Abstract
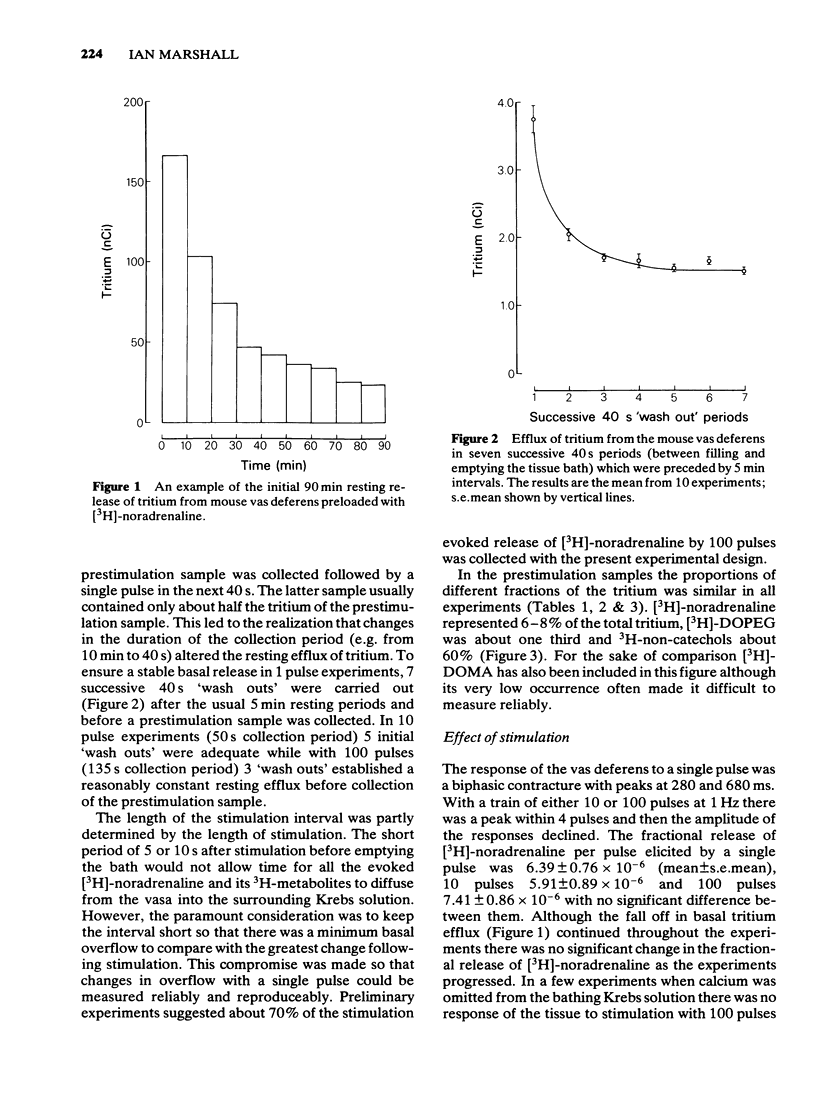
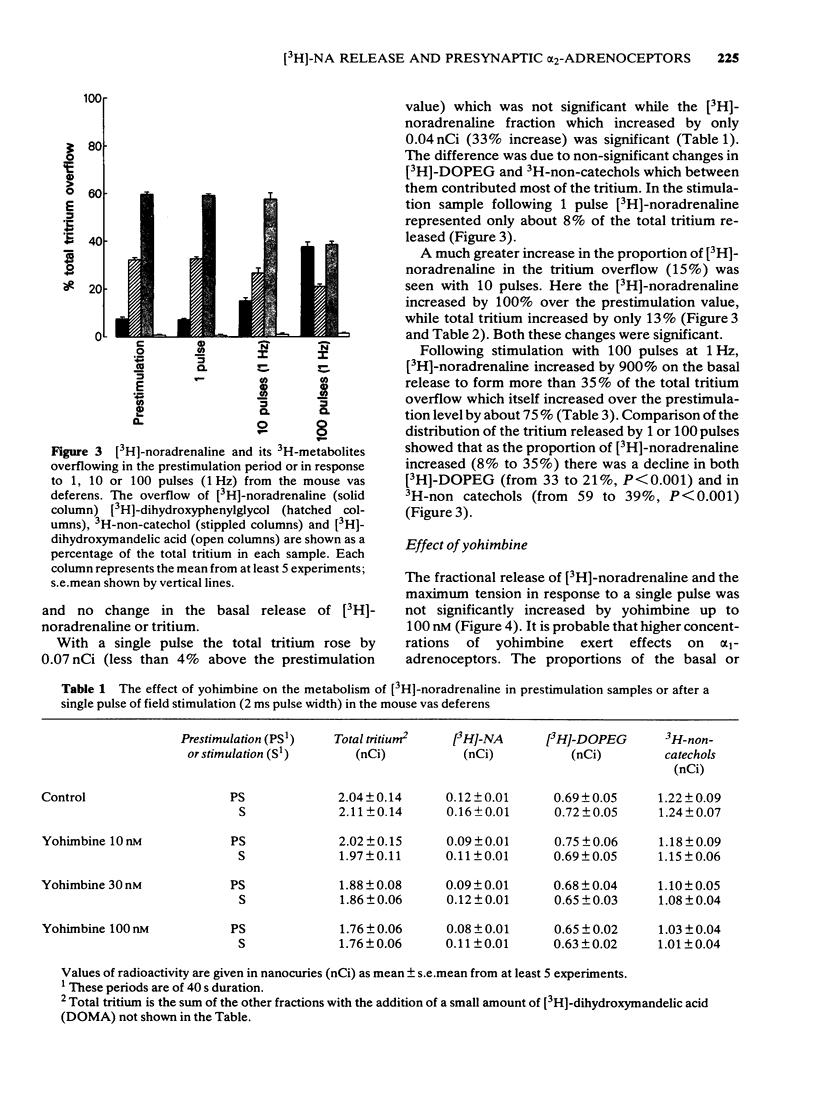
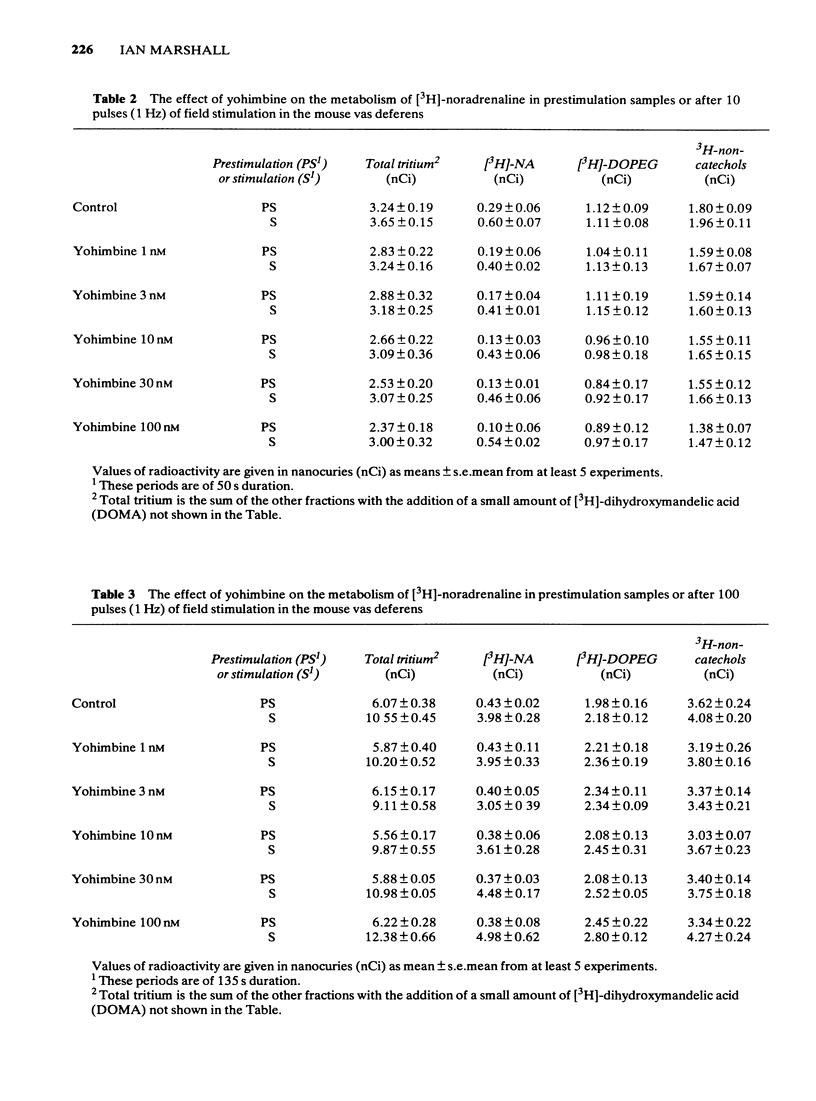
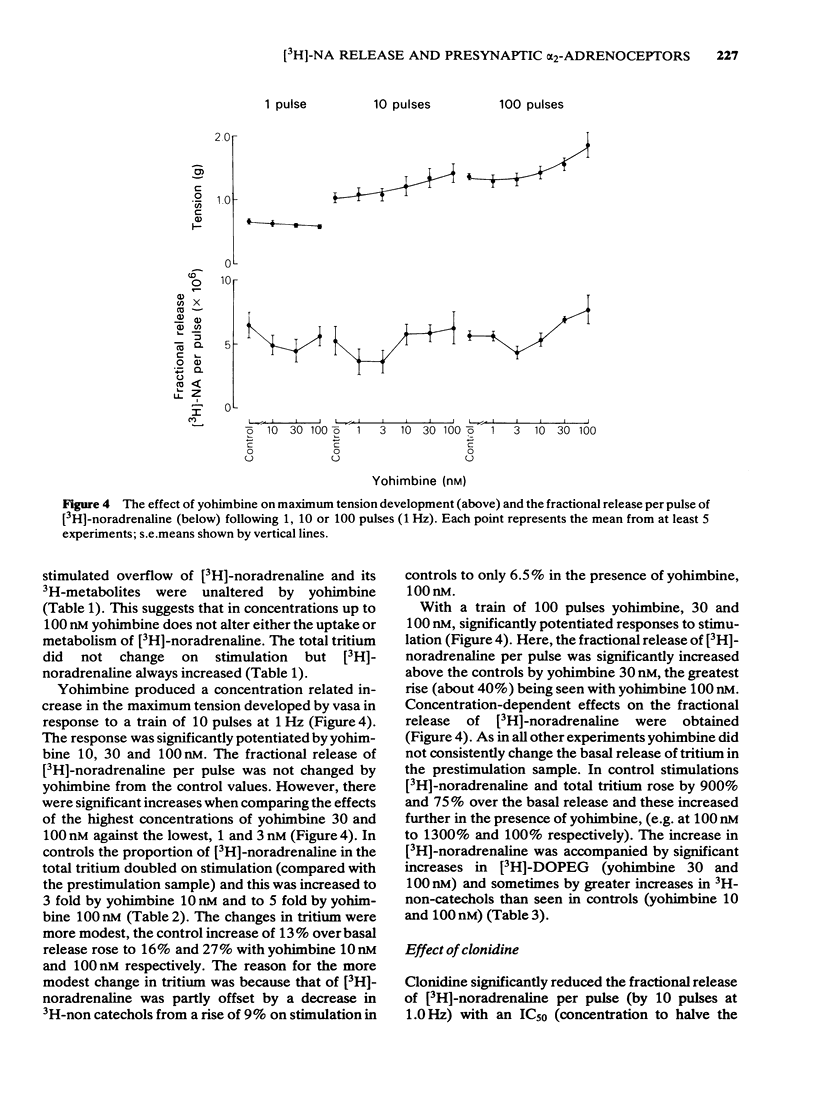
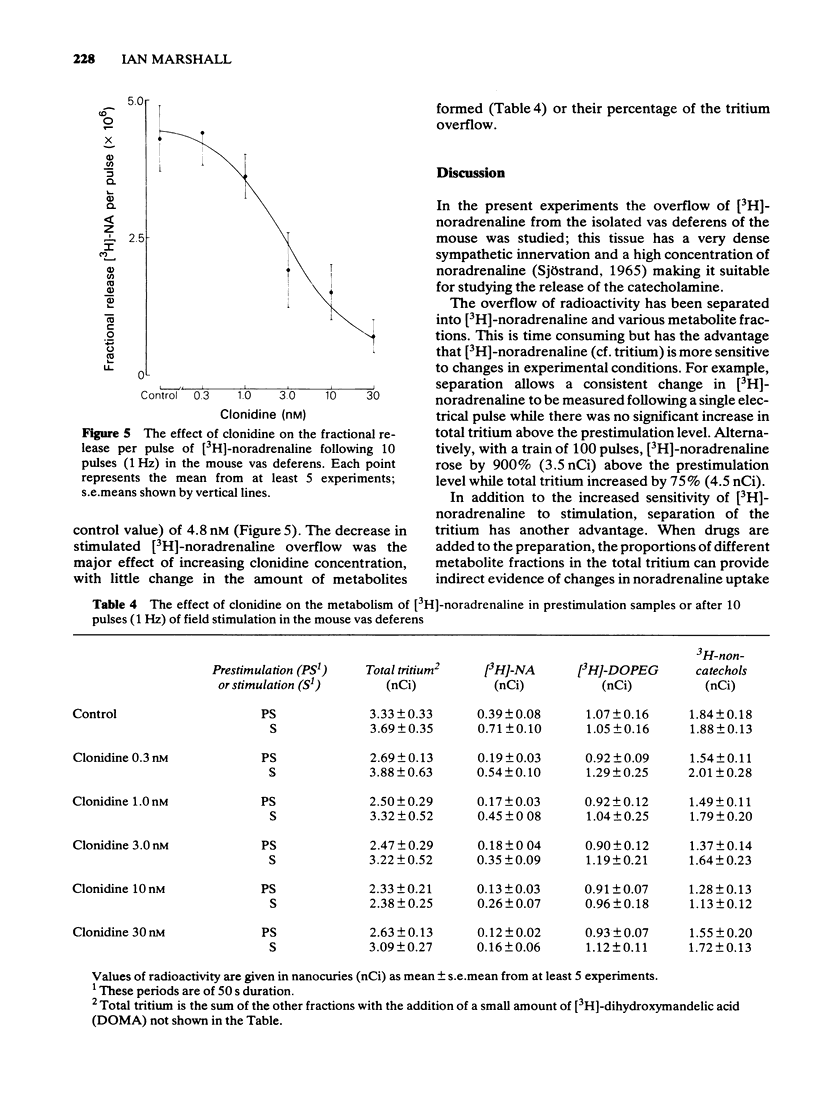
1 Mice isolated vasa deferentia were loaded with 1-[7,8-3H]-noradrenaline and subsequently field stimulated with 1, 10 or 100 pulses (2 ms pulse width, 1 Hz). The tritium overflow was separated into [3H]-noradrenaline and its 3H-metabolites. 2 The resting release of tritium contained about 7% [3H]-noradrenaline, 33% [3H]-3, 4-dihydroxyphenylglycol ([3H]-DOPEG) and 60% 3H-non-catechols with usually less than 1% [3H]-dihydroxymandelic acid ([3H]-DOMA). The proportion of the tritium as [3H]-noradrenaline increased with stimulation train length to 35% with 100 pulses; this increase in [3H]-noradrenaline was associated with falls in [3H]-DOPEG and 3H-non-catechols. Generally the proportional increase in [3H]-noradrenaline on stimulation was about 10 x total tritium when compared with the resting release. 3 The fractional release of [3H]-noradrenaline per pulse was independent of train length, averaging about 6 x 10(-6). This was reduced by the alpha 2-adrenoceptor agonist clonidine (0.3 - 30 nM) with an IC50 of 4.8 nM (10 pulses at 1 Hz). 4 The alpha 2-adrenoceptor antagonist, yohimbine (10 - 100 nM), did not alter the fractional release of [3H]-noradrenaline elicited by 1 pulse. The antagonist did not change the amount or composition of the resting or evoked tritium overflow. However, yohimbine (1 - 100 nM) increased the fractional release of [3H]-noradrenaline per pulse for trains of 10 or 100 pulses (1 Hz) in a concentration-dependent fashion. An increase above controls was significant only with 100 pulses and yohimbine, 30 nM. 5 The results show that the release of noradrenaline during trains of pulses in the mouse vas deferens can be regulated through presynaptic alpha 2-adrenoceptors. There was no evidence of inhibition by noradrenaline of its own release following a single pulse.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Dubocovich M. L., Langer S. Z. Influence of the frequency of nerve stimulation on the metabolism of 3H-norepinephrine released from the perfused cat spleen: differences observed during and after the period of stimulation. J Pharmacol Exp Ther. 1976 Jul;198(1):83–101. [PubMed] [Google Scholar]
- Dubocovich M. L., Langer S. Z. Negative feed-back regulation of noradrenaline release by nerve stimulation in the perfused cat's spleen: differences in potency of phenoxybenzamine in blocking the pre- and post-synaptic adrenergic receptors. J Physiol. 1974 Mar;237(3):505–519. doi: 10.1113/jphysiol.1974.sp010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Malamfors T. 3 H-noradrenaline release and mechanical response in the field stimulated mouse vas deferens. Acta Physiol Scand Suppl. 1971;371:1–18. doi: 10.1111/j.1748-1716.1971.tb05210.x. [DOI] [PubMed] [Google Scholar]
- Graffe K. H., Stefano F. J., Langer S. Z. Preferential metabolism of (-) 3 H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol. 1973 May 15;22(10):1147–1160. doi: 10.1016/0006-2952(73)90231-1. [DOI] [PubMed] [Google Scholar]
- Hughes I. E. The effect of amitriptyline on presynaptic mechanisms in noradrenergic nerves. Br J Pharmacol. 1978 Jun;63(2):315–321. [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Differential labelling of intraneuronal noradrenaline stores with different concentrations of (-)-3H-noradrenaline. Br J Pharmacol. 1973 Feb;47(2):428–430. doi: 10.1111/j.1476-5381.1973.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Evaluation of mechanisms controlling the release and inactivation of the adrenergic transmitter in the rabbit portal vein and vas deferens. Br J Pharmacol. 1972 Mar;44(3):472–491. doi: 10.1111/j.1476-5381.1972.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggendal J., Malmfors T. The effect of nerve stimulation on the uptake of noradrenaline into the adrenergic nerve terminals. Acta Physiol Scand. 1969 Jan-Feb;75(1):28–32. doi: 10.1111/j.1748-1716.1969.tb04351.x. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Limitations of presynaptic adrenoceptor theory: the characteristics of the effects of noradrenaline and phenoxybenzamine on stimulation-induced efflux of [3H]noradrenaline in vas deferens. J Pharmacol Exp Ther. 1980 Feb;212(2):232–239. [PubMed] [Google Scholar]
- Kalsner S. Single pulse stimulation of guinea-pig vas deferens and the presynaptic receptor hypothesis. Br J Pharmacol. 1979 Jun;66(2):343–349. doi: 10.1111/j.1476-5381.1979.tb13686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I., Nasmyth P. A., Nicholl C. G., Shepperson N. B. alpha-Adrenoceptors in the mouse vas deferens and their effects on its response to electrical stimulation. Br J Pharmacol. 1978 Jan;62(1):147–151. doi: 10.1111/j.1476-5381.1978.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I., Nasmyth P. A., Shepperson N. B. Presynaptic alpha-adrenoceptors and [3H]-noradrenaline overflow from the mouse was deferens [proceedings]. Br J Pharmacol. 1978 Mar;62(3):382P–383P. [PMC free article] [PubMed] [Google Scholar]
- Starke K., Borowski E., Endo T. Preferential blockade of presynaptic alpha-adrenoceptors by yohimbine. Eur J Pharmacol. 1975 Dec;34(2):385–388. doi: 10.1016/0014-2999(75)90268-x. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H., Gayk W., Merker R. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Alpha-sympathomimetic inhibition of Neurogenic vasoconstriction. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):133–150. doi: 10.1007/BF00501149. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1981;21:7–30. doi: 10.1146/annurev.pa.21.040181.000255. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Clonidine enhances the secretion of sympathetic neurotransmitter from isolated guineq-pig tissues. Acta Physiol Scand. 1975 Jan;93(1):142–144. doi: 10.1111/j.1748-1716.1975.tb05801.x. [DOI] [PubMed] [Google Scholar]
- Story D. F., McCulloch M. W., Rand M. J., Standford-Starr C. A. Conditions required for the inhibitory feedback loop in noradrenergic transmission. Nature. 1981 Sep 3;293(5827):62–65. doi: 10.1038/293062a0. [DOI] [PubMed] [Google Scholar]


