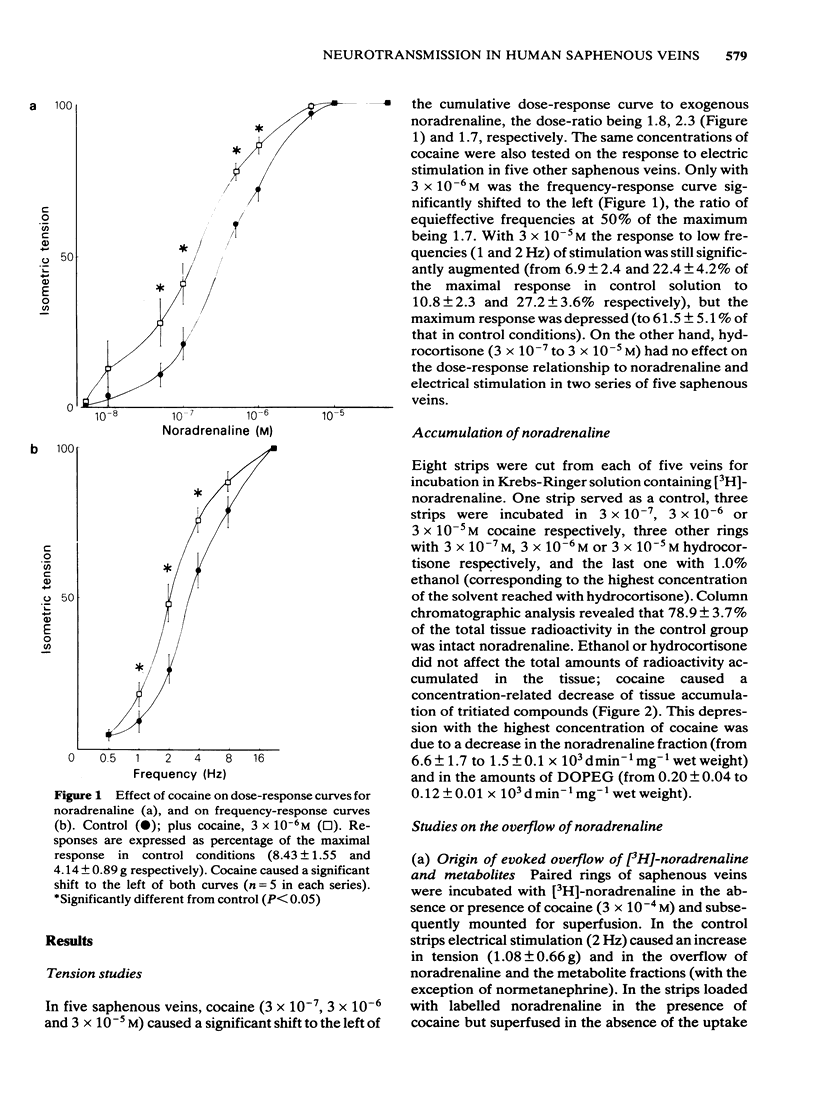
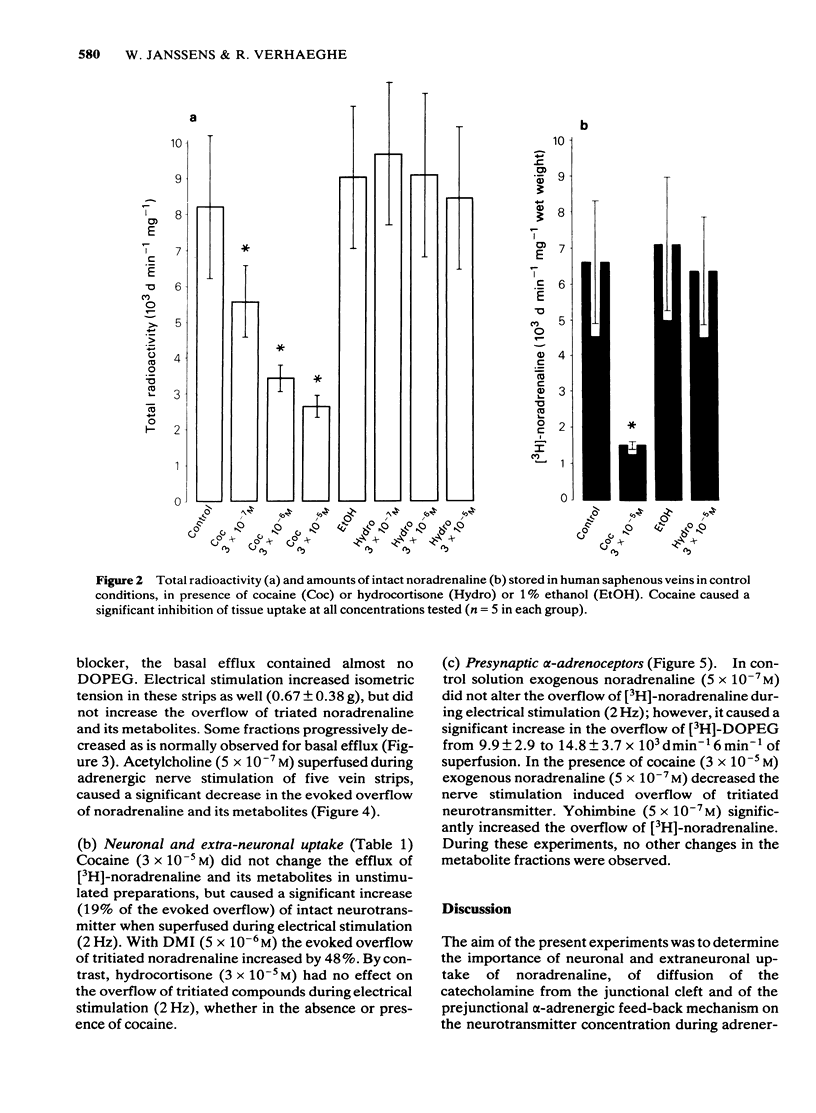
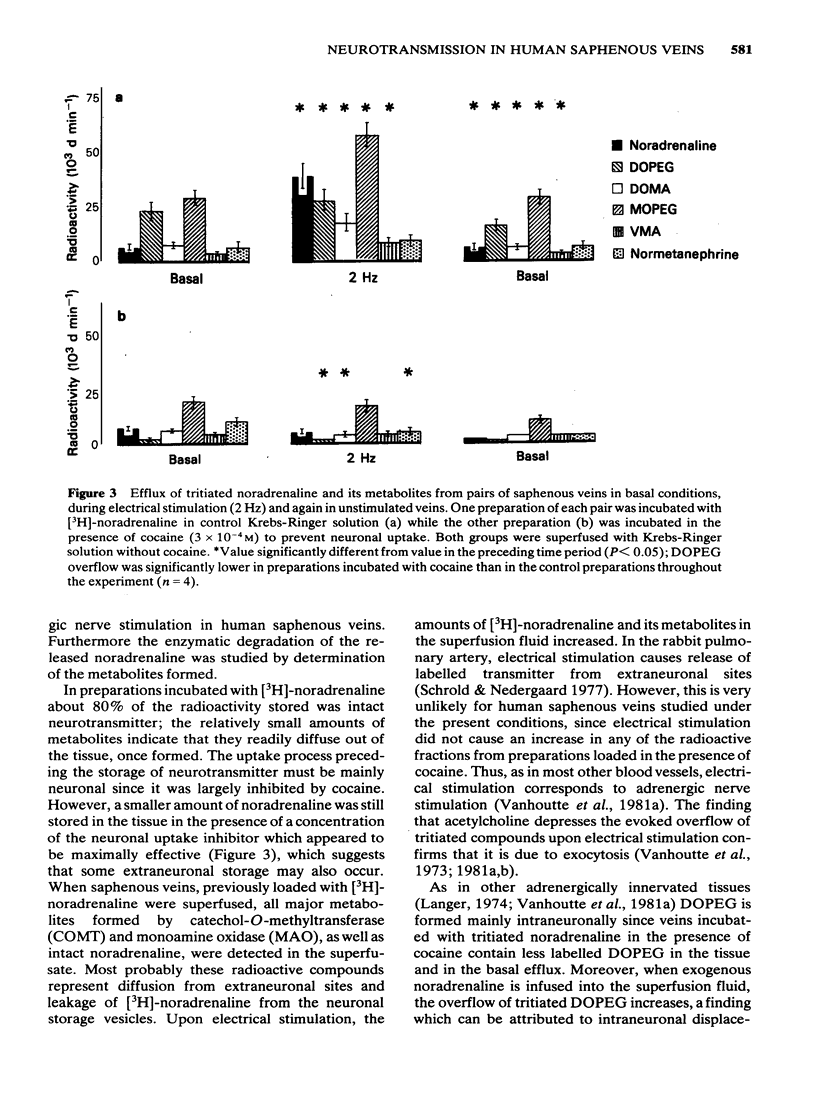
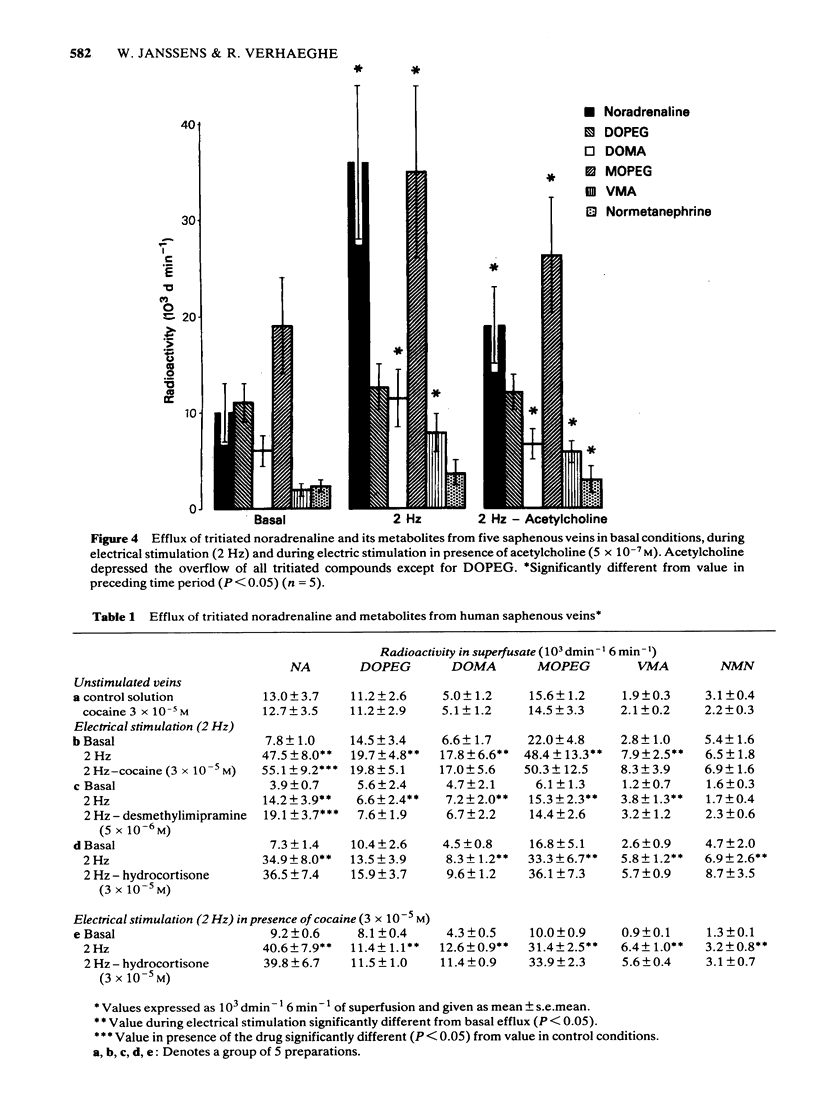
Abstract
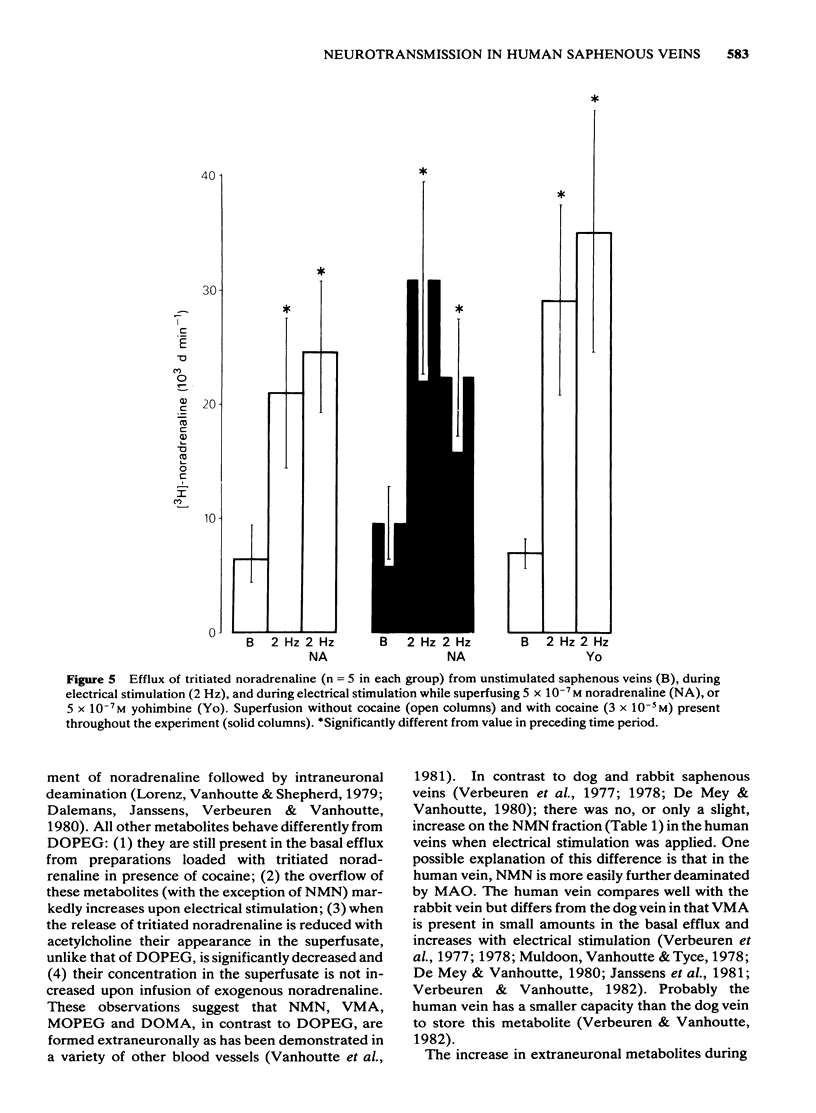
We studied the importance of neuronal and extraneuronal uptake and of the pre-junctional alpha-adrenergic feed-back mechanism for the junctional noradrenaline concentration in the human saphenous vein. All major metabolites of the enzymatic breakdown of noradrenaline were detected in the overflow of superfused veins loaded with [3H]-noradrenaline. The efflux of 3,4-dihydroxyphenylglycol (DOPEG) was drastically reduced in preparations labelled after neuronal uptake blockade indicating its neuronal origin; the other metabolites are formed extraneuronally since they behaved distinctly differently from DOPEG under several experimental conditions. Extraneuronal uptake followed by enzymatic breakdown removes the same amount of noradrenaline from the biophase during nerve activity as that diffusing intact out of the tissue, whereas neuronal uptake appears only half as effective since the overflow of intact noradrenaline increases by only 48% in the presence of desmethylimipramine (DMI). However, in preparations mounted for isometric tension recording, neuronal uptake blockade potentiated contractions to alpha-adrenergic activation, emphasizing the functional importance of the neuronal disposition mechanism. By contrast, no evidence was found for a hydrocortisone-sensitive extraneuronal uptake compartment, suggesting that extraneuronal removal may have little, if any, functional importance. During nerve stimulation, yohimbine increased the amount of labelled noradrenaline present in the superfusate, while exogenously added noradrenaline decreased it in the presence of cocaine. Thus, prejunctional alpha-adrenoceptors can modulate the junctional concentration of neurotransmitter in the human saphenous vein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Mey J. G., Vanhoutte P. M. Comparison of the responsiveness of cutaneous veins of dog and rabbit to adrenergic and cholinergic stimulation. Blood Vessels. 1980;17(1):27–43. doi: 10.1159/000158232. [DOI] [PubMed] [Google Scholar]
- Graefe K. H., Trendelenburg U. The effect of hydrocortisone on the sensitivity of the isolated nictitating membrane to catecholamines: Relationship to extraneuronal uptake and metabolism. Naunyn Schmiedebergs Arch Pharmacol. 1974;286(1):1–48. doi: 10.1007/BF00499103. [DOI] [PubMed] [Google Scholar]
- Janssens W. J., Verbeuren T. J., Vanhoutte P. M. Effect of moderate cooling on adrenergic neuroeffector interaction in canine cutaneous veins. Blood Vessels. 1981;18(6):281–295. doi: 10.1159/000158361. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Enero M. A. The potentiation of responses to adrenergic nerve stimulation in the presence of cocaine: its relationship to the metabolic fate of released norepinephrine. J Pharmacol Exp Ther. 1974 Dec;191(3):431–443. [PubMed] [Google Scholar]
- Langer S. Z. Selective metabolic pathways for noradrena-line in the peripheral and in the central nervous system. Med Biol. 1974 Dec;52(6):372–383. [PubMed] [Google Scholar]
- Muldoon S. M., Vanhoutte P. M., Tyce G. M. Norepinephrine metabolism in canine saphenous vein: prevalence of glycol metabolites. Am J Physiol. 1978 Mar;234(3):H235–H243. doi: 10.1152/ajpheart.1978.234.3.H235. [DOI] [PubMed] [Google Scholar]
- Paiva M. Q., Guimarães S. A comparative study of the uptake and metabolism of noradrenaline and adrenaline by the isolated saphenous vein of the dog. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jul;303(3):221–228. doi: 10.1007/BF00498047. [DOI] [PubMed] [Google Scholar]
- Rorie D. K., Muldoon S. M., Tyce G. M. Disposition of norepinephrine during nerve stimulation in dog saphenous vein. Am J Physiol. 1980 Aug;239(2):H238–H246. doi: 10.1152/ajpheart.1980.239.2.H238. [DOI] [PubMed] [Google Scholar]
- Rorie D. K., Rusch N. J., Shepherd J. T., Vanhoutte P. M., Tyce G. M. Prejunctional inhibition of norepinephrine release caused by acetylcholine in the human saphenous vein. Circ Res. 1981 Aug;49(2):337–341. doi: 10.1161/01.res.49.2.337. [DOI] [PubMed] [Google Scholar]
- Schrold J., Nedergaard O. A. Neuronal and extraneuronal outflow of 3H-noradrenaline induced by electrical-field stimulation of an isolated blood vessel. Acta Physiol Scand. 1977 Oct;101(2):129–143. doi: 10.1111/j.1748-1716.1977.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Brudin J. Prostaglandin E2- and alpha- or beta-adrenoceptor mediated interferences with 3H-noradrenaline secretion from human vasomotor nerves: comparison between effects on omental arteries and veins. Acta Physiol Scand. 1977 Jun;100(2):267–269. doi: 10.1111/j.1748-1716.1977.tb05948.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Brundin J. Affinity of Noradrenaline and dopamine for neural alpha-receptors mediating negative feedback control of noradrenaline secretion in human vasoconstrictor nerves. Acta Physiol Scand. 1975 Sep;95(1):89–94. doi: 10.1111/j.1748-1716.1975.tb10029.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Brundin J. Frequency-dependence of 3H-noradrenaline secretion from human vasoconstrictor nerves: modification by factors interfering with alpha-or beta-adrenoceptor or prostaglandin E2 mediated control. Acta Physiol Scand. 1977 Oct;101(2):199–210. doi: 10.1111/j.1748-1716.1977.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U., Graefe K. H. Supersensitivity to catecholamines after impairment of extraneuronal uptake or catechol-O-methyl transferase. Fed Proc. 1975 Sep;34(10):1971–1974. [PubMed] [Google Scholar]
- Vanhoutte P. M., Collis M. G., Janssens W. J., Verbeuren T. J. Calcium dependence of prejunctional inhibitory effects of adenosine and acetylcholine on adrenergic neurotransmission in canine saphenous veins. Eur J Pharmacol. 1981 Jun 19;72(2-3):189–198. doi: 10.1016/0014-2999(81)90273-9. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]
- Verbeuren T. J., Coen E., Vanhoutte P. M. Determination of 3H-norepinephrine and its metabolites in superfusate from isolated blood vessels. Arch Int Pharmacodyn Ther. 1977 May;227(1):171–174. [PubMed] [Google Scholar]
- Verbeuren T. J., Janssens W. J., Vanhoutte P. M. Effects of moderate acidosis on adrenergic neurotransmission in canine saphenous veins. J Pharmacol Exp Ther. 1978 Jul;206(1):105–114. [PubMed] [Google Scholar]
- Verbeuren T. J., Vanhoutte P. M. Deamination of released 3H-noradrenaline in the canine saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):148–157. doi: 10.1007/BF00500474. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Vanhoutte P. M. Effect of blockade of neuronal uptake on the evoked release of 3-H-norepinephrine in the saphenous vein of the dog. Arch Int Pharmacodyn Ther. 1978 Aug;234(2):349–350. [PubMed] [Google Scholar]


