions, and both are required for normal mouse development.
A strategy for genetic characterization of cell-cycle regulators using hypomorphic mutant mice
Tissue-specific gene targeting can be achieved by excision of a specific DNA segment, which is flanked by loxP sites, upon expression of Cre recombinase in a tissue of interest. Although this approach is commonly used, it has been shown that Cre expression in primary MEFs or developing spermatids can cause growth-inhibitory and genotoxic effects (Schmidt et al., 2000; de Alboran et al., 2001; Loonstra et al., 2001; Silver and Livingston, 2001). Such Cre toxicity may confuse interpretation of the phenotypic analysis of the conditional deletion, especially when the gene of interest is involved in cell growth. Our approach utilizes mutant mice that express a given protein only from transgene under the control of a heterologous promoter. By modifying the tissue specificity and the strength of the promoter used for the transgene, one would be able to generate mutant mice with a tissue-specific hypomorphic allele.
In conclusion, our genetic studies point, for the first time, to the critical role for an appropriate protein level of an essential cell-cycle regulator in mouse development. Possible involvement of hypomorphic mutations in cell-cycle-regulatory genes in human diseases remains to be examined. Our study also disclosed the pivotal roles of Cdc7 in the early stages of spermatogenesis and oogenesis. It will be interesting to examine whether hypomorphic Cdc7 mutations are associated with individuals suffering from infertility.
Materials and methods
Generation of Cdc7–/–tg mice
muCdc7–/–tg ES cells were injected into C57BL/6 blastocysts. Chimeric mice were crossed with C57BL/6 females to produce Cdc7+/–tg mice, which were subsequently crossed with Cdc7+/– mice to generate Cdc7–/–tg mice. Cdc7–/–tg/tg mice with the homozygous transgene were generated by intercrossing Cdc7+/–tg mice.
Antibodies
Rabbit polyclonal anti-mouse Cdc7 antibody was described previously (Kim et al., 1998). Polyclonal mouse anti-COR1 (anti-SCP3) antibody that recognizes mouse SCP3 and rabbit anti-SYN1 (anti-SCP1) antibody that recognizes mouse SCP1 were kindly provided by Dr P.B.Moens (York University). Rat monoclonal antibodies TRA98, anti-calmegin (TRA369), and BC7 were generously provided by Dr Y.Nishimune (Osaka University). The antibodies against PCNA and α-tubulin were purchased from Santa Cruz and Sigma, respectively.
Isolation of MEFs
Cdc7+/–tg males were mated with Cdc7+/– females, and embryos were isolated at E13.5. MEFs were prepared according to standard procedures and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin G/streptomycin sulfate.
BrdU labeling of MEFs
Passage 2 MEFs were incubated for 2 h in the presence of 10 µM BrdU. Cells were stained with FITC-conjugated anti-BrdU antibody (PharMingen) and subjected to FACS analysis.
Immunostaining with anti-PCNA antibody
Cells were fixed for 5 min in methanol:acetone solution (1:1) and incubated with anti-PCNA antibody (1:200 dilution) for 20 min at 37°C. Alexa 488-conjugated anti-mouse IgG (Molecular Probes) was used as a secondary antibody.
Synchronization of MEFs
Passage 3 MEFs were incubated in DMEM containing 0.1% FBS for 48 h, and were restimulated in culture medium containing 15% FBS. For [3H]thymidine incorporation, the cells were incubated with 1 µCi of [3H]thymidine for 2 h at each time point after serum release. For BrdU incorporation, cells were pulse-labeled with 100 µM BrdU for 30 min. For aphidicolin block, cells serum-starved for 48 h were subsequently incubated in medium containing 15% FBS and aphidicolin (1 µg/ml) for 24 h. After release from the aphidicolin block, cells were harvested at various times for FACS analysis.
Histological analysis
Tissues were fixed in 10% buffered neutral formalin, dehydrated and embedded in paraffin. Sections (5 µm) from each tissue were stained with hematoxylin–eosin. Apoptotic cells were detected using an in situ Cell Death Detection Kit (Roche).
Immunohistochemistry
Testes were fixed in Bouin’s solution for 24 h and embedded in paraffin. Sections were incubated with TRA98, anti-calmegin and BC7 at 1:400, and anti-SCP3 and anti-SCP1 antibodies at 1:200. Horseradish peroxidase (HRP)-conjugated secondary antibodies were used at 1:100, and the detection was performed by HRP reaction with DAB as a substrate.
RT–PCR
Total RNA was extracted from adult testes of the wild-type, Cdc7+/–tg and Cdc7–/–tg mice using TRIZOL reagent (Gibco-BRL). Single-stranded cDNAs were prepared by reverse transcription using 5 µg of testes RNA. The following sequences were used as the primers: Hsp70-2, sense, 5′-AGTCATCACTGTTCCTGCCTA-3′, antisense, 5′-TAGAGCGAGTC GATCTCTATG-3′; Tnp1, sense, 5′-ATGTCGACCAGCCGCAAGCTA-3′, antisense, 5′-CGAATTTCGTCACGATGGCAT-3′; Tnp2, sense, 5′-ATGGACACCAAGATGCAGAGC-3′, antisense, 5′-TCACTTGTATC TTCGCCCTGA-3′; Prm1, sense, 5′-ATGGCCAGATACCGATGCTGC-3′, antisense, 5′-CTAGTATTTTTTACACCTTAT-3′; Prm2, sense, 5′-ATGGTTCGCTACCGAATGAGG-3′, antisense, 5′-TTAGTGATGGT GCCTCCTACA-3′; G3pdh, sense, 5′-AGTGGAGATTGTTGCCA TCAACGA-3′, antisense, 5′-TCATACTTGGCAGGTTTCTCCAGG-3′. The sequences of the primers for A-myb, Scp1 and Hox1.4 were described previously (Tanaka et al., 2000).
Western blot analysis
MEFs were lysed in a lysis buffer [50 mM Tris pH 7.6, 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml leupeptine and 10 µg/ml aprotinin]. Freshly prepared mouse organs were homogenized in a lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 2 mM EDTA, 1 mM DTT, 1 mM PMSF, 20 µg/ml leupeptine and 20 µg/ml aprotinin). Lysates were cleared by centrifugation and were used for western blot analysis. TRA98 and anti-calmegin rat monoclonal antibodies were diluted 1:1000, and polyclonal rabbit anti-SCP1 antibody was diluted 1:500. The detection was performed by chemiluminescence ECL kit using HRP-conjugated secondary antibodies.
Activation of thymocytes and proliferation assay
Thymocytes (2 × 105) were placed into 96-well plates and were stimulated using anti-CD3ε (200 ng/ml) antibodies and varying doses of IL-2 (0.1–100 U/ml). Cells were pulsed at 48 h with 1 µCi of [3H]thymidine, and harvested 12 h later.
Acknowledgments
Acknowledgements
We thank Miho Nagoya for devoted technical assistance in experiments with mice, Etsuko Matsui and Chika Taniyama for help in preparing MEFs, and Osamu Hatano for advice on preparation of testicular sections for histological analysis. J.M.K. was supported in part by the Japan Society for the Promotion of Science.
References
- Allen R.L., O’Brien,D.A. and Eddy,E.M. (1988) A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol. Cell. Biol., 8, 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.P. and Dutta,A. (2002) DNA replication in eukaryotic cells. Annu. Rev. Biochem., 71, 333–374. [DOI] [PubMed] [Google Scholar]
- Beumer T.L., Roepers-Gajadien,H.L., Gademan,I.S., Kal,H.B. and de Rooij,D.G. (2000) Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol. Reprod., 63, 1893–1898. [DOI] [PubMed] [Google Scholar]
- Brown G.W. and Kelly,T.J. (1999) Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl Acad. Sci. USA, 96, 8443–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J.W. and Johnston,L.H. (1989) The yeast gene, DBF4, essential for entry into S phase is cell cycle regulated. Exp. Cell Res., 180, 419–428. [DOI] [PubMed] [Google Scholar]
- Connor F., Bertwistle,D., Mee,P.J., Ross,G.M., Swift,S., Grigorieva,E., Tybulewicz,V.L. and Ashworth,A. (1997) Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat. Genet., 17, 423–430. [DOI] [PubMed] [Google Scholar]
- Copp A.J., Brook,F.A. and Roberts,H.J. (1988a) A cell-type-specific abnormality of cell proliferation in mutant (curly tail) mouse embryos developing spinal neural tube defects. Development, 104, 285–295. [DOI] [PubMed] [Google Scholar]
- Copp A.J., Crolla,J.A. and Brook,F.A. (1988b) Prevention of spinal neural tube defects in the mouse embryo by growth retardation during neurulation. Development, 104, 297–303. [DOI] [PubMed] [Google Scholar]
- de Alboran I.M., O’Hagan,R.C., Gartner,F., Malynn,B., Davidson,L., Rickert,R., Rajewsky,K., DePinho,R.A. and Alt,F.W. (2001) Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity, 14, 45–55. [DOI] [PubMed] [Google Scholar]
- de Rooij D.G. and van Dissel-Emiliani,F.M.F. (1997) Regulation of proliferation and differentiation of stem cells in the male germ line. In Potten,C.S. (ed.), Stem Cells. Academic Press, San Diego, CA, pp. 283–313. [Google Scholar]
- Dobson M.J., Pearlman,R.E., Karaiskakis,A., Spyropoulos,B. and Moens,P.B. (1994) Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J. Cell Sci., 107, 2749–2760. [DOI] [PubMed] [Google Scholar]
- Dutta A. and Bell,S.P. (1997) Initiation of DNA replication in eukaryotic cells. Annu. Rev. Cell. Dev. Biol., 13, 293–332. [DOI] [PubMed] [Google Scholar]
- Embury S., Seller,M.J., Adinolfi,M. and Polani,P.E. (1979) Neural tube defects in curly-tail mice. I. Incidence, expression and similarity to the human condition. Proc. R. Soc. Lond. B Biol. Sci., 206, 85–94. [DOI] [PubMed] [Google Scholar]
- Gruneberg H. (1954) Genetical studies on the skeleton of the mouse: VIII. curly-tail. J. Genet., 52, 52–67. [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hollingsworth R.E. Jr and Sclafani,R.A. (1990) DNA metabolism gene CDC7 from yeast encodes a serine (threonine) protein kinase. Proc. Natl Acad. Sci. USA, 87, 6272–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima J., Yasui,H., Morishima,M. and Shiroishi,T. (1998) Dominant lethality of the mouse skeletal mutation tail-short (Ts) is determined by the Ts allele from mating partners. Genomics, 49, 341–350. [DOI] [PubMed] [Google Scholar]
- Jackson A.L., Pahl,P.M., Harrison,K., Rosamond,J. and Sclafani,R.A. (1993) Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol. Cell. Biol., 13, 2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W. and Hunter,T. (1997) Identification and characterization of a human protein kinase related to budding yeast Cdc7p. Proc. Natl Acad. Sci. USA, 94, 14320–14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., McDonald,D., Hope,T.J. and Hunter,T. (1999) Mammalian Cdc7–Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J., 18, 5703–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.H. and Thomas,A.P. (1982a) A further two mutants defective in initiation of the S phase in the yeast Saccharomyces cerevisiae. Mol. Gen. Genet., 186, 445–448. [DOI] [PubMed] [Google Scholar]
- Johnston L.H. and Thomas,A.P. (1982b) The isolation of new DNA synthesis mutants in the yeast Saccharomyces cerevisiae. Mol. Gen. Genet., 186, 439–444. [DOI] [PubMed] [Google Scholar]
- Johnston L.H., Masai,H. and Sugino,A. (1999) First the CDKs, now the DDKs. Trends Cell Biol., 9, 249–252. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Sato,N., Yamada,M., Arai,K. and Masai,H. (1998) Growth regulation of the expression of mouse cDNA and gene encoding a serine/threonine kinase related to Saccharomyces cerevisiae CDC7 essential for G1/S transition. Structure, chromosomal localization and expression of mouse gene for S. cerevisiae CDC7-related kinase. J. Biol. Chem., 273, 23248–23257. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Nakao,K., Nakamura,K., Saito,I., Katsuki,M., Arai,K. and Masai,H. (2002) Inactivation of Cdc7 kinase in mouse ES cells results in S-phase arrest and p53-dependent cell death. EMBO J., 21, 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K.C., Distel,R.J. and Hecht,N.B. (1984) Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev. Biol., 105, 71–79. [DOI] [PubMed] [Google Scholar]
- Koshimizu U., Watanabe,D., Sawada,K. and Nishimune,Y. (1993) A novel stage-specific differentiation antigen is expressed on mouse testicular germ cells during early meiotic prophase. Biol. Reprod., 49, 875–884. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Sato,N., Yamada,M., Mahony,D., Seghezzi,W., Lees,E., Arai,K. and Masai,H. (1999) A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells. Mol. Cell. Biol., 19, 5083–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra A., Vooijs,M., Beverloo,H.B., Allak,B.A., van Drunen,E., Kanaar,R., Berns,A. and Jonkers,J. (2001) Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl Acad. Sci. USA, 98, 9209–9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Miyake,T. and Arai,K. (1995) hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J., 14, 3094–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J.R. (1993) Development of the germ cell. In Ewing,L.L. (ed.), Cell and Molecular Biology of the Testis. Oxford University Press, Oxford, UK, pp. 58–89. [Google Scholar]
- Meistrich M.L. (1989) Histone and basic nuclear protein transitions in mammalian spermatogenesis. In Hnilica,L.S., Stein,G.S., Stein,J.L., Rauterberg,J. and Jaeger,E. (eds), Histones and Other Basic Nuclear Proteins. CRC, Boca Raton, FL, pp. 165–182. [Google Scholar]
- Mettus R.V., Litvin,J., Wali,A., Toscani,A., Latham,K., Hatton,K. and Reddy,E.P. (1994) Murine A-myb: evidence for differential splicing and tissue-specific expression. Oncogene, 9, 3077–3086. [PubMed] [Google Scholar]
- Meuwissen R.L., Offenberg,H.H., Dietrich,A.J., Riesewijk,A., van Iersel,M. and Heyting,C. (1992) A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J., 11, 5091–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S.G., Dubus,P., Mettus,R.V., Galbreath,E.J., Boden,G., Reddy,E.P. and Barbacid,M. (1999) Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet., 22, 44–52. [DOI] [PubMed] [Google Scholar]
- Ravnik S.E., Rhee,K. and Wolgemuth,D.J. (1995) Distinct patterns of expression of the D-type cyclins during testicular development in the mouse. Dev. Genet., 16, 171–178. [DOI] [PubMed] [Google Scholar]
- Regnier C.H., Masson,R., Kedinger,V., Textoris,J., Stoll,I., Chenard,M.P., Dierich,A., Tomasetto,C. and Rio,M.C. (2002) Impaired neural tube closure, axial skeleton malformations and tracheal ring disruption in TRAF4-deficient mice. Proc. Natl Acad. Sci. USA, 99, 5585–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M.R., Toth,L.E., Patel,M.D., D’Eustachio,P. and Nguyen-Huu,M.C. (1986) A mouse homeo box gene is expressed in spermatocytes and embryos. Science, 233, 663–667. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. (2002) Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science, 296, 2176–2178. [DOI] [PubMed] [Google Scholar]
- Sato N., Arai,K. and Masai,H. (1997) Human and Xenopus cDNAs encoding budding yeast Cdc7-related kinases: in vitro phosphorylation of MCM subunits by a putative human homologue of Cdc7. EMBO J., 16, 4340–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.E., Taylor,D.S., Prigge,J.R., Barnett,S. and Capecchi,M.R. (2000) Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl Acad. Sci. USA, 97, 13702–13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R.A. and Jackson,A.L. (1994) Cdc7 protein kinase for DNA metabolism comes of age. Mol. Microbiol., 11, 805–810. [DOI] [PubMed] [Google Scholar]
- Sclafani R.A., Patterson,M., Rosamond,J. and Fangman,W.L. (1988) Differential regulation of the yeast CDC7 gene during mitosis and meiosis. Mol. Cell. Biol., 8, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D.P. and Livingston,D.M. (2001) Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell, 8, 233–243. [DOI] [PubMed] [Google Scholar]
- Stillman B. (1996) Cell cycle control of DNA replication. Science, 274, 1659–1664. [DOI] [PubMed] [Google Scholar]
- Takeda T., Ogino,K., Matsui,E., Cho,M.K., Kumagai,H., Miyake,T., Arai,K. and Masai,H. (1999) A fission yeast gene, him1+/dfp1+, encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol., 19, 5535–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Ogino,K., Tatebayashi,K., Ikeda,H., Arai,K.-i. and Masai,H. (2001) Regulation of initiation of S phase, replication checkpoint signaling and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast. Mol. Biol. Cell., 12, 1257–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Pereira,L.A., Nozaki,M., Tsuchida,J., Sawada,K., Mori,H. and Nishimune,Y. (1997) A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int. J. Androl., 20, 361–366. [DOI] [PubMed] [Google Scholar]
- Tanaka S.S., Toyooka,Y., Akasu,R., Katoh-Fukui,Y., Nakahara,Y., Suzuki,R., Yokoyama,M. and Noce,T. (2000) The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev., 14, 841–853. [PMC free article] [PubMed] [Google Scholar]
- Trauth K., Mutschler,B., Jenkins,N.A., Gilbert,D.J., Copeland,N.G. and Klempnauer,K.H. (1994) Mouse A-myb encodes a trans-activator and is expressed in mitotically active cells of the developing central nervous system, adult testis and B lymphocytes. EMBO J., 13, 5994–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Hesabi,B., Moons,D.S., Pandolfi,P.P., Hansel,K.S., Koff,A. and Kiyokawa,H. (1999) Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol., 19, 7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D., Sawada,K., Koshimizu,U., Kagawa,T. and Nishimune,Y. (1992) Characterization of male meiotic germ cell-specific antigen (Meg 1) by monoclonal antibody TRA 369 in mice. Mol. Reprod. Dev., 33, 307–312. [DOI] [PubMed] [Google Scholar]
- Watanabe D., Yamada,K., Nishina,Y., Tajima,Y., Koshimizu,U., Nagata,A. and Nishimune,Y. (1994) Molecular cloning of a novel Ca2+-binding protein (calmegin) specifically expressed during male meiotic germ cell development. J. Biol. Chem., 269, 7744–7749. [PubMed] [Google Scholar]
- Wolgemuth D.J. and Watrin,F. (1991) List of cloned mouse genes with unique expression patterns during spermatogenesis. Mamm. Genome, 1, 283–288. [DOI] [PubMed] [Google Scholar]
- Zakeri Z.F., Wolgemuth,D.J. and Hunt,C.R. (1988) Identification and sequence analysis of a new member of the mouse HSP70 gene family and characterization of its unique cellular and developmental pattern of expression in the male germ line. Mol. Cell. Biol., 8, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang,X. and Wolgemuth,D.J. (1999) Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology, 140, 2790–2800. [DOI] [PubMed] [Google Scholar]