Abstract
Retinal phosducin is known to sequester transducin Gβγ, thereby modulating transducin activity. Phos ducin is a member of a family of phosducin-like proteins (PhLP) found in eukaryotes. Phylogeny of 33 phosducin-like proteins from metazoa, plants and lower eukaryotes identified three distinct groups named phosducin-I–III. We discovered three phlp genes in Dictyostelium, each encoding a phosducin-like protein of a different group. Disruption of the phlp1 gene strongly impaired G-protein signalling, apparently due to mislocalization of Gβγ in phlp1-null cells. GFP-Gβ and GFP-Gγ are membrane associated in wild-type cells, but cytosolic in phlp1-null cells. Phlp2 disruption is lethal due to a synchronous collapse of the cells after 16–17 cell divisions. Phlp3 disruptants show no abnormal phenotype. These results establish a role for phosducin-like proteins in facilitating folding, localization or function of proteins, in addition to modulating G-protein signalling.
Keywords: chaperone/Dictyostelium/G protein/signal transduction/phosducin
Introduction
Heterotrimeric GTP-binding proteins (G proteins) are signal transducers that couple heptahelical transmembrane receptors to their intracellular effectors. G proteins are composed of Gα, Gβ and Gγ subunits. Upon activation by their receptors, bound GDP is exchanged for GTP. This results in a conformational change that induces dissociation of GTP-bound Gα from the Gβγ complex. The GαGTP and free Gβγ subunits both interact with downstream effectors. The intrinsic GTPase activity of Gα leads to the formation of inactive GαGDP, which reassociates with Gβγ resulting in the reformation of the trimeric G protein (Clapham and Neer, 1997; Hamm, 1998). Signalling via G proteins is modulated by several proteins, such as regulators of G-protein signalling (RGS proteins), which activate the GTPase activity of Gα (Ross and Wilkie, 2000), and by phosducin, which sequesters Gβγ (Bauer et al., 1992).
Phosducin, a cytosolic 28 kDa protein, is composed of two domains: the N-terminal 13 kDa is mostly helical, while the C-terminal 15 kDa folds like thioredoxin. Gβγ-binding studies and the X-ray structure of the phosducin retinal–Gβγ complex show that both domains contribute to the interaction with Gβγ. The N-terminal helical domain of phosducin binds extensively to the loops of Gβ that also provide the interaction with Gα, while the C-terminal domain of phosducin binds to the region of Gβγ that can associate with the membrane surface (Gaudet et al., 1996; Loew et al., 1998; Savage et al., 2000). Therefore phosducin is thought to modulate Gβγ activity by binding to free Gβγ and blocking Gβγ association with Gα subunits, effectors or membranes (Bauer et al., 1992; Bluml et al., 1997).
Phosducin was first discovered at high concentrations in the retina (Lee et al., 1990) and the developmentally related pineal gland (Reig et al., 1990). Additional studies revealed three splice variants of phosducin in human retina (Craft et al., 1998). Besides expression in the retina, phosducin is also detected at lower levels in many other mammalian tissues (Bauer et al., 1992; Danner and Lohse, 1996). Many phosducin-related proteins have been discovered in vertebrates and in lower eukaryotes, suggesting that retinal phosducin is a member of a phosducin family of proteins. The phosducin-like protein PhLP shows 41% amino acid identity with phosducin (Miles et al., 1993). In contrast with phosducin, PhLP has a similar expression level in a wide variety of tissue (Schroder and Lohse, 2000). All phosducin and PhLP variants have been reported to bind to Gβγ in vitro, with the exception of splice variants leading to N-terminal truncations of phosducin (Schroder and Lohse, 1996, 2000; Craft et al., 1998). In addition to these two genes, we recognized three additional phosducin-like proteins in the sequence databases of the Human Genome Project; this large number of phosducin isoforms complicates the functional analysis of phosducin-like proteins in vertebrates.
In unicellular eukaryotes G-protein signal-transduction pathways mediate processes as diverse as mating in yeast (Dohlman, 2002) and morphogenesis and chemotaxis in Dictyostelium (Parent and Devreotes, 1999; Firtel and Chung, 2000). Proteins of the phosducin family appear to be involved in the regulation of Gβ activity in lower eukaryotes. In the fungus Cryphonectria parasitica, the bdm-1 gene encodes a phosducin-like protein (Kasahara et al., 2000). Disruption of this gene demonstrates a role of the BDM-1 protein in Gβγ function and Gα accumulation. The Saccharomyces cerevisiae genome encodes two phosducin-like proteins, Plp1 and Plp2, that are able to bind Gβγ and regulate Gβγ-dependent signalling (Flanary et al., 2000). Furthermore, disruption of the plp2 gene is lethal, indicating that Plp2 must have an essential function in the cell. We discovered three genes in Dictyostelium encoding phosducin-like proteins. Dictyostelium is a soil amoeba which undergoes a developmental program upon starvation. Individual amoebae chemotax towards each other and aggregate to form multicellular structures composed of stalk cells and spores. In this organism the function of G-protein signalling, mediating chemotaxis and multicellular development, has been well established (Wu et al., 1995; Parent and Devreotes, 1999; Firtel and Chung, 2000; Janetopoulos et al., 2001; Zhang et al., 2001). We exploited the genetics of Dictyostelium to investigate the function of the three phosducin-like proteins.
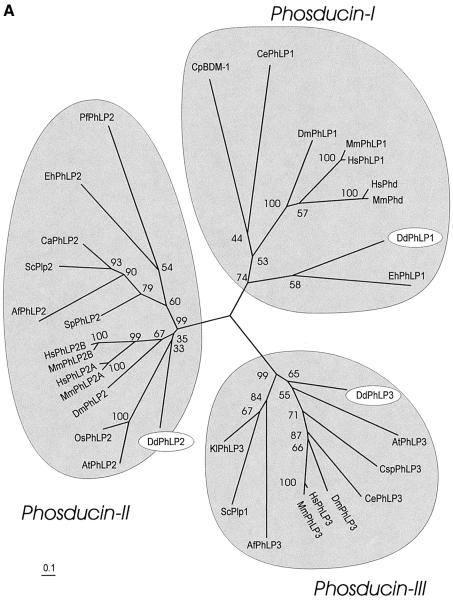
Based on phylogenetic analysis of 33 protein sequences from mammals, invertebrates, plants and unicellular eukaryotes, we show that the phosducin family consists of three subgroups, which we named phosducin-I, phosducin-II and phosducin-III. The phosducin-I subgroup contains retinal phosducin and several phosducin-like proteins. The Gβγ-binding motif of retinal phosducin in the N-terminal domain is highly conserved within this subfamily. In proteins of the phosducin-II subgroup this motif is replaced by another highly conserved motif, while this part of the N-terminal domain is absent in the phosducin-III subgroup. Some organisms, such as S.cerevisiae and Arabidopsis, contain only two phosducin-like proteins, while vertebrates have many members in each subgroup. The three Dictyostelium phosducin-like proteins each belong to a different group, giving the unique opportunity of investigating the function of the phosducin subgroups. Dictyostelium PhLP1, retinal phosducin and the C.parasitica BDM-1 all belong to the phosducin-I group. Disruption of phlp1 in Dictyostelium results in a phenotype resembling that of Gβ-null cells, similar to disruption of bdm-1 in C.parasitica (Kasahara et al., 2000). Interestingly, we observed that GFP-Gβ and GFP-Gγ are both cytosolic in the Dictyostelium phlp1 disruptant, while associated with the plasma membrane in wild-type cells. Disruption of phlp2 is lethal, as for yeast Plp2 that belongs to the same phosducin-II group. The phlp3 disruptant displayed no abnormal phenotype, as was the case after inactivation of the yeast phosducin-III homologue Plp1 (Flanary et al., 2000).
Results
Identification of phosducin-like proteins in Dictyostelium
To identify phosducin family proteins, the Dictyostelium discoideum genomic and cDNA databases were screened with a collection of phosducin-like protein sequences from different organisms (see Materials and methods). This revealed three genes encoding putative proteins sharing a significant degree of identity with phosducin. Therefore we denoted the genes as phlp1, phlp2 and phlp3, respectively. Sequence analysis of genomic DNA and cDNA revealed that each phlp gene consists of two exons, separated by a single intron of 172, 94 and 118 bases in phlp1, phlp2 and phlp3, respectively. The introns are short and AT rich, which is common for Dictyostelium. The position of the intron is not conserved within the Dictyostelium phlp genes. The exons upstream of the introns in phlp1, phlp2 and phlp3 encode 206, 121 and 34 amino acids, and the downstream exons encode 110, 118 and150 amino acids, giving a total predicted size of 316, 239 and 184 amino acids for PhLP1, PhLP2 and PhLP3, respectively. Like mammalian retinal phosducins, the Dictyostelium PhLP1, PhLP2 and PhLP3 are acidic proteins with predicted pI values of 4.68, 4.94 and 5.62, respectively.
Phylogeny of phosducin family proteins
Genomic databases of many organisms were screened to identify members of the phosducin superfamily: the organisms include human, mouse, Caenorhabditis elegans, Drosophila, Arabidopsis and other plants, S.cerevisiae and all other unicellular eukaryotes as far as databases were available. The assembly consists of 33 putative phosducin genes; the protein coding sequence in the database was incomplete for only one sequence (see Supplementary data available at The EMBO Journal Online for DDBJ/EMBL/GenBank entries and sequence alignment). A phylogenetic tree was constructed using the deduced amino acid sequences of these 33 phosducin isoforms (Figure 1A). Two interesting observations can be made. First, the analysis reveals that the phosducin family consists of three monophyletic groups, designated phosducin-I, phosducin-II and phosducin-III, respectively. Secondly, the predicted gene products of Dictyostelium phlp1, phlp2 and phlp3 each belong to a different phosducin group. Apparently, proteins belonging to each of the three subgroups are present only in mammals, Drosophila and Dictyostelium. Human retinal phosducin (HsPhd) and the phosducin-like protein HsPhLP belong to the phosducin-I group. We detected three additional phosducin homologues in the Human Genome Sequence databases. Two phosducin-like proteins (named HsPhLP2A and HsPhLP2B) are incorporated in the phosducin-II group. One phosducin-like protein belonged to the phosducin-III group and is annotated as a putative ATP-binding protein (many sequences of the phosducin-III group have this annotation, which is derived from a distant homologue that has an additional ATP-binding domain).
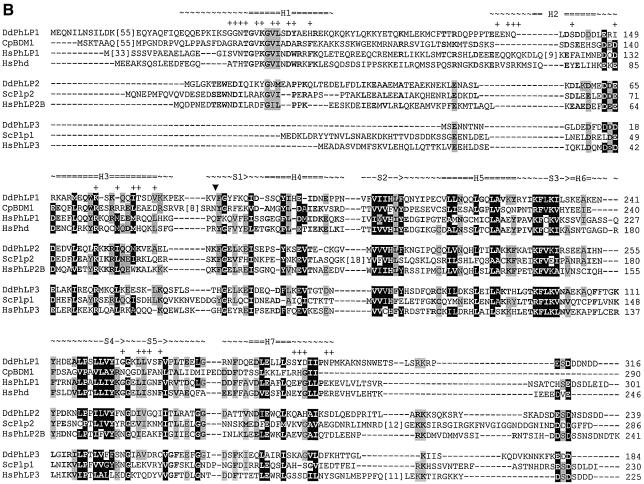
Fig. 1. The phosducin family consists of three defined subgroups. The protein sequences of 33 phosducin homologues were obtained from different organisms. These sequences were aligned (see Supplementary data for protein sequences, DDBJ/EMBL/GenBank entries and complete alignment). (A) Phylogenetic tree. The alignment was used as input for the Phylip program to construct the tree. The numbers indicate bootstrap values. (B) Alignment of three or four members of each subgroup. Residues shaded in black are conserved in 80–100% of all sequences; residues shaded in grey are conserved in 60–70% of all sequences; bold characters are conserved in 75–100% of the sequences of a subgroup only. Substitutions within the following groups were considered as conservative: DE, RK, NQ, ST, FWY and MAILV. The structural elements of HsPhd are indicated as follows: ∼∼∼, flexible loop; ===, α-helix; →, β-strand. The Gβ-contacting residues of HsPhd are denoted by + above the aligned sequences. The start of the thioredoxin-like C-terminal domain is marked by a filled triangle.
Sequence analysis of phosducin family proteins
An alignment of the derived amino acid sequences of phosducin and phosducin-like proteins of Dictyostelium, S.cerevisiae, C.parasitica and Homo sapiens is provided in Figure 1B, along with secondary structural characteristics and Gβγ-binding residues of mammalian retinal phosducin. The archetypal retinal phosducin is composed of an N-terminal domain, containing α-helices 1 to 3, and a C-terminal thioredoxin-like domain, consisting of a five-stranded β-sheet and four α-helixes. The N-terminal domain binds extensively to the loops of Gβγ that provide the interaction with Gα, while the C-domain binds to the membrane association surface of Gβγ (Gaudet et al., 1996; Loew et al., 1998; Savage et al., 2000).
As can be seen in the alignments, all proteins of the three subgroups have extensive homology at the C-terminal thioredoxin domains (Figure 1B and the complete alignment in the Supplementary data). In contrast, each subfamily has distinctive features in its N-terminal domains. The characteristic Gβγ-binding motif TGPK GVINDWR in helix 1 (Gaudet et al., 1996) is highly conserved in all proteins of the phosducin-I group. Within the phosducin-II subgroup, only the residues GVI of this Gβ binding motif are conserved as GIL. However, two motifs adjoining the GIL residues are unique and highly conserved, yielding the phosducin-II-specific signature sequence TEWNDILRxxGILPPK. Phosducin-III in fact lacks helix1.
The amino acids of Gβ that interact with retinal phosducin have been identified in the crystal structure of the complex (Gaudet et al., 1996). Of the 27 amino acids of transducin Gβ that interact with phosducin, 25 are conserved in Dictyostelium Gβ (data not shown). The amino acids of retinal phosducin that interact with Gβ are indicated in Figure 1B. In Dictyostelium PhLP1, 16 of the 32 Gβ-Interacting amino acids are conserved. In PhLP2 and PhLP3, only ten and eight, respectively, of the Gβ-Interacting amino acids are conserved.
Inactivation of phlp genes in Dictyostelium
To establish the functions of the three Dictyostelium phosducin-like proteins, and of the phosducin subgroups in general, each of the Dictyostelium phlp genes was inactivated by homologous recombination. Linear phlp DNA fragments, in which part of the open reading frame (ORF) was replaced by the bsr selection cassette (Sutoh, 1993), were electroporated into the Dictyostelium wild-type strain AX3. Disruption of phlp1 and phlp3 was confirmed by Southern blotting using genomic DNA of blasticidin-resistant cell lines (data not shown).
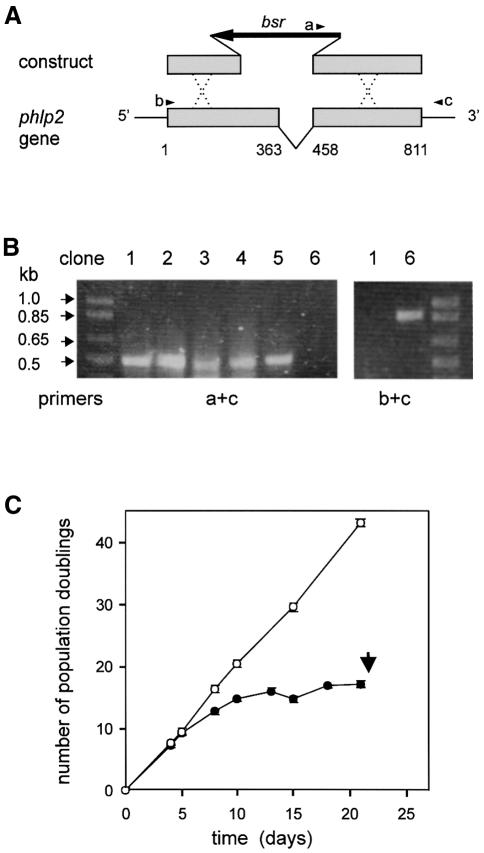
Isolation of phlp2 disruptants appeared to be problematic. We repeatedly obtained two types of blasticidin-resistant clones: normal growers and clones that initially grew well but died ∼3 weeks after transformation. DNA was isolated from several clones ∼5 days before we expected death in the non-viable clones. PCR was performed with primers recognizing the bsr cassette and part of the 3′ untranslated region of the phlp2 gene not present in the knockout construct (Figure 2A). We observed that dying clones gave the expected 0.5 kb PCR product, while none of the normally growing cells gave a PCR product (Figure 2B). Using primers recognizing the 5′ and 3′ ends of the phlp2 gene, the normal growers yielded a wild-type size band, indicating that they are random integrants. This demonstrates that the phlp2 disruption obtained is lethal.
Fig. 2. Lethal phenotype of phlp2– cells. (A) Schematic of phlp2 gene disruption. The arrowheads denote the three primers used for PCR analysis. PCR primer c recognizes part of the 3′ untranslated region that is not present in the knockout construct. (B) Identification of phlp2 disruptants by PCR analysis. DNA was isolated from several clones ∼15 days after transformation; some of these clones died around day 22 (clones 1–5), while others remained viable (clone 6). PCR analysis using primer set a + c is predicted to yield a 503 bp product for a disrupted phlp2 gene and no product for an intact gene, while PCR analysis with primers b + c will yield an 893 bp product for the intact phlp2 gene. (C) Cell growth curve. The amount of cells was estimated at different days after transformation to calculate the number of population doublings. Data shown are the means and standard deviations of six phlp2– strains; the clones were identified as phlp2 gene disruptant by PCR on day 18–20 as shown in (B). The population-doubling kinetics for random integrants are identical with those of wild-type AX3 cells and are shown for comparison. The results reveal that initially the doubling time of phlp2– cells (closed circles) is about the same as that of random integrants (open circles) but gradually increases until cells stop dividing and die after 3 weeks (arrow).
Phenotypes of phlp null cells
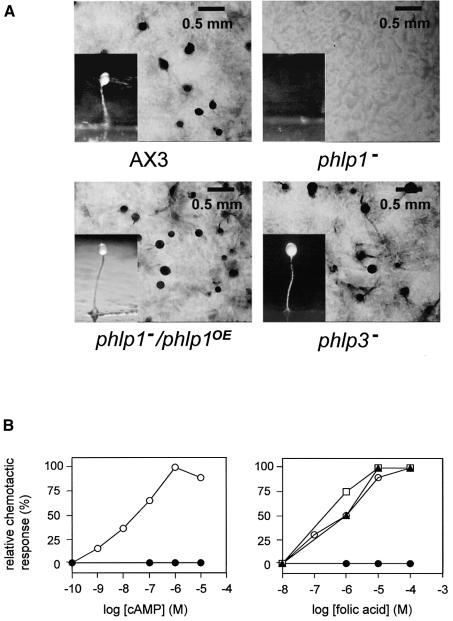
The mutants obtained by targeted disruption of phlp genes behaved quite differently from each other. For phlp3– mutants we did not find any abnormal phenotype. Growth rates were normal and the disruptants aggregated and developed normally into fruiting bodies on non-nutrient agar plates (Figure 3A). Also chemotaxis assays did not reveal a difference from wild-type AX3 cells (Figure 3B).
Fig. 3. Phenotype of Dictyostelium phlp1– and phlp3– disruptants. The genes were inactivated by homologous recombination. (A) Phenotype of wild-type AX3, phlp1–, phlp1–/phlp1OE and phlp3– cells on non-nutrient agar plates. Photographs were taken after 30 h of starvation. (B) Dose–response curves of chemotaxis towards cAMP and folic acid. The response to a range of cAMP concentrations was measured in droplets using the small- population assay. An agar cutting assay was used to score for chemotaxis to different concentrations of folic acid: AX3 (open circles), phlp1– (closed circles), phlp1–/phlp1OE (squares) and phlp3– (triangles).
Disruption of phlp2 resulted in a pronounced phenotype showing a loss of cell viability. The phlp2– cells initially proliferated well (Figure 2C). However, ∼5 days after transformation the growth rate declined, and cells stopped proliferating after ∼18 days when a maximum of ∼105 cells was obtained. Between days 20 and 22 the cells died synchronously (Figure 2C). Cells showed the normal amoeboid appearance and movement until 2 days before death, when they became round and small, and finally lysed. When some of the cells were transferred to medium without blasticidin selection or to plates with bacteria 7 days before their expected death, they died on approximately the same day as the cells in the original medium. The data strongly suggest that PhLP2 is essential in Dictyostelium.
Targeted disruption of phlp1 also resulted in a very strong phenotype. First, the ability of phlp1– cells to grow on bacterial lawns was severely affected. While wild-type AX3 cells formed plaques, aggregated and produced fruiting bodies within a few days, the phlp1– disruptants cleared the lawns much more slowly, forming 2- to 3-fold smaller plaques than AX3. Secondly, the phlp1– cells did not aggregate on non-nutrient agar plates or bacterial lawns, even after several weeks (Figure 3A). Some Dictyostelium mutants that are aggregation deficient because they cannot produce or secrete cAMP, do aggregate if they are supplied with exogenous cAMP pulses or mixed with cAMP-secreting wild-type cells. However, the phlp1– cells failed to coaggregate with wild-type cells in mixtures with AX3 cells, and also remained aggregation deficient after pulsing with cAMP. Thirdly, the mutant cells did not display chemotaxis towards a wide range of cAMP and folic acid concentrations (Figure 3B), and did not respond to bacterial extracts which are a rich source of chemoattractants binding to different surface receptors (data not shown). Finally, the doubling time in axenic medium, both on plates and in shaking cultures, was increased (18–20 h) compared with the doubling time of AX3 cells (10–12 h). Except for the reduced growth rate, these phenotypic properties of phlp1-null cells are very similar to those of gβ– cells (Lilly et al., 1993; Wu et al., 1995). A gβ-null mimicking phenotype has also been reported for disruption of the bdm1 gene from C.parasitica which, like Dictyostelium phlp1, encodes a phosducin-I family member (Kasahara et al., 2000).
To confirm that the phenotype of phlp1– cells was due to disruption of the phlp1 gene, an extrachromosomal plasmid containing the ORF of phlp1 was transformed into the phlp1– cells. Expression of phlp1 in these phlp1–/phlp1OE cells rescued all phenotypic defects; developmental morphology, growth rate and chemotactic responses returned to those of wild-type AX3 (Figure 3). The presence of phlp1 mRNA in phlp1–/phlp1OE was confirmed in a northern blot (data not shown). Although the amount of phlp1 mRNA was much elevated compared with the level in wild-type cells, no adverse effects were observed; it is not known if PhLP1 protein levels are elevated in the phlp1–/phlp1OE cells.
Biochemical assays in phlp1– cells
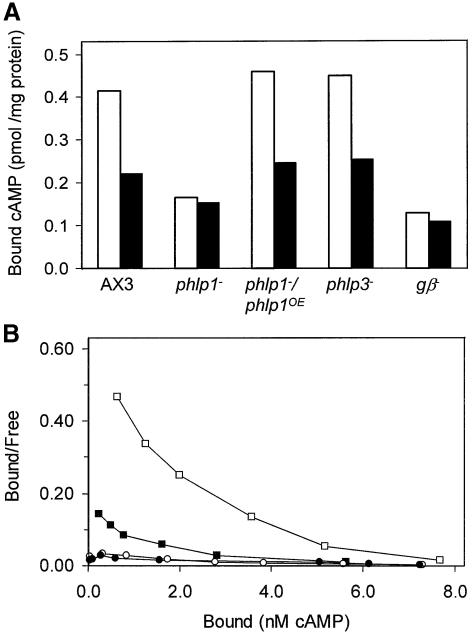
To investigate why phlp1-null cells have a phenotype that is similar to that of gβ-null cells, we analysed receptor– G-protein effector interactions in phlp1-null cells. First, we studied the interaction between cAMP surface receptors and G proteins. Membranes of wild-type AX3 cells contain both high- and low-affinity cAMP binding sites; high-affinity sites represent cAMP receptors that are coupled to functional G proteins. Addition of GTPγS causes the release of G proteins from the receptors and hence a conversion of cAMP receptors from a high-affinity to a low-affinity form (Van Haastert, 1984; Van Haastert et al., 1986). We measured the effect of GTPγS on the binding of 10 nM cAMP to membranes from wild-type, phlp1-null and gβ-null cells. GTPγS induces a strong inhibition of cAMP binding to AX3 membranes (Figure 4A). In phlp1-null cells, the basal level of cAMP binding was substantially diminished and no effect of GTPγS was observed. The gβ-null cells showed essentially the same cAMP binding properties as phlp1-null cells, while the rescue phlp1–/phlp1OE cells displayed the high cAMP binding and strong GTPγS-mediated inhibition of wild-type cells (Figure 4A).
Fig. 4. Defective receptor–G protein interaction in phlp1– cells. (A) GTPγS inhibition of 3H-cAMP binding to membranes of AX3, phlp1–, phlp1–/phlp1OE and gβ– cells. Membranes were prepared from cells that were starved for 5 h. Binding assays were performed in the absence (open bars) or presence (black bars) of 30 µM GTPγS. (B) To determine the number and affinity of the cAMP-binding sites, Scatchard analysis was carried out by including different concentrations of cAMP in the binding assays. The results for membranes of phlp1–/phlp1OE (squares) or phlp1– cells (circles) in the absence (open symbols) or presence (closed symbols) of 30 µM GTPγS are shown. One nanomole of bound cAMP is equivalent to 6000 binding sites per cell. Data were fitted using the program FigP. The data for phlp1–/phlp1OE in the absence of GTPγS were fitted with a two-receptor model; the data for the other conditions were fitted statistically better with a one-receptor model. The kinetic data are as follows: for phlp1–/phlp1OE without GTPγS, Kd1 = 4.07 ± 3.68 nM, B1d = 15 600 ± 2600 sites/cell, Kd2 = 557 ± 107 nM and B2 = 84 000 ± 17 000 sites/cell; for phlp1–/phlp1OE with GTPγS, Kd = 507 ± 92 nM and B = 80 500 ± 6000 sites/cell; for phlp1– without GTPγS, Kd = 480 ± 35 nM and B = 60 000 ± 2500 sites/cell; for phlp1– with GTPγS, Kd = 491 ± 31 nM and B = 65 000 ± 2000 sites/cell.
Next, we investigated whether the diminished basal level of cAMP binding in phlp1– cells was due to a reduction of the total number of receptor sites, or whether the affinity of the cAMP receptors was affected. Scatchard analysis was performed on membranes from phlp1– and phlp1–/phlp1OE cells. As shown in Figure 4B, cAMP binding to phlp1–/phlp1OE membranes displayed curvilinear Scatchard plots showing ∼16 000 high-affinity binding sites per cell with a Kd of 4 nM and ∼84 000 low-affinity binding sites per cell with a Kd of 550 nM. The high-affinity binding sites disappear upon addition of GTPγS, while the total number of sites is unaffected. These cAMP-binding properties of phlp1–/phlp1OE membranes are essentially identical with cAMP binding to wild-type membranes (Van Haastert et al., 1986). Membranes from phlp1-null cells displayed only low-affinity binding sites and GTPγS had no effect; the number of binding sites was ∼60 000 per cell which is ∼70% of that for wild type or phlp1–/phlp1OE. The disappearance of high-affinity cAMP binding and the absence of GTPγS-mediated inhibition of cAMP binding in phlp1-null cells were also described for gβ– cells (Lilly et al., 1993; Wu et al., 1995), suggesting that a functional coupling between G proteins and cAMP receptors is abolished in phlp1 disruptants.
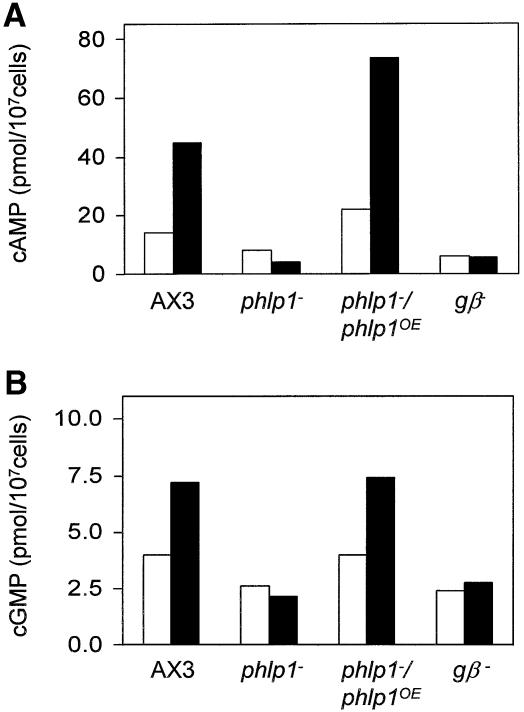
Agonist binding to cAMP receptors induces the accumulation of several second messengers, including cAMP and cGMP (Van Haastert and Kuwayama, 1997). Figure 5 shows that phlp1– cells starved for 5 h did not display a significant cAMP or cGMP accumulation in response to the agonist. Pulsing of the cells with cAMP at 5 min intervals during the starvation period has been shown to rescue some mutants, but did not have any affect on the responses of phlp1– cells (data not shown). The phlp1–/phlp1OE cells displayed wild-type patterns of cAMP and cGMP accumulation (Figure 5). In summary, the data demonstrate that the interactions between cAMP receptors and G proteins, and between G proteins and effector enzymes are absent in phlp1– cells. The absence of functional heterotrimeric G proteins explains the defects of chemotaxis and cell aggregation. These combined properties of phlp1– cells are very similar to the phenotype described for gβ– cells (Lilly et al., 1993; Wu et al., 1995).
Fig. 5. Defective G-protein-mediated responses in phlp1– cells. cAMP and cGMP response of AX3, phlp1–, phlp1–/phlp1OE and gβ– cells. (A) Cells were starved and pulsed with cAMP for 5 h and stimulated with 10 µM 2′-deoxy-cAMP and 10 mM dithiothreitol for the detection of the cAMP response. Prior to stimulation (open bars) and 90 s after stimulation (black bars), cells were lysed and assayed for cAMP. (B) For measurement of the cGMP response, starved cells were stimulated with 1 µM cAMP. Prior to stimulation (open bars) and 15 s after stimulation (black bars), cells were lysed and assayed for cGMP.
Localization of GFP-Gβ and GFP-Gγ in phlp1-null cells
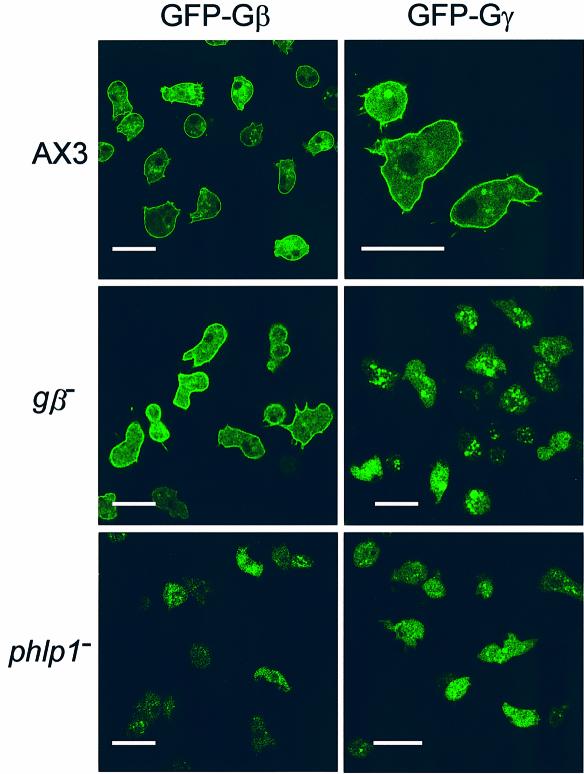
Because the phenotype of phlp1– is very similar to that of gβ– cells, the effect of phlp1 disruption on subcellular localization of GFP-Gβ and GFP-Gγ was examined. In wild-type cells both GFP-Gβ and GFP-Gγ are enriched at the plasma membrane (Figure 6). Expression of GFP-Gβ in gβ– cells rescued the aggregation-defective phenotype (data not shown), and resulted in membrane-enriched localization as in wild-type cells. In contrast, overexpression of either GFP-Gβ or GFP-Gγ did not restore aggregation competence in phlp1– cells (data not shown). Moreover, disruption of phlp1 caused GFP-Gβ and GFP-Gγ to become localized in the cytosol (Figure 6). In gβ– cells, localization of GFP-Gγ was also restricted to the cytosol (Figure 6). The fluorescence of cytosolic GFP-Gβ and GFP-Gγ in phlp1– and gβ– cells displayed a rather granular pattern. Western blots using a rabbit polyclonal anti-GFP antiserum confirmed that the complete fusion proteins were expressed in all cell lines (data not shown). In summary, expression of GFP-tagged Gβ and Gγ showed that Gβγ is mislocalized to the cytosol in phlp1– cells, which explains their gβ– -like phenotype.
Fig. 6. Expression of GFP-Gβ and GFP-Gγ. Confocal images of GFP-Gβ and GFP-Gγ in AX3, phlp1– and gβ– cells. Cells were incubated in phosphate buffer. GFP was excited with a 488 nm laser and the GFP fluorescence was filtered through a BP 505–550 nm filter. Cell strains and expressed GFP-fusion proteins are marked at the left and the top of the figure, respectively. Scale bar, 20 µm.
Discussion
The phosducin family appears to comprise many members found in different eukaryotes. The prototype is retinal phosducin which consists of an N-terminal helical domain and a C-terminal thioredoxin-like domain (Gaudet et al., 1996; Loew et al., 1998; Savage et al., 2000). Phosducin and phosducin-like proteins have been reported to bind to Gβγ in vitro (Schroder and Lohse, 1996, 2000; Craft et al., 1998). G-protein signalling has been studied in great detail in Dictyostelium (Wu et al., 1995; Parent and Devreotes, 1999; Firtel and Chung, 2000; Janetopoulos et al., 2001; Zhang et al., 2001). In order to investigate a possible role of phosducin-like proteins in regulation of G-protein activity in Dictyostelium, we screened the D.discoideum cDNA and genomic databases for phosducin isoforms. We discovered three novel genes belonging to the phosducin family of proteins. In order to investigate the diversity of the phosducin family, we compared the deduced protein sequences of 33 phosducin family proteins from a variety of eukaryotes, including Dictyostelium. Phylogenetic analysis revealed that they can be classified into three distinct groups denoted phosducin-I, phosducin-II and phosducin-III. These three distinct phylogenetic groups were also obtained when only the more conserved C-terminal thioredoxin-like domains of the proteins were used in the phylogenetic analysis. The Dictyostelium proteins, called PhLP1, PhLP2 and PhLP3, each belong to one of the three groups.
The phosducin-I group harbours both retinal phosducins and the mammalian phosducin-like protein PhLP. No phosducins of subgroup I were detected in the completed genomes of the plants Arabidopsis and Oryza sativa (rice), and the yeasts S.cerevisiae and Schizosaccharomyces pombe. PhLP and the phosducin-I proteins of lower eukaryotes contain an N-terminal extension compared with retinal phosducin. In all members of group I, the Gβγ binding motif in the N-terminal domain (TGPKGVINDWRK) is highly conserved. This suggests that all members of the phosducin-I group may interact with Gβγ (Bauer et al., 1992; Danner and Lohse, 1996; Bluml et al., 1997; Flanary et al., 2000; Kasahara et al., 2000; Savage et al., 2000).
To test the function of PhLP1 in Dictyostelium, we dis rupted the phlp1 gene. This resulted in a strong phenotype, nearly indistinguishable from the D.discoideum gβ-null phenotype (Lilly et al., 1993; Wu et al., 1995). The phlp1– cells were non-chemotactic and aggregation deficient. Furthermore, cAMP binding in phlp1– cells was insensitive to GTPγS, and did not induce cAMP or cGMP responses in these cells. These results strongly indicate the absence of G-protein signalling in phlp1– cells owing to loss of Gβγ function. A gβ-null mimicking phenotype has also been reported for disruption of bdm1, a homologue of phlp1 in the fungus C.parasitica (Kasahara et al., 2000). In order to examine whether Gβγ was properly localized, we overexpressed GFP-Gβ and GFP-Gγ in wild-type, phlp1– and gβ– cells. In wild-type cells Gβγ was mainly found on the membrane. Expression of GFP-Gβ in the phlp1 disruptant did not restore the phenotype. The fluorescent images of phlp1– cells clearly show localization of GFP-Gβ and GFP-Gγ in the cytosol. Therefore we conclude that the defects in G-protein signalling in phlp1– cells are due to mislocalization of Gβγ. Membrane localization of Gβγ is mediated by isoprenylation at the C-terminus of Gγ (Muntz et al., 1992; Zhang et al., 2001). Our observation that GFP-Gγ in gβ– cells is cytosolic indicates that membrane localization of Gγ requires functional Gβ, which has also been described for mammalian Gγ (Pronin and Gautam, 1993). This suggests that the primary defect of G-protein signalling in the phlp1 disruptant is due to either non-functional Gβ or Gγ, or the inability to make a functional Gβγ complex. Several studies indicate that chaperones may be important for correct folding of Gβ and other proteins of the family of WD repeat proteins (Clapham and Neer, 1997; Garcia-Higuera et al., 1998). In addition, mammalian PhLP1 has been reported to have the capacity to interact with the proteasomal protein SUG1 (Barhite et al., 1998) and the cytosolic chaperonin complex (CCT) (McLaughlin et al., 2002). We propose that Dictyostelium PhLP1 facilitates proper folding of Gβ or assembly of Gβ into a Gβγ complex.
Members of the phosducin-III group lack most of the N-terminal domain, but their C-terminal domain shares extensive homology with phosducin homologues from the other two groups. The phlp3– mutants in Dictyostelium did not show an abnormal phenotype under the conditions tested. A similar result was described for disruption of the yeast homologue plp1 (Flanary et al., 2000). Apparently, PhLP3 is not required for growth, G-protein signalling or development. Further investigation is needed to provide more information about the function of PhLP3.
Phosducin-II proteins lack part of the N-terminal domain compared with members of the phosducin-I group. Their N-terminal domain contains a different motif: (TEWNDILRxxGILPPK). Members of the phosducin-II group are present in lower eukaryotes and plants; mammals possess two different isoforms named PhLP2A and PhLP2B. Unexpectedly, no phosducin-II homologue could be found in C.elegans. Like plp2 in S.cerevisiae (Flanary et al., 2000), phlp2 appears to be an essential gene in Dictyostelium. Disruption of phlp2 resulted in a gradually decreasing growth rate, leading to a synchronous collapse after 16–17 population doublings. Cell death might be caused by dilution of an essential factor due to subsequent cell divisions until the concentration declines below a threshold level. This essential factor may be either PhLP2 itself or another factor requiring PhLP2 for functioning. It is unlikely that PhLP2 is expressed at a high level; the number of plp2 transcripts in S.cerevisiae has been estimated to be less than one per cell (Flanary et al., 2000). Therefore we suggest that PhLP2 functions in a cascade and fulfils a catalytic role, for example as a chaperone for one or more essential proteins of the family of WD repeat proteins. The homologous mouse germ-cell-specific phosducin-like protein (MgcPhLP or MmPhLP2B) is proposed to be involved in germ-cell maturation (Lopez et al., 2003). When expressed in yeast plp2– cells, MgcPhLP complemented the defect caused by plp2 disruption, suggesting an evolutionarily conserved function of PhLP2.
In summary, phylogenetic analysis reveals that members of the phosducin family of proteins can be classified into three distinct groups: phosducin-I, phosducin-II and phosducin–III. Having three distinct phosducin-like proteins, Dictyostelium may serve as a model system to investigate the specific functions of phosducin isoforms. While phosducin in the visual system is thought to downregulate G-protein signalling, this may not be the only function of members of the phosducin family of proteins. Indeed, phenotypic analyses of phosducin-like protein disruption mutants in Dictyostelium, C.parasitica and S.cerevisiae strongly suggest that phosducin homologues from different groups have distinct cellular functions.
Materials and methods
Cell growth and development
AX3 (wild-type) and phlp– cells (see below) were grown in HG5 medium, which was supplemented with 10 µg/ml blasticidinn S (ICN) for phlp– cells. Rescued phlp1–/phlp1OE cells and cells expressing GFP-Gβ and GFP-Gγ were supplemented with 30 µg/ml G418 (GibcoBRL). Cells were starved by shaking for up to 5 h in 10 mM phosphate buffer pH 6.5 (PB) at a density of 107 cells/ml. To observe developmental phenotypes, cells were deposited on non-nutrient (NN) agar (1.5% agar in PB) and incubated at 22°C. To study growth on bacteria, cells were plated with Klebsiella aerogenes on SM medium and incubated at 22°C for several days.
Bioinformatics
BLAST searches in databases representing organisms from different phyla (see below) yielded in total ∼30 phosducin homologues (see Supplementary data for abbreviations, DDBJ/EMBL/GenBank accession numbers, sequences and alignments of the protein sequences). Using these phosducin sequences, we identified sequences in the Dictyostelium cDNA and genomic databases representing three phosducin homologues, named phlp1, phlp2 and phlp3. The complete ORFs were assembled from raw sequence data of the Dictyostelium genome project (http://genome.imb-jena.de/dictyostelium/). The sequences of phlp1, phlp2 and phlp3 have been deposited at DDBJ/EMBL/GenBank under accession numbers AF540058, AF540059 and AF540060, respectively, and the encoded amino acid sequences are presented in Figure 1B.
Blast searches were carried out in the general DDBJ/EMBL/GenBank (http://www.ncbi.nlm.nih.gov), and several specific databases for Drosophila (http://www.fruitfly.org), C.elegans (http://www.wormbase.org), human (http://www.ncbi.nlm.nih.gov/genome/seq/HsBlast.html and http://publication.celera.com), yeast (http://www.ncbi.nlm.nih.gov), Plasmodium (http://www.ncbi.nlm.nih.gov/Malaria/plasmodiumbl.html), Dictyostelium (http://www.sdsc.edu/mpr/dicty/) and Arabidopsis (http://www.arabidopsis.org).
Multiple sequence alignments were constructed using the CLUSTAL W program (Thompson et al., 1994), followed by manual optimization. Distance matrices were constructed from the alignments with the PROTDIST program of the PHYLIP package, which uses the Dayhoff PAM 100 matrix for the calculation of evolutionary distances (Phylip 3.5) (Felsenstein, 1996). Phylogenetic trees were generated using the FITCH program of the PHYLIP package, with 100 bootstrap replications to assess the reliability of the nodes. Programs from the ExPASy Molecular Biology server (http://www.expasy.ch/) were used for the analysis of protein sequences.
Plasmid construction
The coding sequences for phlp1, phlp2 and phlp3 were amplified by PCR (Expand Long Template System, Roche) from Dictyostelium genomic DNA or by RT-PCR (M-MLV Reverse Transcriptase, Promega) from mRNA. The complete phlp1 coding sequence was amplified with the primers 5′-TCTCAGATCTAAAGAATGGAACAAAACATTTTAAATAG-3′ and 5′-AGAGGGATCCTTAATCGTCATTATCATCATCGGAC-3′). The primers 5′-GAGGATCCAAAAATGGGTTTAGGTAA AACAGAATG-3′ and 5′-GAGGATCCTCATCAGAATCAGAATTATCAG-3′ yielded the complete coding sequence of phlp2. For amplification of phlp3, primers 5′-GGAAGATCTAAAAATGTCAGAAAATAATACCAATAATG-3′ and 5′-GCTAGATCTATCATCTTCTTTAAATTTATTATTCTTAACATC-3′ were used. The PCR products were cloned into the TA cloning vector pGEM T-easy (Promega). All constructs were sequenced to confirm the nucleotide sequences of the phosducin inserts and the position of the introns, and subcloned using the restriction sites underlined in the primers above in Dictyostelium extrachromosomal expression vector AH2, an MB12neo derivative (Linskens et al., 1999).
For the creation of expression plasmids encoding GFP-Gβ and GFP-Gγ fusion proteins, the coding sequences of Gβ and Gγ genes were PCR-amplified from plasmids carrying the ORFs of Gβ or Gγ (Janetopoulos et al., 2001) with Pfu polymerase (Promega). For Gβ, the primers 5′-TTTAGATCTAAAATGTCATCAGATATTTCAGAAAAAATTCAACAAGCAAG-3′ and 5′-TTTGGATCCTTAAGCCCAAATCTTGAGGAGAGAATCC-3′ were used. For Gγ, we employed the primers 5′-ATATAGATCTATAATGTCCGAATCACAATTAAAAAAAGTTTTAAAAG-3′ and 5′-ATTACTAGTTTATAACACAGAACATCCATTTCCTTTGAGTGGTT-3′. The resultant PCR products were cloned in pGEM-T-easy, sequenced and subcloned in the Dictyostelium expression vector LB5Neo, an MB12neo derivative with an N-terminal GFP cloning cassette (a gift from L.Bosgraaf).
Gene inactivation
The phlp genes have small coding regions (∼550–950 bp), each interrupted by an AT-rich intron. We PCR amplified fragments upstream (5′) and downstream (3′) of the intron of each phlp gene. The 5′ and 3′ fragments of each gene were cloned sequentially around the bsr selection cassette (Sutoh, 1993), such that the bsr cassette is in the reverse orientation with respect to the phlp fragments.
The 5′ phlp1 fragment was amplified using primers 5′-GTACGCGTAGCTCGTATGGAACAATG-3′ and 5′-GTCTGCAGCGAAAACATTTGGTGGTTC-3′; this fragment was cloned in the bsr-containing plasmid pUC21/bsr using the restriction sites PstI and MluI. The 3′ phlp1 fragment was amplified using primers 5′-ATAGAATTCTATATACCAGAATGTG-3′ and 5′-GTATCGATCCTAGTTCTTCAGTGAG-3′; this fragment was cloned in the TA cloning vector pGEM T-easy, and subsequently cloned into the 5′ phlp1/bsr-containing plasmid using the EcoRI sites.
To obtain the phlp2 disruption construct, the EcoRI fragment from pGEMT-easy/phlp2 (see above) was used as the 5′ fragment. The 3′ fragment was obtained by PCR using the primers 5′-GATCTAGATTCCTCAATGTCAATTAGTAAATC-3′ and 5′-GAGGATCCTCATCAGAATCAGAATTATCAG-3′. These fragments were cloned into the bsr containing plasmid using BamHI and XbaI.
For the phlp3 disruption plasmid, the 5′ fragment was amplified using the primers 5′-GGAAGATCTAAAAATGTCAGAAAATAATACCAATAATG-3′ and 5′-GAGGATCCCACCATGTGTTGATAAAAAG-3′. The primer pair 5′-GTTCTAGAGCAAAAACACATTTAGGTAC-3′ and 5′-GCTAGATCTATCATCTTCTTTAAATTTATTATTCTTAACATC-3′ was used to amplify the 3′ fragment of phlp3.
A linear fragment with the bsr cassette and phlp flanking sequences was obtained from each disruption construct by PCR, using the 5′-sense and 3′-antisense outer primers. Dictyostelium AX3 cells were transformed by electroporation using 5 µg of linear DNA fragment. Blasticidin-resistant clones were screened for homologous recombination using PCR and Southern blot analysis.
For rescue experiments of phlp1– cells, the ORF of phlp1 was cloned into the BglII site of the extrachromosomal expression plasmid AH2.
Assays
All biochemical assays were carried out with cells that were starved for 5 h by shaking in PB at a density of 107 cells/ml. When indicated, cells were pulsed with 10 nM cAMP at 5 min intervals during the starvation period. To assay for 3H-cAMP binding to membranes, starved cells were harvested and resuspended to 108/ml in PB. Preparation of membranes and the binding assays were performed as described (Snaar-Jagalska and Van Haastert, 1994). Each binding assay was performed in triplicate in the presence or absence of 30 µM guanosine 5′-O-(3-thiotriphosphate) (GTPγS). To determine the number and affinity of the cAMP-binding sites, Scatchard analysis was carried out by including different concentrations of cAMP in the binding assays.
The receptor-mediated responses of the cells were measured as the amount of cGMP produced upon stimulation by 1 µM cAMP, and the amount of cAMP produced upon stimulation by 10 µM 2′-deoxy-cAMP and 10 mM dithiotreitol; cGMP and cAMP levels were determined by isotope dilution assays as described (Snaar-Jagalska and Van Haastert, 1994).
Chemotaxis towards cAMP was measured in droplets using the small-population assay (Konijn, 1970). An agar cutting assay was used to score for chemotaxis to folic acid (Kuwayama et al., 1993).
Fluorescence microscopy
For microscopy, cells were washed twice with phosphate buffer (17 mM, pH 6.5) and starved for 1 h. Images were obtained from cells incubated in buffer using a confocal laser scanning microscope (ConfoCor 2, LSM 510 combination setup; Carl-Zeiss, Germany). GFP was excited with the 488 nm argon ion laser controlled by an acousto-optical tunable filter (AOTF). A dichroic beam splitter (HFT 488) separated the excitation from the emission. The GFP fluorescence was filtered through a BP 505–550 nm filter. The objective used was a 40× oil–immersion Plan-Neofluar with a numerical aperture of 1.3. The pinhole was set at 73 µm. Images were analysed with the Zeiss LSM Image Browser software package.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Chris Janetopoulos and Leonard Bosgraaf for providing plasmids and Annelies Heidekamp for constructing plasmid AH2. We are indebted to the Japanese Dictyostelium cDNA consortium (Hokkaido University, University of Tsukuba and Kinki University), and to the Dictyostelium genomic DNA consortium (University of Cologne, the Institute of Molecular Biotechnology in Jena, the Baylor College of Medicine in Houston and the Sanger Centre in Hinxton). This research was supported by the Netherlands Organization for Scientific Research.
References
- Barhite S., Thibault,C. and Miles,M.F. (1998) Phosducin-like protein (PhLP), a regulator of G beta gamma function, interacts with the proteasomal protein SUG1. Biochim. Biophys. Acta, 1402, 95–101. [DOI] [PubMed] [Google Scholar]
- Bauer P.H., Muller,S., Puzicha,M., Pippig,S., Obermaier,B., Helmreich,E.J. and Lohse,M.J. (1992) Phosducin is a protein kinase A-regulated G-protein regulator. Nature, 358, 73–76. [DOI] [PubMed] [Google Scholar]
- Bluml K., Schnepp,W., Schroder,S., Beyermann,M., Macias,M., Oschkinat,H. and Lohse,M.J. (1997) A small region in phosducin inhibits G-protein βγ-subunit function. EMBO J., 16, 4908–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. and Neer,E.J. (1997) G protein βγ subunits. Annu. Rev. Pharmacol. Toxicol., 37, 167–203. [DOI] [PubMed] [Google Scholar]
- Craft C.M., Xu,J., Slepak,V.Z., Zhan-Poe,X., Zhu,X., Brown,B. and Lolley,R.N. (1998) PhLPs and PhLOPs in the phosducin family of G βγ binding proteins. Biochemistry, 37, 15758–15772. [DOI] [PubMed] [Google Scholar]
- Danner S. and Lohse,M.J. (1996) Phosducin is a ubiquitous G-protein regulator. Proc. Natl Acad. Sci. USA, 93, 10145–10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H.G. (2002) G proteins and pheromone signaling. Annu. Rev. Physiol., 64, 129–152. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1996) Inferring phylogenies from protein sequences by parsimony, distance and likelihood methods. Methods Enzymol., 266, 418–427. [DOI] [PubMed] [Google Scholar]
- Firtel R.A. and Chung,C.Y. (2000) The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. Bioessays, 22, 603–615. [DOI] [PubMed] [Google Scholar]
- Flanary P.L., DiBello,P.R., Estrada,P. and Dohlman,H.G. (2000) Functional analysis of Plp1 and Plp2, two homologues of phosducin in yeast. J. Biol. Chem., 275, 18462–18469. [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I., Gaitatzes,C., Smith,T.F. and Neer,E.J. (1998) Folding a WD repeat propeller. Role of highly conserved aspartic acid residues in the G protein β subunit and Sec13. J. Biol. Chem., 273, 9041–9049. [DOI] [PubMed] [Google Scholar]
- Gaudet R., Bohm,A. and Sigler,P.B. (1996) Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell, 87, 577–588. [DOI] [PubMed] [Google Scholar]
- Hamm H.E. (1998) The many faces of G protein signaling. J. Biol. Chem., 273, 669–672. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Jin,T. and Devreotes,P. (2001) Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science, 291, 2408–2411. [DOI] [PubMed] [Google Scholar]
- Kasahara S., Wang,P. and Nuss,D.L. (2000) Identification of bdm-1, a gene involved in G protein β-subunit function and α-subunit accumulation. Proc. Natl Acad. Sci. USA, 97, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn T.M. (1970) Microbiological assay of cyclic 3′,5′-AMP. Experientia, 26, 367–369. [DOI] [PubMed] [Google Scholar]
- Kuwayama H., Ishida,S. and Van Haastert,P.J.M. (1993) Non-chemotactic Dictyostelium discoideum mutants with altered cGMP signal transduction. J. Cell Biol., 123, 1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.H., Fowler,A., McGinnis,J.F., Lolley,R.N. and Craft,C.M. (1990) Amino acid and cDNA sequence of bovine phosducin, a soluble phosphoprotein from photoreceptor cells. J. Biol. Chem., 265, 15867–15873. [PubMed] [Google Scholar]
- Lilly P., Wu,L., Welker,D.L. and Devreotes,P.N. (1993) A G-protein β-subunit is essential for Dictyostelium development. Genes Dev., 7, 986–995. [DOI] [PubMed] [Google Scholar]
- Linskens M.H., Grootenhuis,P.D., Blaauw,M., Huisman-de Winkel,B., Van Ravestein,A., Van Haastert,P.J. and Heikoop,J.C. (1999) Random mutagenesis and screening of complex glycoproteins: expression of human gonadotropins in Dictyostelium discoideum. FASEB J., 13, 639–645. [DOI] [PubMed] [Google Scholar]
- Loew A., Ho,Y.K., Blundell,T. and Bax,B. (1998) Phosducin induces a structural change in transducin βγ. Structure, 6, 1007–1019. [DOI] [PubMed] [Google Scholar]
- Lopez P., Yaman,R., Lopez-Fernandez,L.A., Vidal,F., Puel,D., Clertant,P., Cuzin,F. and Rassoulzadegan,M. (2003) A novel germ line-specific gene of the phosducin-like protein (PhLP) family. A meiotic function conserved from yeast to mice. J. Biol. Chem., 278, 1751–1757. [DOI] [PubMed] [Google Scholar]
- McLaughlin J.N., Thulin,C.D., Hart,S.J., Resing,K.A., Ahn,N.G. and Willardson,B.M. (2002) Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc. Natl Acad. Sci. USA, 99, 7962–7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles M.F., Barhite,S., Sganga,M. and Elliott,M. (1993) Phosducin-like protein: an ethanol-responsive potential modulator of guanine nucleotide-binding protein function. Proc. Natl Acad. Sci. USA, 90, 10831–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz K.H., Sternweis,P.C., Gilman,A.G. and Mumby,S.M. (1992) Influence of gamma subunit prenylation on association of guanine nucleotide-binding regulatory proteins with membranes. Mol. Biol. Cell, 3, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1999) A cell’s sense of direction. Science, 284, 765–770. [DOI] [PubMed] [Google Scholar]
- Pronin A.N. and Gautam,N. (1993) Proper processing of a G protein gamma subunit depends on complex formation with a β subunit. FEBS Lett., 328, 89–93. [DOI] [PubMed] [Google Scholar]
- Reig J.A., Yu,L. and Klein,D.C. (1990) Pineal transduction. Adrenergic–cyclic AMP-dependent phosphorylation of cytoplasmic 33-kDa protein (MEKA) which binds βγ-complex of transducin. J. Biol. Chem., 265, 5816–5824. [PubMed] [Google Scholar]
- Ross E.M. and Wilkie,T.M. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem., 69, 795–827. [DOI] [PubMed] [Google Scholar]
- Savage J.R., McLaughlin,J.N., Skiba,N.P., Hamm,H.E. and Willardson,B.M. (2000) Functional roles of the two domains of phosducin and phosducin-like protein. J. Biol. Chem., 275, 30399–30407. [DOI] [PubMed] [Google Scholar]
- Schroder S. and Lohse,M.J. (1996) Inhibition of G-protein βγ-subunit functions by phosducin-like protein. Proc. Natl Acad. Sci. USA, 93, 2100–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder S. and Lohse,M.J. (2000) Quantification of the tissue levels and function of the G-protein regulator phosducin-like protein (PhlP). Naunyn Schmiedebergs Arch. Pharmacol., 362, 435–439. [DOI] [PubMed] [Google Scholar]
- Snaar-Jagalska B.E. and Van Haastert,P.J. (1994) G-protein assays in Dictyostelium. Methods Enzymol., 237, 387–408. [DOI] [PubMed] [Google Scholar]
- Sutoh K. (1993) A transformation vector for Dictyostelium discoideum with a new selectable marker bsr. Plasmid, 30, 150–154. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P.J.M. (1984) Guanine nucleotides modulate cell surface cAMP-binding sites in membranes from Dictyostelium discoideum. Biochem. Biophys. Res. Commun., 124, 597–604. [DOI] [PubMed] [Google Scholar]
- Van Haastert P.J.M. and Kuwayama,H. (1997) cGMP as second messenger during Dictyostelium chemotaxis. FEBS Lett., 410, 25–28. [DOI] [PubMed] [Google Scholar]
- Van Haastert P.J.M., De Wit,R.J., Janssens,P.M., Kesbeke,F. and DeGoede,J. (1986) G-protein-mediated interconversions of cell-surface cAMP receptors and their involvement in excitation and desensitization of guanylate cyclase in Dictyostelium discoideum. J. Biol. Chem., 261, 6904–6911. [PubMed] [Google Scholar]
- Wu L., Valkema,R., Van Haastert,P.J. and Devreotes,P.N. (1995) The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J. Cell Biol., 129, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Long,Y. and Devreotes,P.N. (2001) Ggamma in Dictyostelium: its role in localisation of gbetagamma to the membrane is required for chemotaxis in shallow gradients. Mol. Biol. Cell, 12, 3204–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]