Abstract
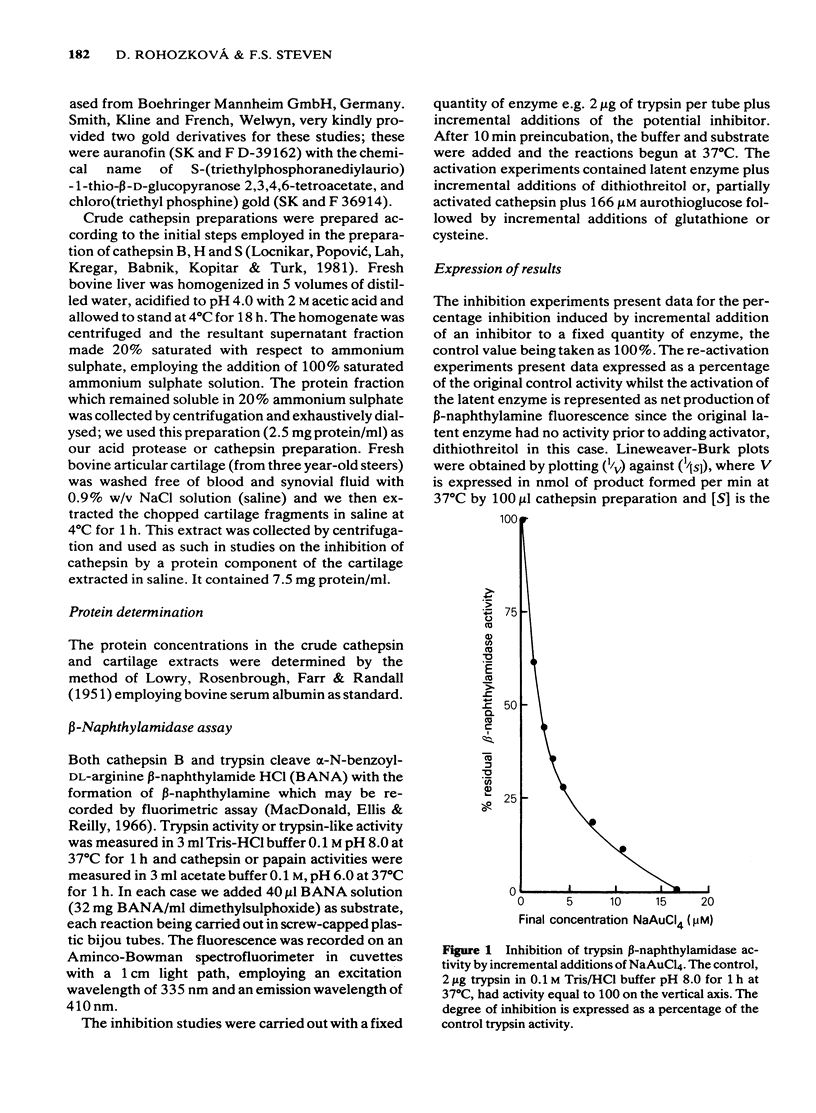
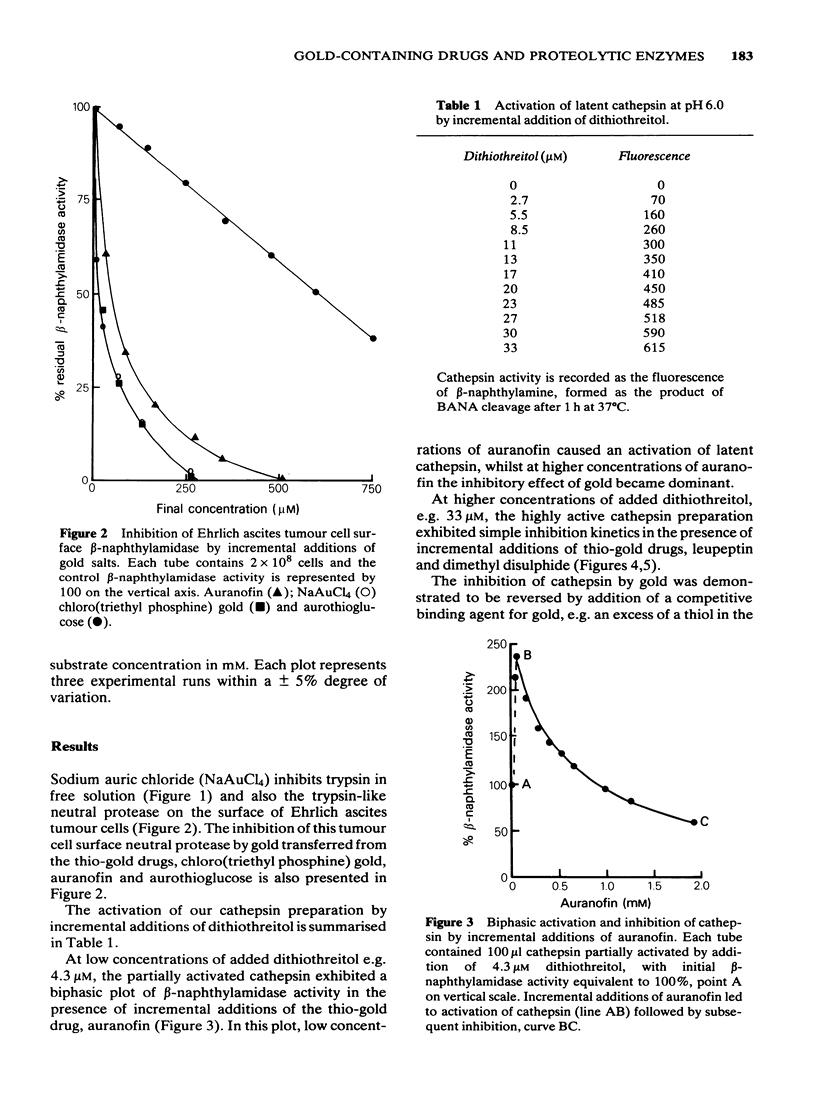
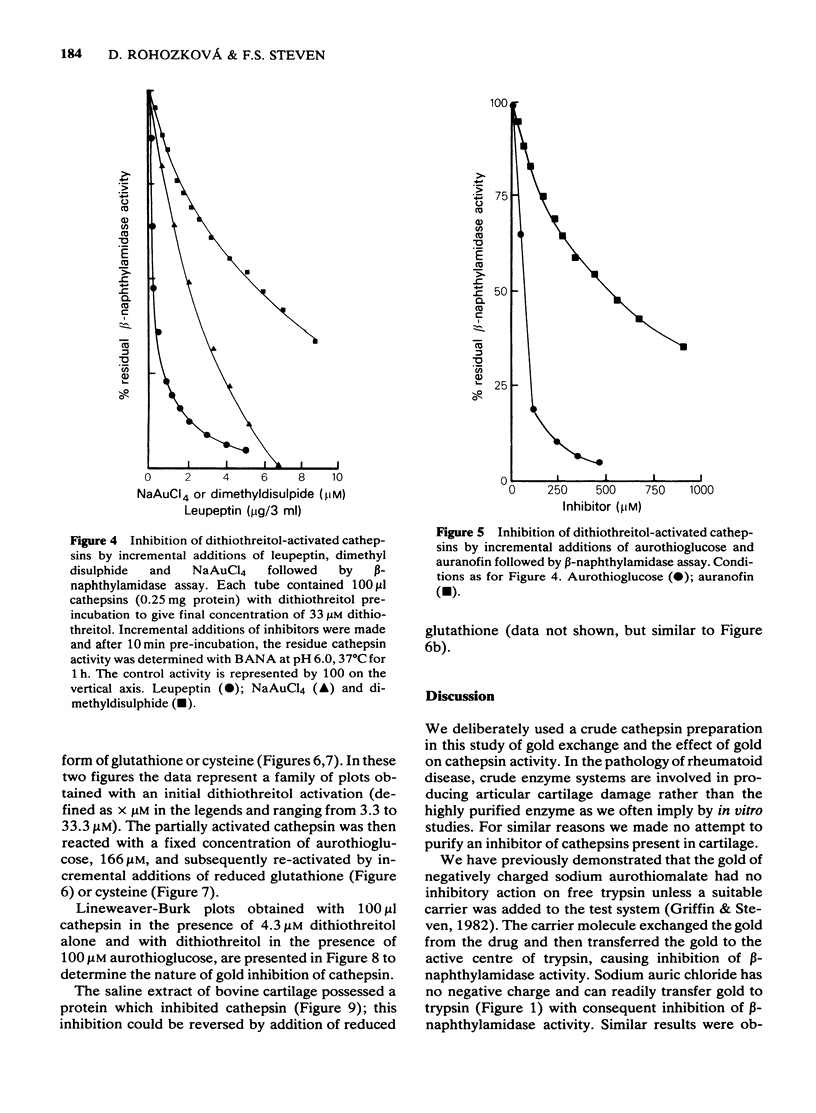
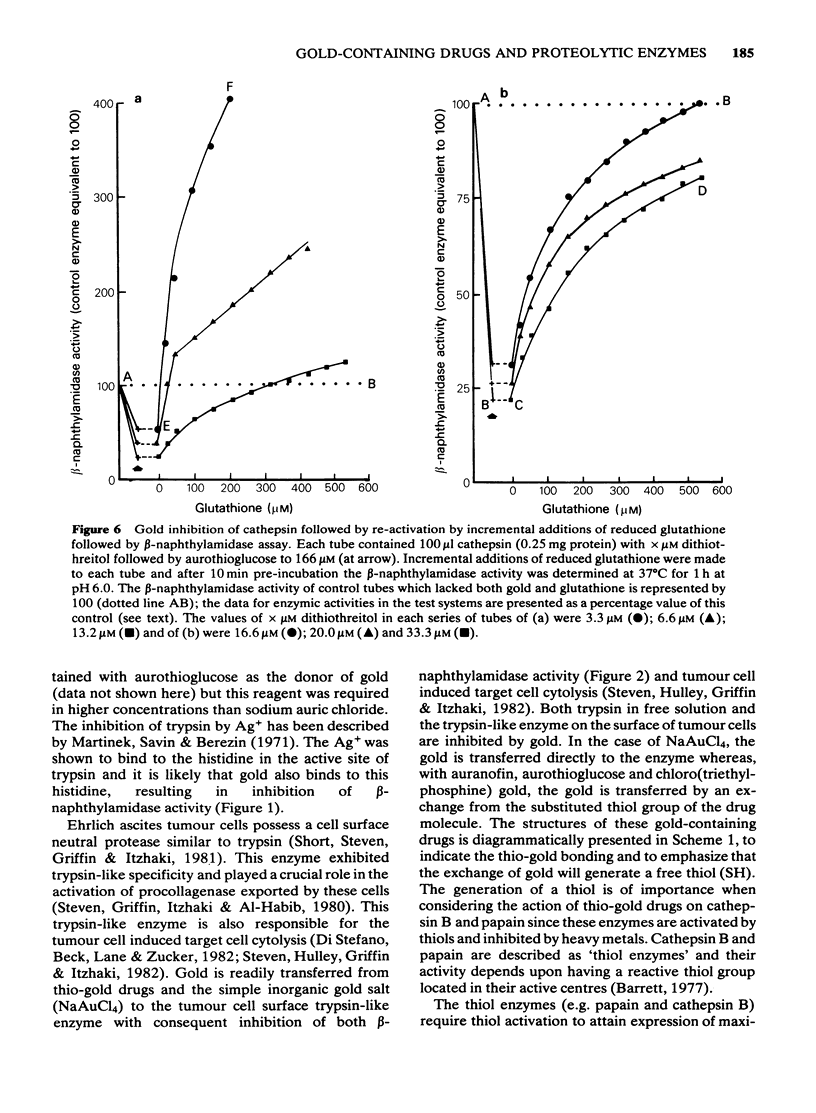
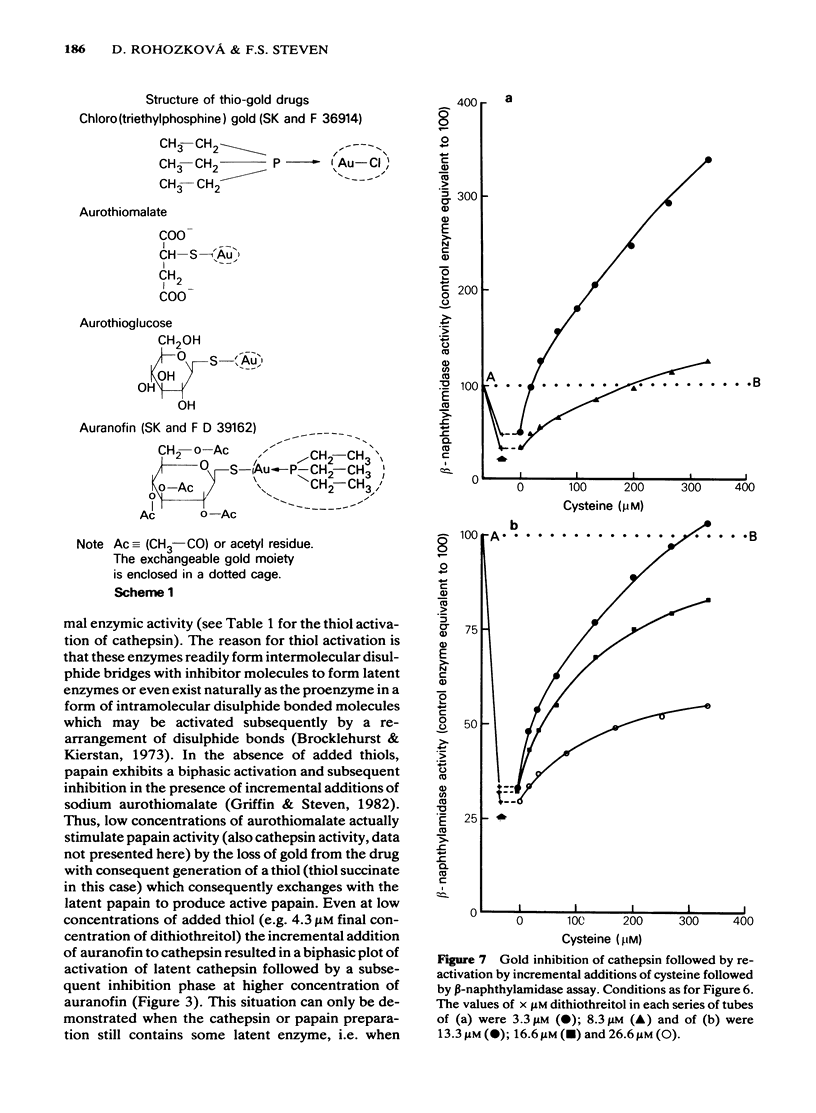
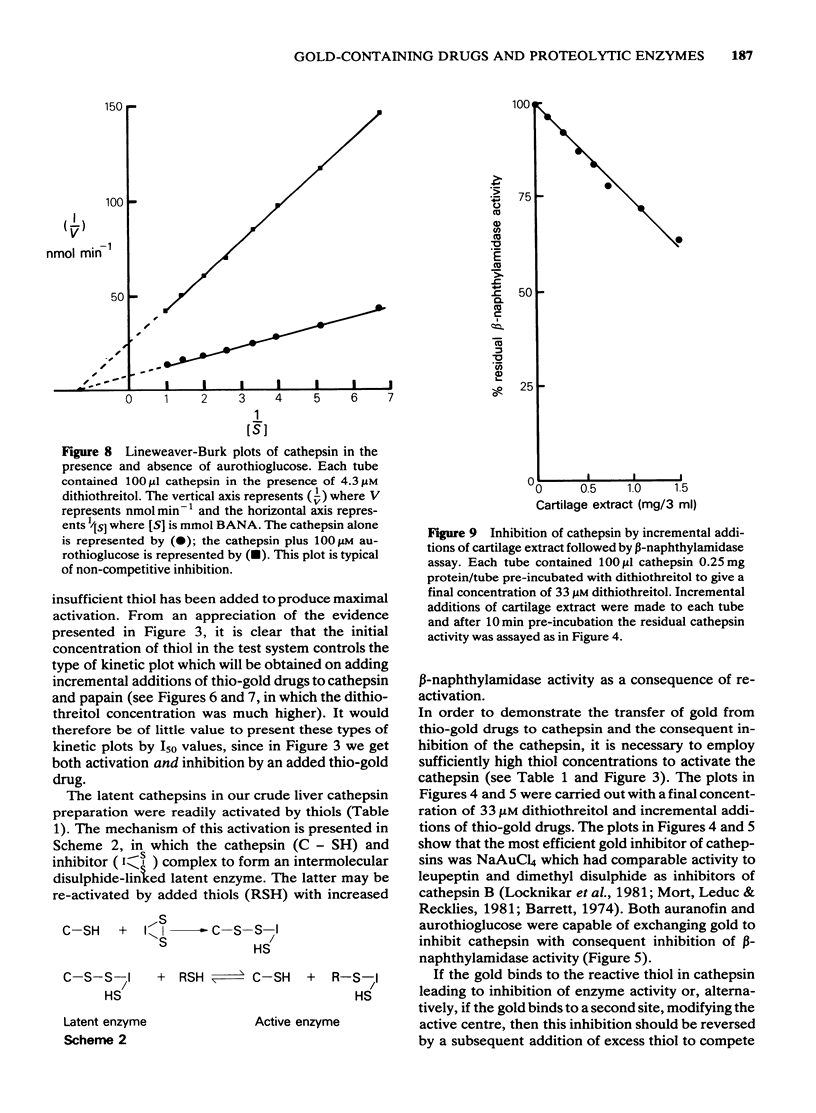
1 NaAuCl4 and aurothioglucose inhibited trypsin in free solution without the need of a carrier molecule. 2 NaAuCl4, aurothioglucose, aurothiomalate, auranofin and chloro-triethyl phosphine) gold all inhibited the trypsin-like neutral protease on the surfaces of Ehrlich ascites tumour cell membranes equally well. 3 Crude cathepsin preparations were activated by low concentrations of dithiothreitol and also by aurothioglucose, due to the displacement of an inhibitor. 4 Thiol-activated cathepsins were inhibited by each of the gold derivatives. The gold could be withdrawn from the enzyme by incremental additions of thiols such as reduced glutathione and cysteine with regeneration of enzymic activity. 5 Lineweaver-Burk plots of kinetic data indicated that gold acted as a non competitive inhibitor of cathepsins. 6 A naturally occurring inhibitor of cathepsins was extracted from cartilage. The mechanism of inhibition was again shown to be a thiol-disulfide exchange, the disulphide being provided by the inhibitor and the thiol being provided by the enzyme. 7 The role of gold in the attempted control of proteolysis in the rheumatoid arthritis is briefly discussed in terms of reversible exchange reactions involving gold thiols, disulphides and cartilage inhibitors of proteolytic enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brocklehurst K., Kierstan M. P. Propapain and its conversion to papain: a new type of zymogen activation mechanism involving intramolecular thiol-disulphide interchange. Nat New Biol. 1973 Apr 11;242(119):167–170. doi: 10.1038/newbio242167a0. [DOI] [PubMed] [Google Scholar]
- DiStefano J. F., Beck G., Lane B., Zucker S. Role of tumor cell membrane-bound serine proteases in tumor-induced target cytolysis. Cancer Res. 1982 Jan;42(1):207–218. [PubMed] [Google Scholar]
- Griffin M. M., Steven F. S. Inhibition of trypsin and papain by sodium aurothiomalate mediated by exchange reactions. Br J Pharmacol. 1982 Feb;75(2):333–339. doi: 10.1111/j.1476-5381.1982.tb08791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halla J. T., Cassady J., Hardin J. G. Sequential gold and penicillamine therapy in rheumatoid arthritis. Am J Med. 1982 Mar;72(3):423–426. doi: 10.1016/0002-9343(82)90509-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald J. K., Ellis S., Reilly T. J. Properties of dipeptidyl arylamidase I of the pituitary. Chloride and sulfhydryl activation of seryltyrosyl-beta-naphthylamide hydrolysis. J Biol Chem. 1966 Apr 10;241(7):1494–1501. [PubMed] [Google Scholar]
- Mort J. S., Leduc M., Recklies A. D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981 Dec 15;662(2):173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M. Isolation of a protease inhibitor from tissues resistant to tumor invasion. Hoppe Seylers Z Physiol Chem. 1977 Dec;358(12):1525–1531. doi: 10.1515/bchm2.1977.358.2.1525. [DOI] [PubMed] [Google Scholar]
- Short A. K., Steven F. S., Griffin M. M., Itzhaki S. beta-Naphthylamidase activity of the cell surface of Ehrlich ascites cells. Reversible control of enzyme activity by metal ions and thiols. Br J Cancer. 1981 Nov;44(5):709–716. doi: 10.1038/bjc.1981.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S., Griffin M. M., Itzhaki S., Al-Habib A. A trypsin-like neutral protease on Ehrlich ascites cell surfaces: its role in the activation of tumour-cell zymogen of collagenase. Br J Cancer. 1980 Nov;42(5):712–721. doi: 10.1038/bjc.1980.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S., Podrazký V. Evidence for the inhibition of trypsin by thiols. The mechanism of enzyme-inhibitor complex formation. Eur J Biochem. 1978 Feb 1;83(1):155–161. doi: 10.1111/j.1432-1033.1978.tb12079.x. [DOI] [PubMed] [Google Scholar]


