Abstract
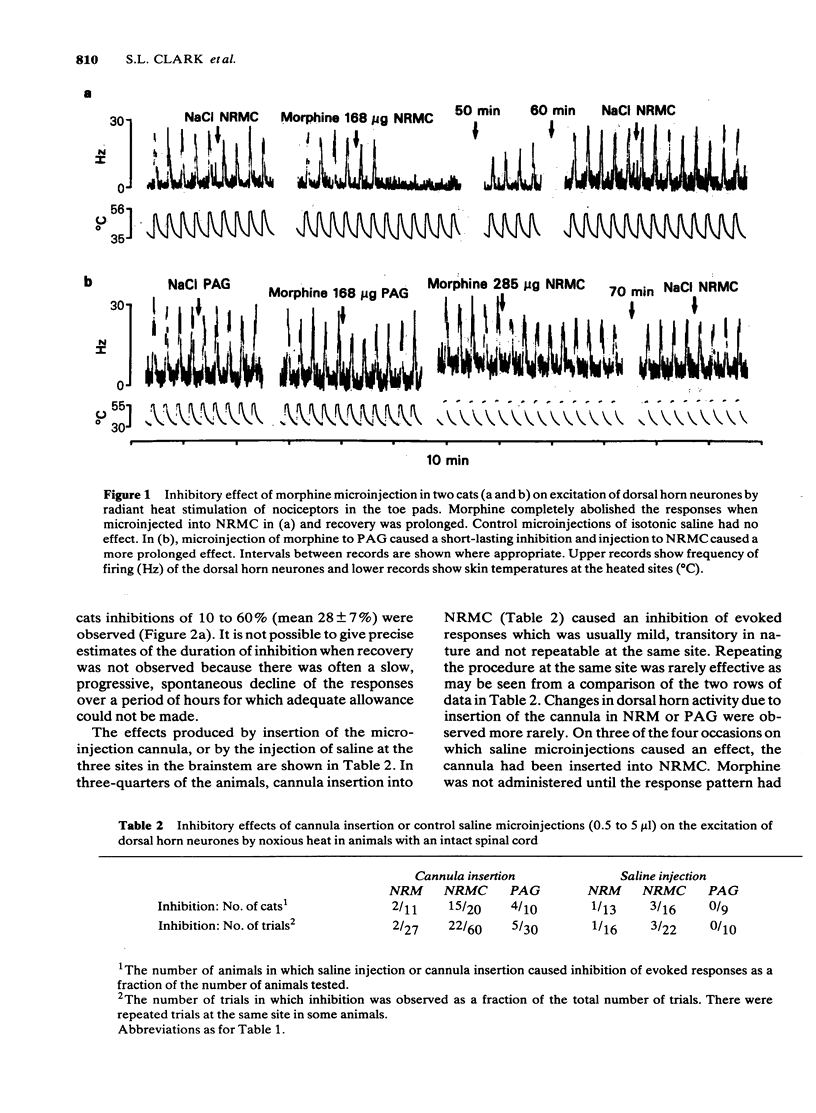
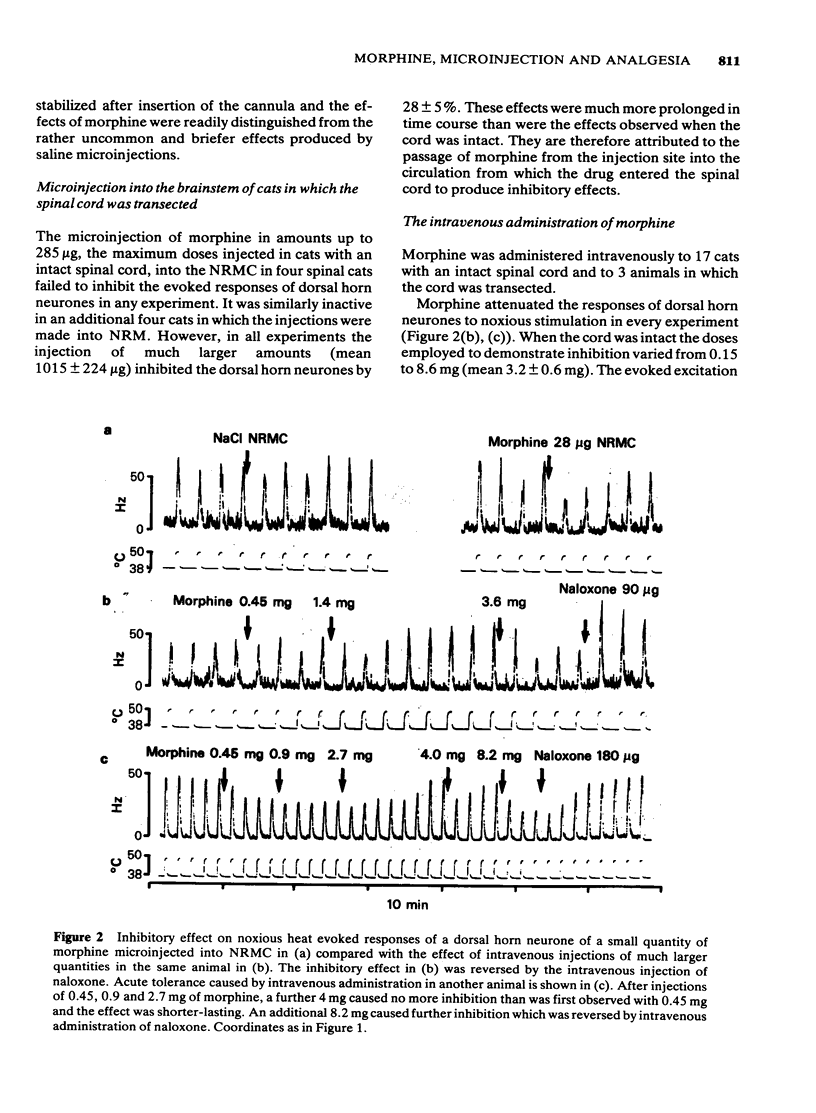
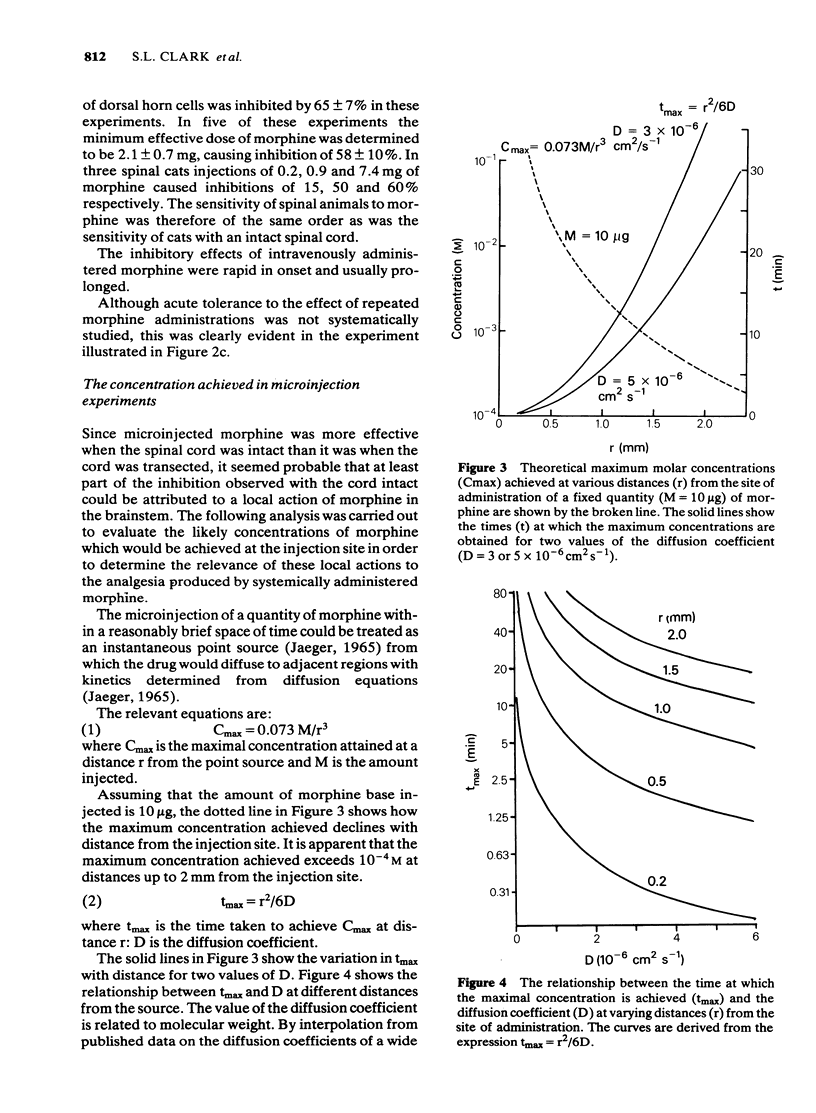
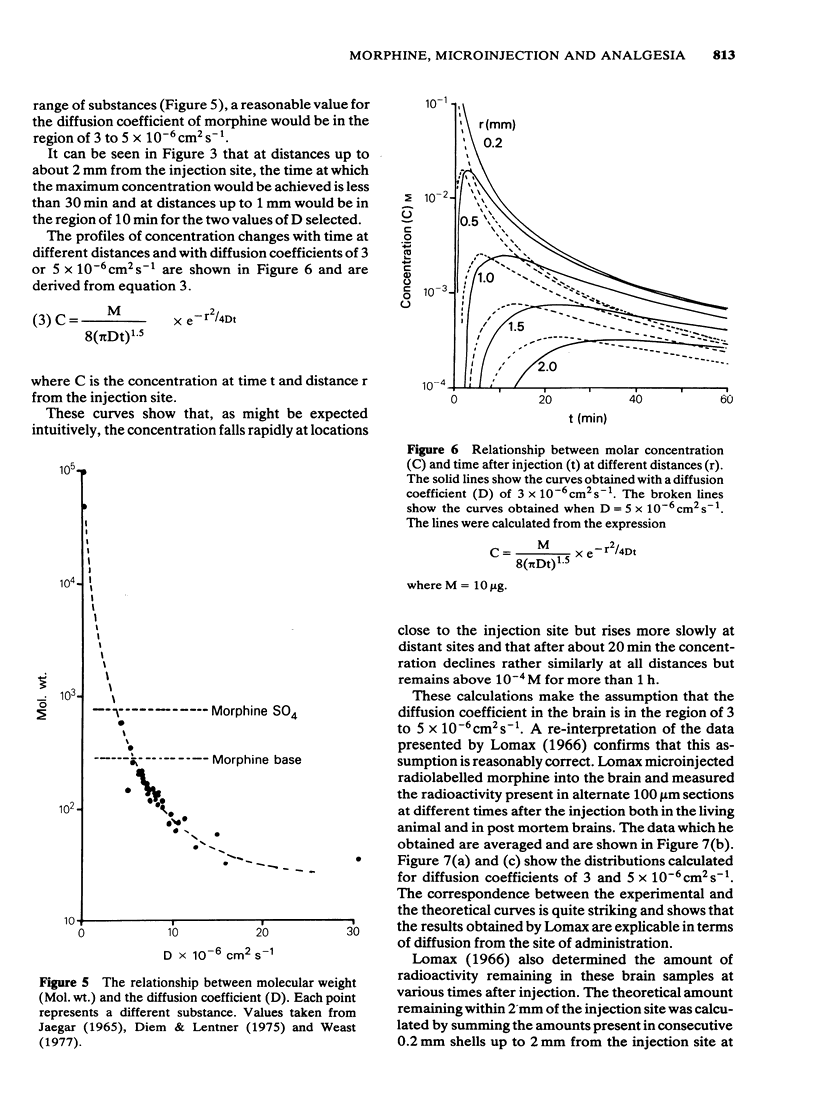
Large quantities of morphine injected directly into the brainstem of spinal anaesthetized cats inhibited the noxious heat-evoked excitation of dorsal horn neurones. The amounts required were similar to those that were required intravenously in cats with the spinal cord intact or transected. When the spinal cord was intact the amount of morphine microinjected into the brainstem required to inhibit the excitation of dorsal horn neurones was about ten fold less than it was in spinal animals. It is concluded that large, but not small doses of morphine microinjected into the brainstem can exert effects on the spinal cord after first entering the circulation. The effects of small doses are attributed to a local action in the brainstem which causes inhibition of spinal neurones either by activating descending inhibitory neuronal systems or by liberating endogenous substances which reach the spinal cord via the cerebro-spinal fluid. The concentrations of morphine achieved at various distances from the site of injection by the microinjection of microgram quantities and the time courses of the concentration changes were calculated from diffusion equations, assuming diffusion coefficients of 3 or 5 X 10(6) cm2 s-1. The curves obtained closely approximated those obtained experimentally. The concentrations achieved at distances up to 2 mm from the site of injection of 10 micrograms of morphine were calculated to exceed 10(-4)M and the time-courses of these concentration changes were compatible with the time course of inhibition of spinal neurones, or the production of analgesia after microinjection. Such concentrations are vastly in excess of those achieved in the brain after the systemic administration of morphine in analgesic doses. It is concluded that the local effects in the brainstem produced by the microinjection of microgram quantities of morphine have no relevance to the mechanism of analgesia produced by systemic administration.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Shibata T., Satoh M., Takagi H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978 Sep;17(9):775–778. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- Azami J., Llewelyn M. B., Roberts M. H. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982 Mar;12(3):229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W. Differential excitatory and inhibitory effects of opiates on non-nociceptive and nociceptive neurones in the spinal cord of the cat. Brain Res. 1978 Apr 28;145(2):303–314. doi: 10.1016/0006-8993(78)90864-8. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Mayer D. J. Inhibition of spinal cord interneurons by narcotic microinjection and focal electrical stimulation in the periaqueductal central gray matter. Brain Res. 1979 Aug 24;172(2):243–257. doi: 10.1016/0006-8993(79)90536-5. [DOI] [PubMed] [Google Scholar]
- Boudier H. A., van Rossum J. M. Clonidine-induced cardiovascular effects after stereotaxic application in the hypothalamus of rats. J Pharm Pharmacol. 1972 May;24(5):410–411. doi: 10.1111/j.2042-7158.1972.tb09019.x. [DOI] [PubMed] [Google Scholar]
- Bryant R. M., Olley J. E., Tyers M. B. Involvement of the median raphe nucleus in antinociception induced by morphine, buprenorphine and tilidine in the rat. Br J Pharmacol. 1982 Dec;77(4):615–624. doi: 10.1111/j.1476-5381.1982.tb09339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo O., Henry J. L., Neuman R. S. Effects of morphine and naloxone on dorsal horn neurones in the cat. Can J Physiol Pharmacol. 1974 Dec;52(6):1207–1211. doi: 10.1139/y74-158. [DOI] [PubMed] [Google Scholar]
- Chan S. H. Participation of the nucleus reticularis gigantocellularis in the morphine suppression of jaw-opening reflex in cats. Brain Res. 1979 Jan 12;160(2):377–378. doi: 10.1016/0006-8993(79)90436-0. [DOI] [PubMed] [Google Scholar]
- Clark S. L., Ryall R. W. The antinociceptive action of etorphine in the dorsal horn is due to a direct spinal action and not to activation of descending inhibition. Br J Pharmacol. 1983 Feb;78(2):307–319. doi: 10.1111/j.1476-5381.1983.tb09396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney R. A., Goodchild A. K., Robertson L. G., Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res. 1982 Oct 14;249(2):223–235. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Dray A. Pharmacological and electrophysiological studies of morphine and enkephalin on rat supraspinal neurones and cat spinal neurones. Br J Pharmacol. 1978 May;63(1):87–96. doi: 10.1111/j.1476-5381.1978.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson A. H., Oliveras J. L., Besson J. M. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979 Jul 6;170(1):95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T., North R. A. Morphine and supraspinal inhibition of spinal neurones: evidence that morphine decreases tonic descending inhibition in the anaesthetized cat. Br J Pharmacol. 1980 Jul;69(3):461–466. doi: 10.1111/j.1476-5381.1980.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Hall J. G., Headley P. M. Suppression of transmission of nociceptive impulses by morphine: selective effects of morphine administered in the region of the substantia gelatinosa. Br J Pharmacol. 1977 Sep;61(1):65–76. [PMC free article] [PubMed] [Google Scholar]
- Epstein A. N., Fitzsimons J. T., Rolls B. J. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970 Sep;210(2):457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K., Ohtani M., Toyooka H., Dohi S., Ghazisaidi K., Taub A., Kitahata L. M. The relative contribution of direct and supraspinal descending effects upon spinal mechanisms of morphine analgesia. J Pharmacol Exp Ther. 1978 Nov;207(2):476–484. [PubMed] [Google Scholar]
- Hipps P. P., Eveland M. R., Meyer E. R., Sherman W. R., Cicero T. J. Mass fragmentography of morphine: relationship between brain levels and analgesic activity. J Pharmacol Exp Ther. 1976 Mar;196(3):642–648. [PubMed] [Google Scholar]
- Jacquet Y. F., Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976 Feb 27;103(3):501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Jacquet Y. F., Wolf G. Morphine and ACTH1-24: correlative behavioral excitations following micro-injections in rat periaqueductal gray. Brain Res. 1981 Aug 24;219(1):214–218. doi: 10.1016/0006-8993(81)90285-7. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Gau B. A., Nemeroff C. B., Prange A. J., Jr Antinociception after microinjection of neurotensin into the central amygdaloid nucleus of the rat. Brain Res. 1982 Jul 15;243(2):279–286. doi: 10.1016/0006-8993(82)90251-7. [DOI] [PubMed] [Google Scholar]
- Kitahata L. M., Kosaka Y., Taub A., Bonikos K., Hoffert M. Lamina-specific suppression of dorsal-horn unit activity by morphine sulfate. Anesthesiology. 1974 Jul;41(1):39–48. doi: 10.1097/00000542-197407000-00008. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Microinjection of morphine within nucleus raphe magnus and dorsal horn neurone activities related to nociception in the rat. Brain Res. 1980 May 12;189(2):467–481. doi: 10.1016/0006-8993(80)90106-7. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Guilbaud G., Chitour D., Besson J. M. Does systemic morphine increase descending inhibitory controls of dorsal horn neurones involved in nociception? Brain Res. 1980 Nov 24;202(1):223–228. doi: 10.1016/0006-8993(80)90659-9. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Menétrey D., Conseiller C., Besson J. M. Depressive effects of morphine upon lamina V cells activities in the dorsal horn of the spinal cat. Brain Res. 1975 Nov 14;98(2):261–277. doi: 10.1016/0006-8993(75)90005-0. [DOI] [PubMed] [Google Scholar]
- Levy R. A., Proudfit H. K. Analgesia produced by microinjection of baclofen and morphine at brain stem sites. Eur J Pharmacol. 1979 Jul 15;57(1):43–55. doi: 10.1016/0014-2999(79)90102-x. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Gebhart G. F. Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Res. 1977 Mar 25;124(2):283–303. doi: 10.1016/0006-8993(77)90886-1. [DOI] [PubMed] [Google Scholar]
- Lomax P. The distribution of morphine following intracerebral microinjection. Experientia. 1966 Apr 15;22(4):249–250. doi: 10.1007/BF01900940. [DOI] [PubMed] [Google Scholar]
- Mantegazza P., Parenti M., Tammiso R., Vita P., Zambotti F., Zonta N. Modification of the antinociceptive effect of morphine by centrally administered diazepam and midazolam. Br J Pharmacol. 1982 Apr;75(4):569–572. doi: 10.1111/j.1476-5381.1982.tb09175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai S. H., Berkowitz B. A., Yang J. C., Hempstead J., Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976 May;44(5):398–401. doi: 10.1097/00000542-197605000-00008. [DOI] [PubMed] [Google Scholar]
- Ohlsson A. E., Fu T. C., Jones D., Martin B. R., Dewey W. L. Distribution of radioactivity in the spinal cord after intracerebroventricular and intravenous injection of radiolabeled opioid peptides in mice. J Pharmacol Exp Ther. 1982 May;221(2):362–367. [PubMed] [Google Scholar]
- Oley N., Córdova C., Kelly M. L., Bronzino J. D. Morphine administration to the region of the solitary tract nucleus produces analgesia in rats. Brain Res. 1982 Mar 25;236(2):511–515. doi: 10.1016/0006-8993(82)90736-3. [DOI] [PubMed] [Google Scholar]
- Patrick G. A., Dewey W. L., Spaulding T. C., Harris L. S. Relationship of brain morphine levels to analgesic activity in acutely treated mice and rats and in pellet implanted mice. J Pharmacol Exp Ther. 1975 Jun;193(3):876–883. [PubMed] [Google Scholar]
- Pert A., Yaksh T. Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res. 1974 Nov 8;80(1):135–140. doi: 10.1016/0006-8993(74)90731-8. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. P., Stocco S. Differential effects of systemic versus intracranial injection of opiates on central, orofacial and lower body nociception: somatotypy in bulbar analgesia systems. Pain. 1980 Dec;9(3):307–318. doi: 10.1016/0304-3959(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Sharpe L. G., Garnett J. E., Cicero T. J. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav Biol. 1974 Jul;11(3):303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Urca G., Nahin R. L., Liebeskind J. C. Glutamate-induced analgesia: blockade and potentiation by naloxone. Brain Res. 1980 Jun 23;192(2):523–530. doi: 10.1016/0006-8993(80)90902-6. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Akil H., Watson S. J. Evidence for homologous actions of pro-opiocortin products. Science. 1980 Dec 12;210(4475):1247–1249. doi: 10.1126/science.6254152. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Nauss L. A., Thomas J. E. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979 Feb;50(2):149–151. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- Willer J. C., Bussel B. Evidence for a direct spinal mechanism in morphine-induced inhibition of nociceptive reflexes in humans. Brain Res. 1980 Apr 7;187(1):212–215. doi: 10.1016/0006-8993(80)90507-7. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977 Aug;202(2):411–428. [PubMed] [Google Scholar]
- Yaksh T. L., Yeung J. C., Rudy T. A. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976 Sep 10;114(1):83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Zorman G., Hentall I. D., Adams J. E., Fields H. L. Naloxone-reversible analgesia produced by microstimulation in the rat medulla. Brain Res. 1981 Aug 24;219(1):137–148. doi: 10.1016/0006-8993(81)90273-0. [DOI] [PubMed] [Google Scholar]
- de Jong W., Nijkamp F. P., Bohus B. Role of noradrenaline and serotonin in the central control of blood pressure in normotensive and spontaneously hypertensive rats. Arch Int Pharmacodyn Ther. 1975 Feb;213(2):272–284. [PubMed] [Google Scholar]



