Abstract
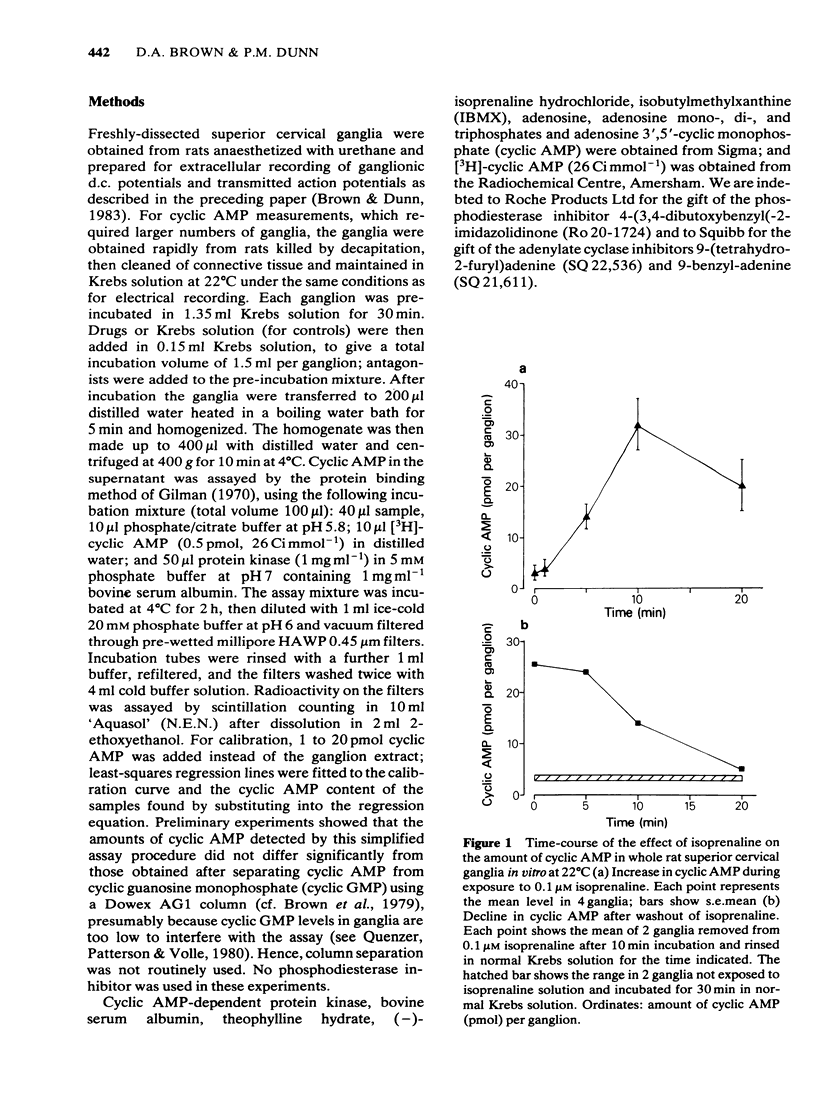
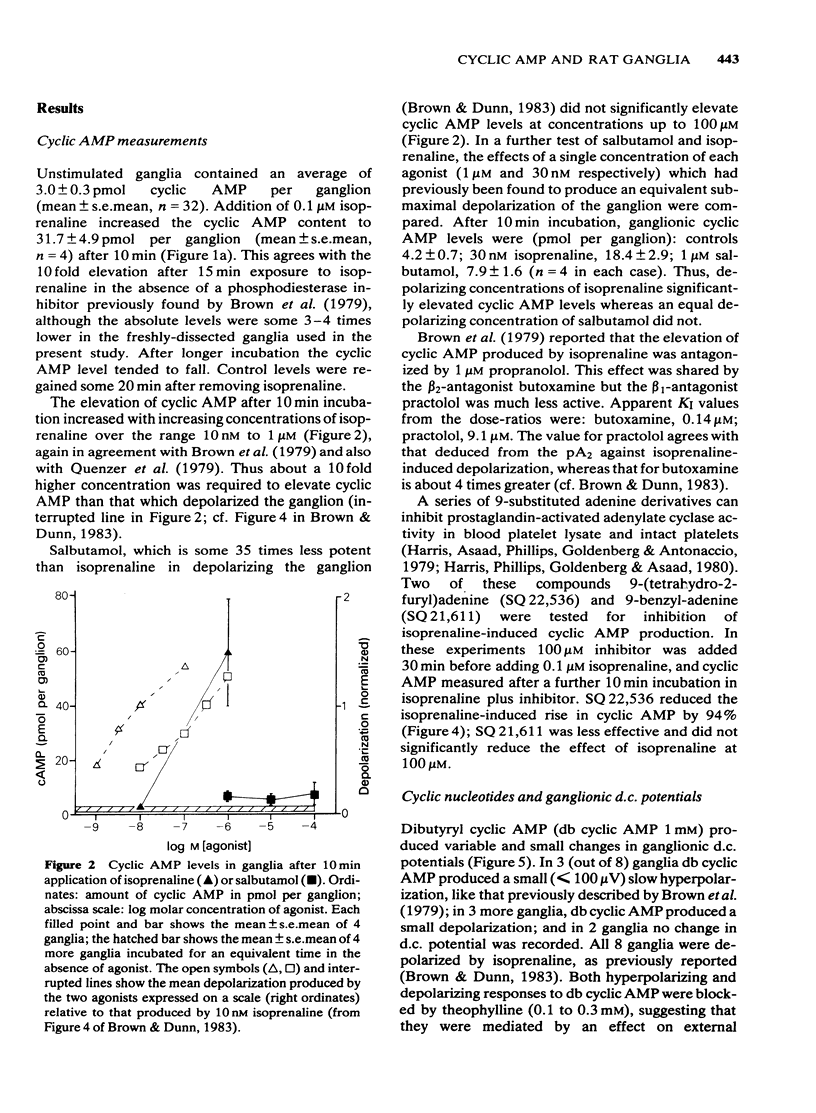
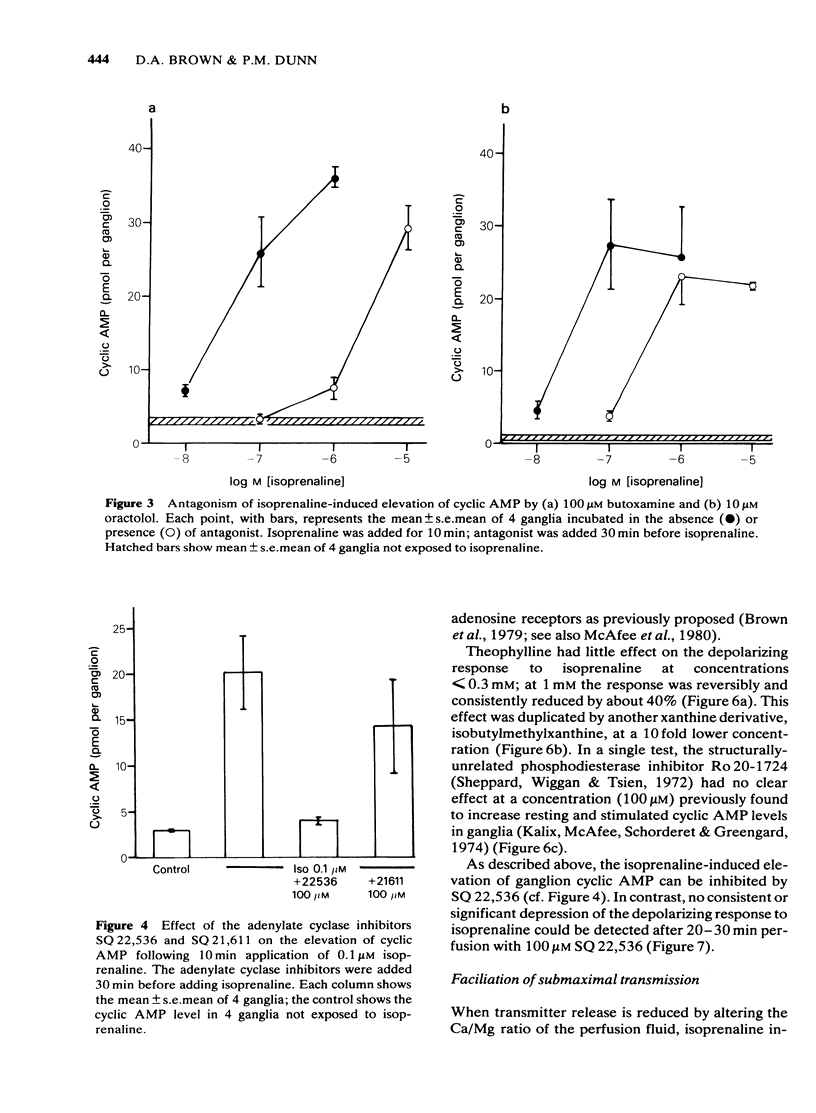
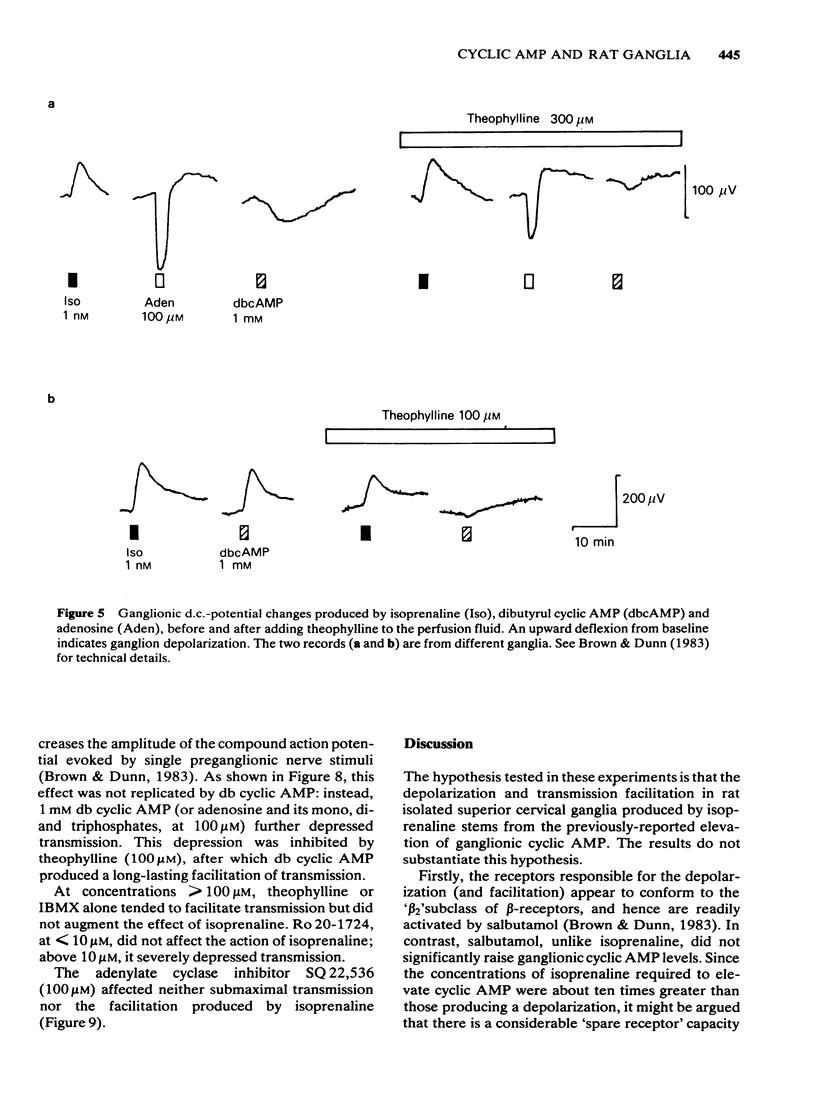
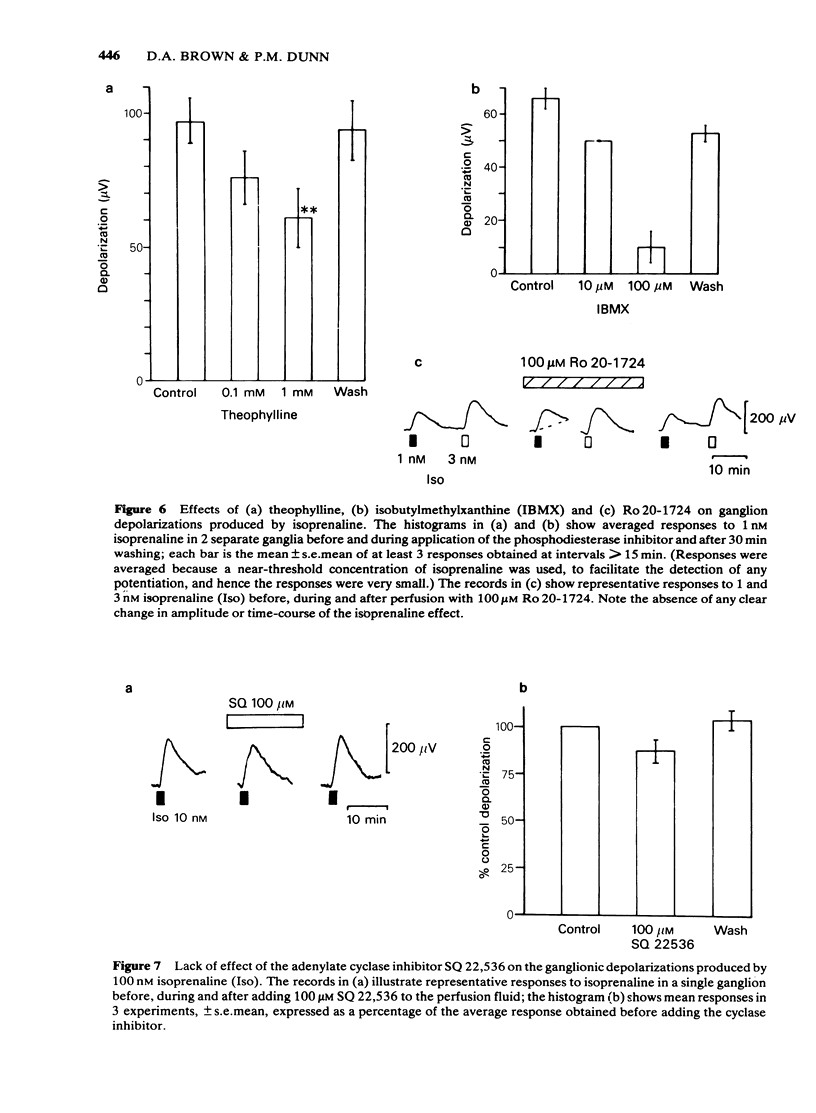
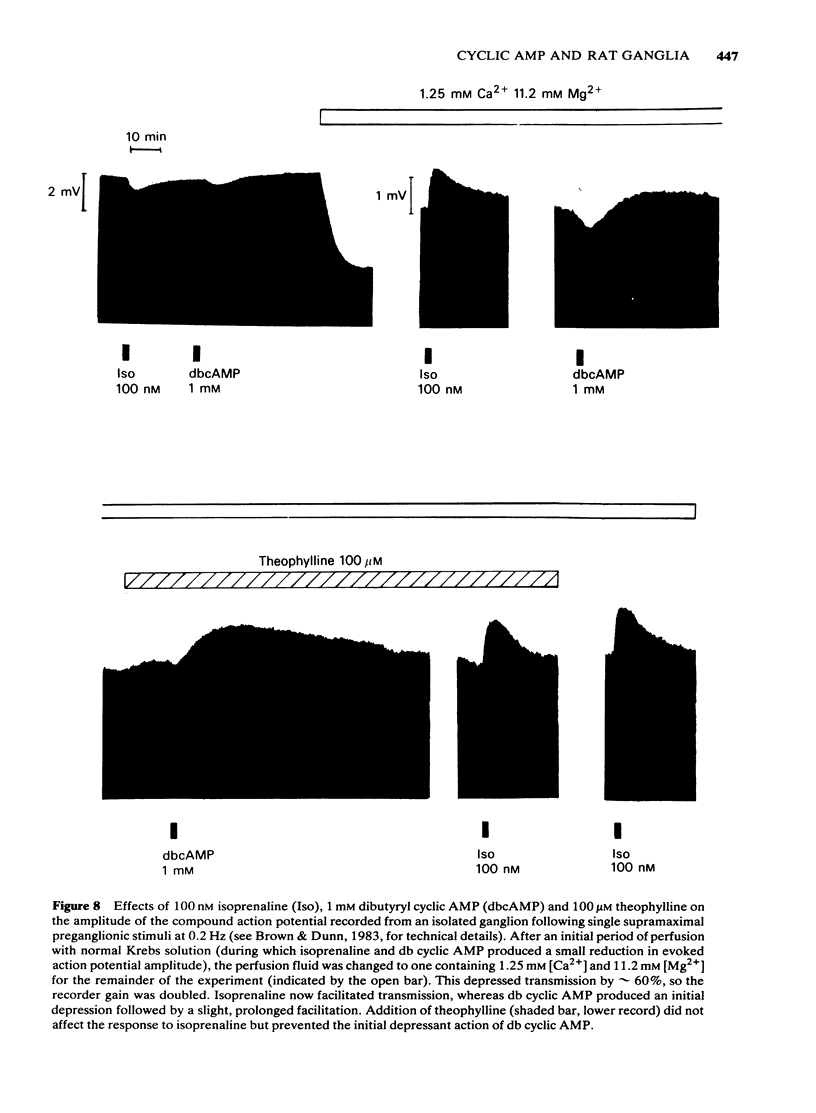
Isoprenaline (0.01-1 microM) increased the amount of cyclic adenosine 3',5'-monophosphate (cyclic AMP) in rat isolated superior cervical ganglia by up to 10 times after 10 min application. Cyclic AMP levels returned to control values after 20 min washing. Salbutamol, in concentrations (1-100 microM) that depolarized the ganglion and facilitated submaximal transmission, did not significantly raise ganglionic cyclic AMP levels. The action of isoprenaline was antagonized by butoxamine (apparent KI approximately equal to 0.14 microM) and weakly by practolol (apparent KI approximately equal to 9.1 microM). The effect of 0.1 microM isoprenaline was also inhibited 94% by 100 microM of the adenylate cyclase inhibitor, 9-(tetrahydro-2-furyl)adenine (SQ 22,536). Exogenous dibutyryl cyclic AMP did not replicate the effects of isoprenaline on ganglionic d.c. potentials or submaximal transmission. The phosphodiesterase inhibitors theophylline, isobutylmethylxanthine or 4-(3,4-dibutoxybenzyl)-2-imidazolidinone (Ro 20-1724) did not potentiate these electrical responses to isoprenaline. The adenylate cyclase inhibitor, SQ 22,536, did not inhibit the electrical responses to isoprenaline. It is concluded that available evidence does not support the view that the ganglion depolarization or facilitation of submaximal transmission in rat isolated ganglia produced by isoprenaline are likely to be mediated by cyclic AMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Koketsu K. Effects of dibutyryl cyclic adenosine 3',5'-monophosphate and theophylline on the bullfrog sympathetic ganglion cells. Br J Pharmacol. 1977 Jul;60(3):331–336. doi: 10.1111/j.1476-5381.1977.tb07504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Ottesen B. Mechanism of action of vasoactive intestinal polypeptide on myometrial smooth muscle of rabbit and guinea-pig. J Physiol. 1981 Sep;318:41–55. doi: 10.1113/jphysiol.1981.sp013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P., Kirby P. J. Relation between catecholamine-induced cyclic AMP changes and hyperpolarization in isolated rat sympathetic ganglia. J Physiol. 1979 May;290(2):441–451. doi: 10.1113/jphysiol.1979.sp012782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F., Smith P. A. Synaptic potentials in sympathetic ganglia: are they mediated by cyclic nucleotides? Science. 1978 Jun 2;200(4345):1079–1081. doi: 10.1126/science.206964. [DOI] [PubMed] [Google Scholar]
- Cramer H., Johnson D. G., Hanbauer I., Silberstein S. D., Kopin I. J. Accumulation of adenosine 3',5'-monophosphate induced by catecholamines in the rat superior cervical ganglion in vitro. Brain Res. 1973 Apr 13;53(1):97–104. doi: 10.1016/0006-8993(73)90769-5. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Kaibara K., Karczmar A. G. Dopamine and adenosine 3',5'-monophosphate responses of single mammalian sympathetic neurons. Science. 1977 Aug 19;197(4305):778–780. doi: 10.1126/science.196332. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. A comparison of the effect of theophylline and cyclic adenosine 3': 5'-monophosphate on the superior cervical ganglion of the rabbit by means of the sucrose-gap method. J Pharmacol Exp Ther. 1977 Jul;202(1):89–96. [PubMed] [Google Scholar]
- Gallagher J. P., Shinnick-Gallagher P. Cyclic nucleotides injected intracellularly into rat superior cervical ganglion cells. Science. 1977 Nov 25;198(4319):851–852. doi: 10.1126/science.199943. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Norepinephrine inhibits calcium-dependent potentials in rat sympathetic neurons. Science. 1979 Jun 15;204(4398):1233–1235. doi: 10.1126/science.221979. [DOI] [PubMed] [Google Scholar]
- Hsu S. Y., McIsaac R. J. Effects of theophylline and N6,O2-dibutyryl adenosine 3':5'-monophosphate on sympathetic ganglionic transmission in rats. J Pharmacol Exp Ther. 1978 Apr;205(1):91–103. [PubMed] [Google Scholar]
- Kalix P., McAfee D. A., Schorderet M., Greengard P. Pharmacological analysis of synaptically mediated increase in cyclic adenosine monophosphate in rabbit superior cervical ganglion. J Pharmacol Exp Ther. 1974 Mar;188(3):676–687. [PubMed] [Google Scholar]
- Kobayashi H., Hashiguchi T., Ushiyama N. Postsynaptic modulation of excitatory process in sympathetic ganglia by cyclic AMP. Nature. 1978 Jan 19;271(5642):268–270. doi: 10.1038/271268a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Kato E., Kumamoto E., Koketsu K., Hirai K. Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature. 1981 Jun 25;291(5817):654–656. doi: 10.1038/291654a0. [DOI] [PubMed] [Google Scholar]
- Lindl T., Cramer H. Evidence against dopamine as the mediator of the rise of cyclic AMP in the superior cervical ganglion of the rat. Biochem Biophys Res Commun. 1975 Jul 22;65(2):731–739. doi: 10.1016/s0006-291x(75)80206-3. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Henon B. K., Whiting G. J., Horn J. P., Yarowsky P. J., Turner D. K. The action of cAMP and catecholamines in mammalian sympathetic ganglia. Fed Proc. 1980 Oct;39(12):2997–3002. [PubMed] [Google Scholar]
- Otten U., Mueller R. A., Oesch F., Thoenen H. Location of an isoproterenol-responsive cyclic AMP pool in adrenergic nerve cell bodies and its relationship to tyrosine 3-monooxygenase induction. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2217–2221. doi: 10.1073/pnas.71.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenzer L. F., Patterson B. A., Volle R. L. The cyclic nucleotide content of the rat superior cervical ganglion. J Pharmacol Exp Ther. 1980 Nov;215(2):297–303. [PubMed] [Google Scholar]
- Quenzer L., Yahn D., Alkadhi K., Volle R. L. Transmission blockade and stimulation of ganglionic adenylate cyclase by catecholamines. J Pharmacol Exp Ther. 1979 Jan;208(1):31–36. [PubMed] [Google Scholar]
- Sheppard H., Wiggan G., Tsien W. H. Structure-activity relationships for inhibitors of phosphodiesterase from erythrocytes and other tissues. Adv Cyclic Nucleotide Res. 1972;1:103–112. [PubMed] [Google Scholar]
- Suzuki T., Volle R. L. Responses of the rat superior cervical ganglion in vitro to isoprenaline and bethanechol. Naunyn Schmiedebergs Arch Pharmacol. 1978 Aug;304(1):15–20. doi: 10.1007/BF00501372. [DOI] [PubMed] [Google Scholar]


